Abstract
Background and Aims
Interest in pollinator-mediated evolutionary divergence of flower phenotype and speciation in plants has been at the core of plant evolutionary studies since Darwin. Specialized pollination is predicted to lead to reproductive isolation and promote speciation among sympatric species by promoting partitioning of (1) the species of pollinators used, (2) when pollinators are used, or (3) the sites of pollen placement. Here this last mechanism is investigated by observing the pollination accuracy of sympatric Pedicularis species (Orobanchacae).
Methods
Pollinator behaviour was observed on three species of Pedicularis (P. densispica, P. tricolor and P. dichotoma) in the Hengduan Mountains, south-west China. Using fluorescent powder and dyed pollen, the accuracy was assessed of stigma contact with, and pollen deposition on, pollinating bumble-bees, respectively.
Key Results
All three species of Pedicularis were pollinated by bumble-bees. It was found that the adaptive accuracy of female function was much higher than that of male function in all three flower species. Although peak pollen deposition corresponded to the optimal location on the pollinator (i.e. the site of stigma contact) for each species, substantial amounts of pollen were scattered over much of the bees' bodies.
Conclusions
The Pedicularis species studied in the eastern Himalayan region did not conform with Grant's ‘Pedicularis Model’ of mechanical reproductive isolation. The specialized flowers of this diverse group of plants seem unlikely to have increased the potential for reproductive isolation or influenced rates of speciation. It is suggested instead that the extreme species richness of the Pedicularis clade was generated in other ways and that specialized flowers and substantial pollination accuracy evolved as a response to selection generated by the diversity of co-occurring congeners.
Keywords: Adaptive accuracy, Bombus, evolution, floral precision, Pedicularis densispica, P. tricolor, P. dichotoma, pollination, reproductive isolation, specialization, speciation
INTRODUCTION
There has been a resurgence in interest in recent years on the causal links between specialized mating systems, reproductive isolation and speciation in plants and animals (e.g. Coyne and Orr, 2004; Johnson, 2006; Kay and Sargent, 2009; Nosil, 2012). In plants, specialized pollination systems have fascinated botanists since Sprengel (1793) and Darwin (1859, 1876, 1877) pointed out the role of flowers in attracting and manipulating pollinators. This led to a later appreciation of floral specialization as a potential mechanism of reproductive isolation between related species (Grant, 1949). Interest in pollinator-driven divergence and speciation in plants has been at the core of plant evolutionary studies ever since (e.g. Stebbins, 1950, 1970, 1974; Baker, 1960; Grant, 1971, and references below).
At least three divergent hypotheses concerning the link between pollination and speciation in plants have emerged. These include (1) pollinator-related reproductive isolation driving speciation (e.g. ethological and mechanical isolation of Grant 1949, 1971, 1994; see Schemske and Bradshaw, 1999; Bradshaw and Schemske, 2003; Ramsey et al., 2003; Kay and Sargent, 2009); (2) allopatric adaptation to new pollinator environments, with isolation as a by-product of floral divergence (Stebbins, 1970; Johnson, 2006, 2010); and (3) allopatric divergence in non-floral traits, with divergence from congeners in pollinators evolving though reinforcement (van der Niet et al., 2006) or character displacement (Armbruster et al., 1994) upon sympatry. Because all of these processes can generate strong associations between speciation and pollinator shifts, it is challenging to distinguish between them using historical and comparative approaches (Johnson, 2006; Armbruster and Muchhala, 2009).
Another approach to evaluating the link between specialized pollination and speciation is to investigate mechanical aspects of pollination, namely processes of pollinator attraction (Ramsey et al., 2003), pollen placement on pollinators and pollen retrieval by stigmas. Assessing the dynamics of pollen movement within and among species can yield new insights into the functional consequences of floral specialization (e.g. Armbruster et al., 2009; Muchhala et al., 2010; Muchhala and Thomson, 2012) and help establish whether or not interactions with pollinators contribute to initial or secondary reproductive isolation (Johnson, 2006).
