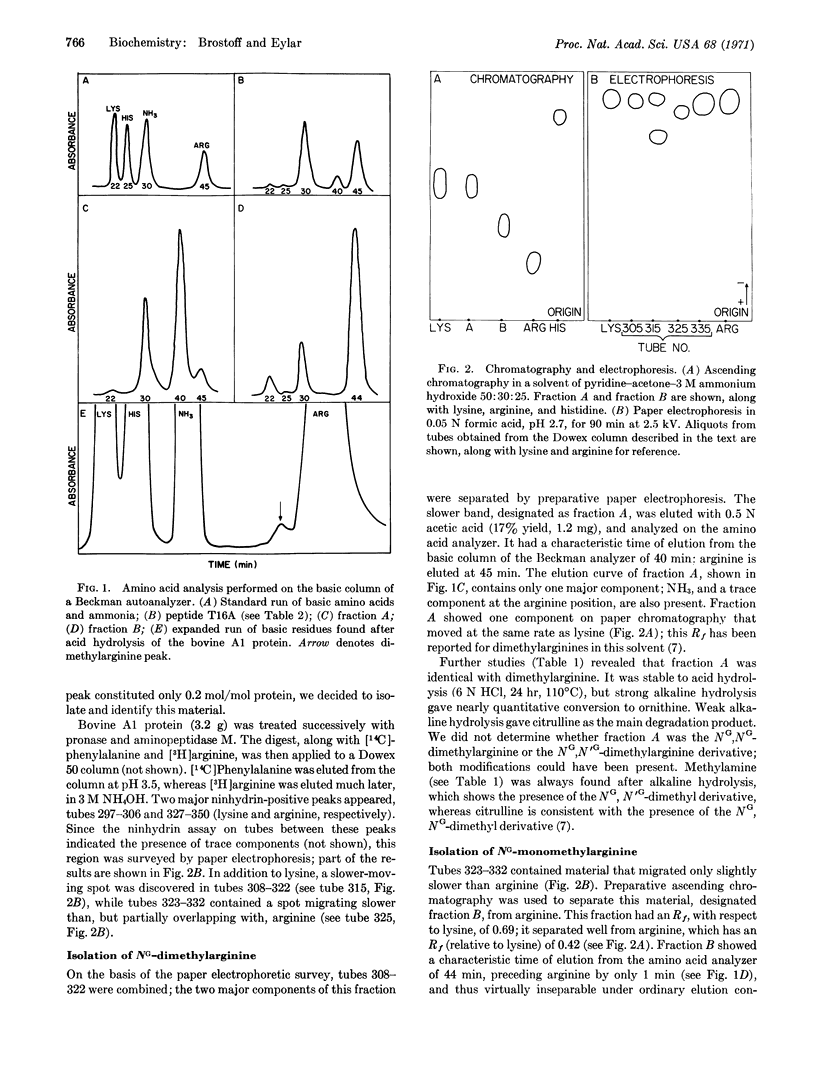
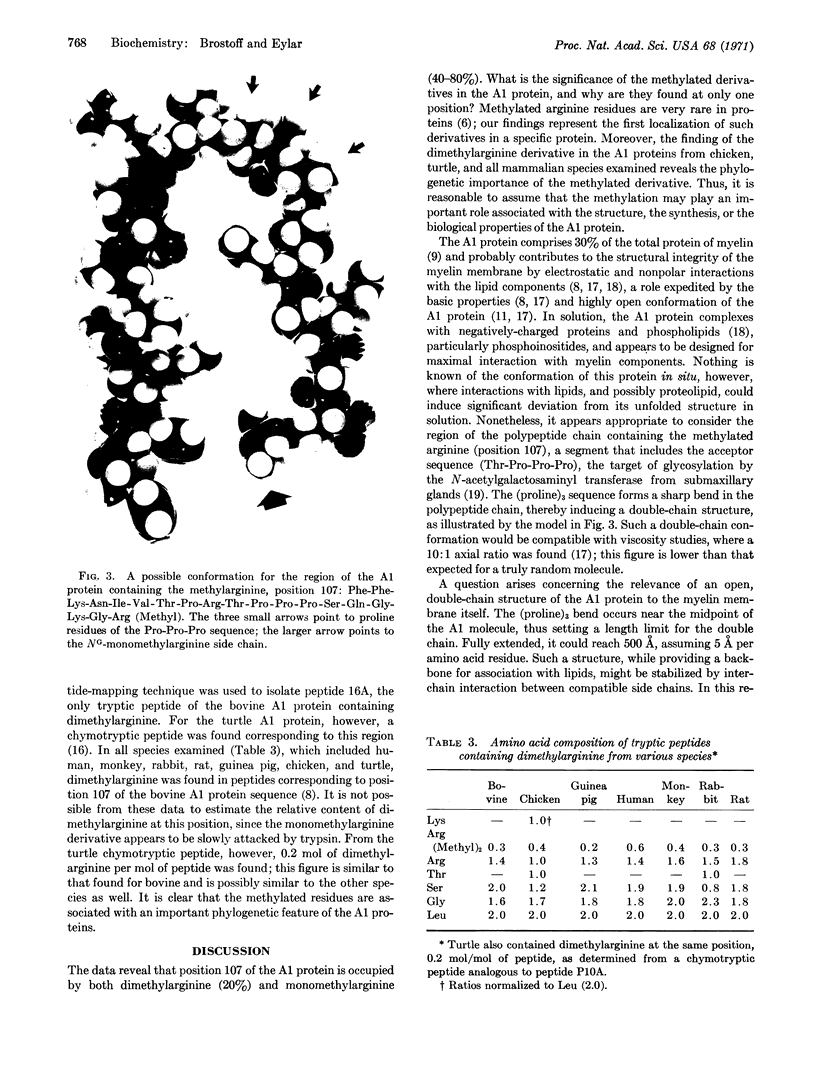
Abstract
Methylated arginine residues are found at only one site (position 107) of the polypeptide chain of the A1 protein, as shown by analysis of tryptic and peptic peptides; these analyses show 0.2 mole of NG-dimethylarginine and 0.4-0.8 mole of NG-monomethylarginine per mole of A1 protein. The methylated arginine residues appeared to be relatively resistant to tryptic attack. Both methylated derivatives were isolated from an enzymatic digest of the A1 protein; they were identified by chromatography, electrophoresis, and degradation to citrulline, methylamine, and ornithine on alkaline hydrolysis. The phylogenetic importance of the methylated derivatives was shown by their presence in the human, monkey, bovine, rabbit, guinea pig, rat, chicken, and turtle A1 proteins at the analogous position to that of the bovine sequence: [Formula: see text] We postulate that the methylated arginine residues may serve an important role in the myelin membrane in situ by stabilization of a double-chain structure for the A1 protein; such a double-chain conformation is induced by a (proline)3 sequence located nearby.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P., REES M. W. Epsilon-N-Methyl-lysine in bacterial flagellar protein. Nature. 1959 Jul 4;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- Asatoor A. M., Armstrong M. D. 3-methylhistidine, a component of actin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):168–174. doi: 10.1016/0006-291x(67)90229-x. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem. 1969 Jan 25;244(2):319–334. [PubMed] [Google Scholar]
- Eylar E. H. Amino acid sequence of the basic protein of the myelin membrane. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1425–1431. doi: 10.1073/pnas.67.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar E. H., Salk J., Beveridge G. C., Brown L. V. Experimental allergic encephalomyelitis. An encephalitogenic basic protein from bovine myelin. Arch Biochem Biophys. 1969 Jun;132(1):34–48. doi: 10.1016/0003-9861(69)90336-1. [DOI] [PubMed] [Google Scholar]