Grant (1949, 1971) and Stebbins (1950, p. 210–213; but see contrasting view in Stebbins 1974, p. 11–13) emphasized the potential for reproductive isolation through divergence in pollinator use (‘floral isolation’). Verne Grant distinguished between two mechanisms: (1) ethological isolation (visitation by different pollinator species or individuals–the latter through constancy; Waser, 1986) and (2) mechanical isolation, relating to the fit of flowers and pollinators. Grant (1994a) recognized two types of mechanical isolation: the ‘Salvia type’, where the form of the flower either precludes access to the reward by some flower visitors or creates a mismatch in fit such that anthers and stigmas fail to contact the ‘wrong’ flower visitors, and the ‘Pedicularis type’, where the form of the flower results in pollen being placed in locations on shared pollinators different from other congeneric species. In this study we assess the likelihood that mechanical isolation of the ‘Pedicularis type’ plays a role in speciation by focusing on the mechanics of pollination of sympatric species of Pedicularis (Orobanchaceae) in the eastern Himalayan region.
Floral specialization and potential reproductive isolation
Some floral specializations lead to potential reproductive isolation but others do not. At least three kinds of floral specialization can be recognized and categorized as: ‘who’ (including ‘what’ and ‘why’), ‘when’ and ‘where’.
Specialization on which species of animals are attracted and used as pollinators (‘who’) occurs through ‘what’ reward and advertisements are produced and thus ‘why’ pollinators visit. Additional specialization comes through restricting access to rewards (e.g. nectar tubes and spurs; see Newman et al., 2014) and size restrictions on pollinators as determined by the size and shape of flowers. This can lead to reproductive isolation (RI) if the difference in pollinators is a qualitative one, i.e. with a binary function. For example, some euglossine-bee-pollinated plants attract only certain bees with specific chemicals (Dressler, 1968; Armbruster et al., 1992; Hentrich et al., 2010). Similarly, specialized pollination by sexual deception is based on specific pheromone chemistry, and small chemical changes in the pheromone mimic can change which insect species visit (Schiestl and Ayasse, 2002; Peakall and Whitehead, 2014).
Specialization in ‘when’ pollination occurs can be manifested through differences in either the season or the time of day that pollination can occur. For example, the blossoms of most Dalechampia species (Euphorbiaceae) are open for pollination most of the day. However, some species are more specialized in that they open only approx. 3 h per day, and one species opens only approx. 1·5 h per day. Such species attract only a small sub-set of the individual (and species of) resin-collecting bees that might otherwise visit (Armbruster, 2006). There are many examples of related species blooming at different times of the year (e.g. Stiles, 1975; Lennartsson, 1997). Seasonal (phenological) divergence can lead to complete reproductive isolation if there is no overlap whatsoever in flowering season. It is more difficult to model isolation through divergence in time of day of pollination because of pollen carryover within and between days (see Stone et al., 1998).
Specialization in ‘where’ pollen is placed on, and stigmas contact, pollinators is a common route of specialization. This was recognized long ago, as reflected in the old pollination-ecological terms ‘nototribic’ and ‘sternotribic’, meaning placement (and pick up) of pollen on the back vs. the ventral side of the pollinator, respectively (Faegri and van der Pijl, 1971; Grant, 1994b). Many other examples of specificity in where pollen is placed on pollinators are seen in the Orchidaceae (e.g. Dressler, 1968). Spatial isolation of pollen and stigma contact on shared pollinators may (or may not) lead to RI. The likelihood of RI is sensitive to the realized precision of pollen placement on and stigma contact with pollinators (see below).
Floral precision in the context of adaptive accuracy
There is considerable published evidence supporting an association between clade species richness and specialized animal pollination (e.g. Dodd et al., 2000; Sargent, 2003; Kay and Sargent, 2009), although the causal mechanism is still not clear (Armbruster and Muchhala, 2009). It is clear, however, that whether or not populations can diverge (ecotypic differentiation or speciation) and avoid introgression upon sympatry depends on the degree of reproductive isolation and, hence, in the context of flowers, just how precise pollen placement on pollinators is.
To understand the proposed connection between floral specialization and reproductive isolation, however, we must assess the ability of specialization to segregate gene flow. This leads to a consideration of pollination precision and accuracy.