- Eylar E. H., Thompson M. Allergic encephalomyelitis: the physico-chemical properities of the basic protein encephalitogen from bovine spinal cord. Arch Biochem Biophys. 1969 Feb;129(2):468–479. doi: 10.1016/0003-9861(69)90204-5. [DOI] [PubMed] [Google Scholar]
- Hashim G. A., Eylar E. H. Allergic encephalomyelitis: enzymatic degradation of the encephalitogenic basic protein from bovine spinal cord. Arch Biochem Biophys. 1969 Feb;129(2):635–644. doi: 10.1016/0003-9861(69)90224-0. [DOI] [PubMed] [Google Scholar]
- Hashim G. A., Eylar E. H. The structure of the terminal regions of the encephalitogenic basic protein from bovine myelin. Arch Biochem Biophys. 1969 Dec;135(1):324–333. doi: 10.1016/0003-9861(69)90546-3. [DOI] [PubMed] [Google Scholar]
- Kakimoto Y., Akazawa S. Isolation and identification of N-G,N-G- and N-G,N'-G-dimethyl-arginine, N-epsilon-mono-, di-, and trimethyllysine, and glucosylgalactosyl- and galactosyl-delta-hydroxylysine from human urine. J Biol Chem. 1970 Nov 10;245(21):5751–5758. [PubMed] [Google Scholar]
- Kaye A. M., Sheratzky D. Methylation of protein (histone) in vitro: enzymic activity from the soluble fraction of rat organs. Biochim Biophys Acta. 1969 Oct 22;190(2):527–538. doi: 10.1016/0005-2787(69)90101-4. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., Eylar E. H. Allergic encephalomyelitis: a comparison of the encephalitogenic A1 protein from human and bovine brain. Arch Biochem Biophys. 1970 Jun;138(2):606–613. doi: 10.1016/0003-9861(70)90387-5. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., Eylar E. H. Allergic encephalomyelitis: preparation of the encephalitogenic basic protein from bovine brain. Arch Biochem Biophys. 1970 Jun;138(2):392–396. doi: 10.1016/0003-9861(70)90361-9. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Omega-N-methylarginine in histones. Biochem Biophys Res Commun. 1970 Jul 13;40(1):224–229. doi: 10.1016/0006-291x(70)91070-3. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylation in rat brain in vitro. J Neurochem. 1969 Aug;16(8):1257–1261. doi: 10.1111/j.1471-4159.1969.tb05974.x. [DOI] [PubMed] [Google Scholar]
- Palmer F. B., Dawson R. M. The isolation and properties of experimental allergic encephalitogenic protein. Biochem J. 1969 Mar;111(5):629–636. doi: 10.1042/bj1110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]