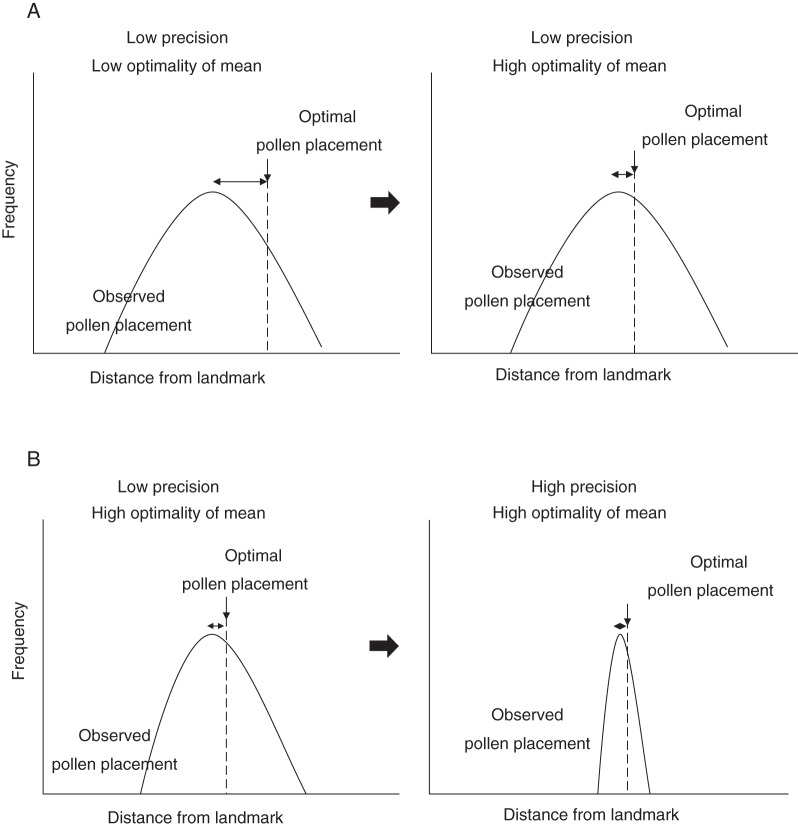
Adaptive accuracy estimates the phenotypic load (maladaptation) that results from morphological departure from the optimum in a population. At the level of the population there are at least two components, which are additive (Armbruster et al., 2004; Hansen et al., 2006): (1) optimality of the mean (= bias), which is how far the mean of events departs from the optimum; and (2) the variance, which is how much individuals vary from the mean (= precision; Fig. 1). [There is a third possible component, the variance in the adaptive target (Armbruster et al., 2009), but we omit this component in the analyses that follow in order to maintain independence of the male and female accuracy estimates.] By extrapolation from measurement theory (Armbruster et al., 2004), these two components sum to the adaptive inaccuracy as:
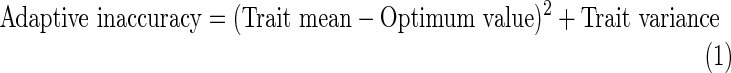 |
(1) |
Fig. 1.
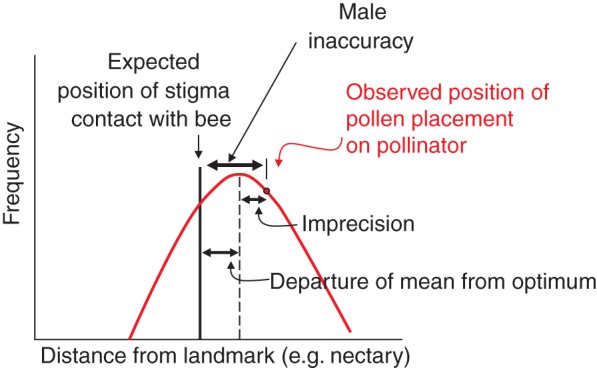
Adaptive inaccuracy of male floral function, as used here, comprises imprecision of pollen placement (variance) and departure of the population mean from the optimum (expected location of stigma contact).
Although natural selection only ‘sees’ relative adaptive inaccuracy of flowers (a component of reproductive fitness; Armbruster et al., 2004, 2009), genetic response to phenotypic selection may occur through reducing the departure from the optimum, increasing the precision, or both (Fig. 2).
Fig. 2.
Response to selection for accuracy can occur through one or both of (A) an increase in optimality (reduced bias) or (B) an increase in precision.
Two new aspects of adaptive accuracy in the context of pollination are introduced and used here. We wish to distinguish between fundamental accuracy and precision and realized accuracy and precision, by analogy to niche concepts. Fundamental pollination accuracy relates to measurements of optimality, precision and accuracy taken from the flower itself. This is only a predictor of the actual accuracy in play ecologically, the realized pollination accuracy. The latter is typically measured on the pollinator itself and reflects the pollinators' behaviour and interaction with the flower (e.g. variation in approach), as well as the effects of florivores, and the distribution and redistribution of pollen on the pollinator. The realized precision of pollination is nearly always lower than the fundamental precision, and therefore the same is likely to be the case for the accuracies.
Floral specialization in relation to the Grant Model of reproductive isolation
The three kinds of floral specialization lead potentially to different kinds of reproductive isolation. Specialization on different species of pollinator may lead to what Grant (1949) termed ethological isolation. If this is manifested through floral ‘fit’ with pollinators, it would be mechanical isolation of the ‘Salvia type’ (Grant, 1994a). Different times of anthesis (flowering) lead potentially to ethological isolation, while placing and picking up pollen on different specific locations on a pollinator leads to mechanical isolation of the ‘Pedicularis type’ (Grant, 1994a). The present study assesses the potential for ‘Pedicularis type’ isolation in sympatric Pedicularis.
The first question to ask is: how precise must pollination be to promote mechanical reproductive isolation of the ‘Pedicularis type’? Grant was clearly of the opinion that pollination was commonly precise enough to provide genetically meaningful isolation. For example, he described pollen deposition and stigma contact on the dorsal surface of the bees' thorax/abdomen (nototriby) vs. on the anterior face of the bees' head by sympatric Pedicularis groenlandica and P. attollans, respectively; he indicated that this difference contributed importantly to reproductive isolation in the Sierra Nevada, California (Grant, 1994b). Interestingly, Ledyard Stebbins in his later years (1974, p. 11 ff.) thought that this kind of difference was very unlikely to lead to reproductive isolation:
‘reproductive isolation … [permitting] … related populations to occupy the same habitat without … [introgression] … cannot be achieved unless the isolation is virtually complete … [and this] … is accomplished only by means of … mechanisms based on internal divergence in the genotypes themselves, particularly those that lead to hybrid inviability or sterility.’
This perspective follows from population genetic theory that assesses the degree of gene flow needed to swamp genetic drift and weak selection. Derivations from Sewall Wight's FST equations (Wright, 1951) show that two populations or morphs will be strongly linked by gene flow and function essentially as a single panmictic population if 4Nm > 1, where N is the population size, and m the fraction of the population mating with other populations per generation (Roughgarden, 1979; Nunney, 2001). Nm is then the number of interpopulation matings per generation, only one of which results in 4Nm >> 1. This means that roughly one intermorph pollination per generation will swamp out differentiation under all but very strong divergent/disruptive selection and that this is true regardless of population size. Therefore, the precision of realized pollen location on pollinators (‘realized precision’) must be very high for two populations/species to use the same individual pollinators and remain reproductively isolated, as argued by Stebbins. The above theoretical issues underscore the importance of understanding the detailed mechanics and precision of pollen placement and retrieval from pollinators, in particular the relationship between the fundamental precision (based on floral structure) and the realized precision (based on flower–pollinator interaction) of pollination.
The above review shows the importance of detailed assessment of the potential for even small amounts of interspecific (/intermorph) pollen flow in studies of floral specialization, ecotypic differentiation and incipient speciation. The goal of our empirical presentation below is to assess the likelihood that reproductive isolation could be maintained by specialized flowers with precise pollinator fit (i.e. potential mechanical isolation), using Pedicularis, a group of plants cited by Grant (1994a) and others (e.g. Sprague, 1962; Macior, 1983) as a model of how mechanical isolation works. We use measures of adaptive accuracy and precision of pollen placement and stigma contact to investigate the dynamics of pollen deposition and pick up by three sympatric species of Pedicularis in the easternmost Himalayan region.
MATERIALS AND METHODS
Study system
Pedicularis (Orobanchaceae) comprises approx. 500 species, with 300+ in the study area (Eastern Himalayan region). Pollination in Asia is largely by bumble-bees, with some species providing nectar and others only pollen rewards. Floral tubes range from moderately short to very long (approx. 5–130 mm). Nectar reward species generally have fairly short floral tubes, 5–10 mm long. Pollen reward (nectarless) species often have longer corolla tubes. They attract pollen-collecting bumble-bees that vibrate the flowers (‘buzz pollination’). Commonly 3–8 species co-flower sympatrically in the Himalayan region. Interspecific pollinator movements occur infrequently, and there is usually some degree of constancy. Primarily pollen-collecting bumble-bees were observed moving between Pedicularis species more commonly than primarily nectar-collecting bees.
Pedicularis flowers are characterized by a tubular corolla base and bilabiate corolla limb with a trilobate lower lip and a galeate upper lip containing the style and four introrse anthers. The punctiform stigma and tip of the style project slightly beyond the upper lip. The upper lip (galea) is formed by the fusion of two upper petals; it can be straight and hood-like (e.g. P. densispica; Fig 1A) or elongated into a curved, tubular ‘beak’ (e.g. P. tricolor, Fig. 1B; P. dichotoma, Fig.1C). The pollen exits the flower through the tip of this beak in buzz-pollinated species (Huang and Shi, 2013).
We studied three sympatric species in montane meadows at the Shangri-La Alpine Botanical Garden field station (27 °54'5''N, 99 °38'17''E, 3300–3350 m a.s.l.), Yunnan Province, south-western China, July–August 2010. These species represent the three major flower types in Pedicularis (see Li, 1951; Ree, 2005): a nectar-producing, short-tubed, beakless species (P. densispica Franchet ex Maximowicz); a nectarless, short-tubed, beaked species (P. dichotoma Bonati); and a nectarless, long-tubed, beaked species (P. tricolor Handel-Mazzetti). There were many hundreds of individuals of the three species in the study area, and their floral phenology overlapped from late July to early August. Pollination was effected primarily by Bombus friseanus Skorikov (previously referred to as B. richardsi; e.g. Huang and Shi, 2013) and secondarily by B. festivus Smith (Fig. 2) obtaining nectar from P. densispica or pollen from all three species. Bee names follow Williams et al. (2009). Plant names follow the online Flora of China (http://www.efloras.org). Additional details about the study species, study sites and methods are reported in Huang and Shi (2013).
Field methods
Pollinator movements in naturally sympatric populations of P. denspica and P. dichotoma were observed at the Shangri-La Alpine Botanical Garden field station on 8–12 August 2012 in order to assess the frequency of interspecific movement by individual bees.
The placement of pollen on bumble-bees visiting P. densispica, P. tricolor and P. dichotoma was studied at the same field site in July–August 2010. The location of pollen placed on bumble-bees was assessed in two ways: (1) by examining the distribution of dyed pollen in the flower; and (2) by examining the distribution of all pollen on bees visiting the target species. The first method suffers from limited representation on the bees, while the second suffers from the problem that we cannot distinguish the pollen of the three Pedicularis species. However, it was clear from detailed observations of the bees visiting that nearly all the pollen on captured bees was that of the Pedicularis species being analysed (S.-Q. Huang and X.O. Shi, unpubl. obs.). For these reasons we report both results. For the first method, we stained the pollen grains in dehisced anthers with 1 % safranin dye (see Huang and Guo, 1999). We stained pollen in the early morning in about 100 flowers of one species in a patch under a mesh tent (2 × 2 m). After allowing the flowers to dry for 0·5 h, we removed the tent and observed pollinator visitation to the patch. After bees had visited several pollen-stained flowers (usually 2–10 flowers), we captured and killed them instantly using two electric mosquito ‘rackets’. We repeated the pollen staining experiments with P. densispica, P. tricolor and P. dichotoma on three, five and four sunny days, respectively. We collected 12, 23 and 15 visiting bumble-bees from each species, respectively. The bumble-bee body was divided into 11 sectors (see the Results), and pollen grains from each sector were removed with gelatin cubes (not containing safranin dye; Kearns and Inouye, 1993) and then transferred to a clean slide. The slides were warmed gently to melt the jelly. We counted all pollen grains (both stained and unstained) from each part under a microscope.
The site of stigma contact was determined by placing fluorescent powder on the stigmas of labelled flowers and examining bees after they visited. We placed fluorescent powder on the stigmas in an experimental population in the early morning, then examined powder placement on the bumble-bee body. We placed 2 × 2 m mesh tents over random patches of each Pedicularis species and then gently coated the stigmas of 100 flowers with fluorescent powder. The powder was mixed with a little water, and was repeatedly added to the stigmas. We removed the tent after 0·5 h and observed pollinator visitation in the patch. The bumble-bee pollinators were collected as in the pollen placement experiment. For each species, the experiment was replicated three times on separate clear days, with only one species studied per day. At the end of each experimental period, all powder-bearing flowers were collected and disposed of. We took photos of the bumble-bees under UV light in order to record the parts of the bumble-bee body bearing fluorescent powder.
Statistical methods
Adaptive inaccuracies were calculated using eqn (1), as further described in Armbruster et al. (2009), using an ordinal distance index for each pollen grain in each sector (e.g. top of head = 1·0, top of thorax = 2·0, etc.) to indicate the approximate distance of each pollen grain from a selected landmark, the bee's clypeus. We calculated adaptive inaccuracies and imprecision from both the dyed pollen and the total pollen detected in P. densispica, but only the latter in P. tricolor and P. dichotoma due to low counts of dyed pollen in these two species (see the Results). We compared these metrics against equiprobable expectations by calculating inaccuracies of a simulated data set with equal proportions of pollen placed on each geographic sector on the bee. Departure from uniform equiprobablity was tested using Kolmogorov–Smirnov two-tailed test for equality of distributions. We analysed dorsal pollen placement and ventral pollen placement separately, because of ambiguities in how to combine the two in calculating distances from the landmark.
RESULTS
Pedicularis accuracy
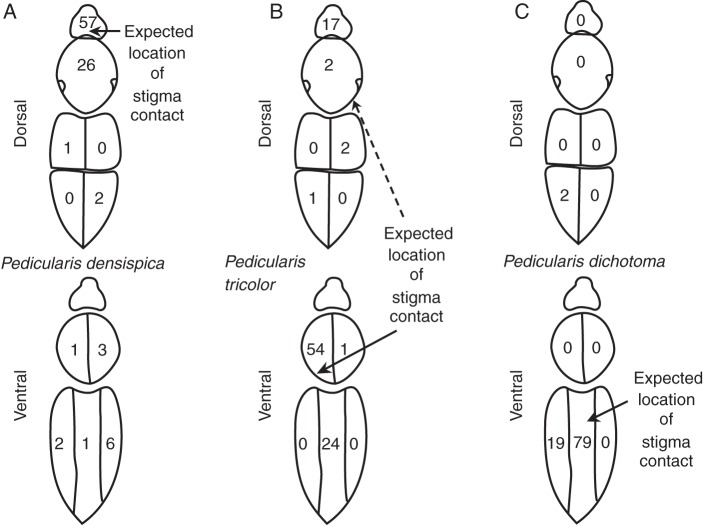
In P. densispica, a species with mostly dorsal stigma contact, 13·4 % of the dyed pollen on 16 bees visiting treatment flowers was on the ventral side of the bee, opposite the site of stigma contact (hereafter the ‘wrong’ side; Fig. 5A), and 86·6 % of the dyed pollen was on the ‘correct’ side. Of all counted pollen on the bees (almost all of which was expected to have been from P. densispica), 39·3 % of the pollen was on the wrong side (Fig. 6A).
Fig. 5.
Distribution of safranin-dyed pollen on bumble bees, as a percentage in each sector of total pollen grains. (A) Pedicularis densispica, n = 16 bumble-bees, 17 650 pollen grains. (B) Pedicularis tricolor, n = 23 bumble-bees, 1169 pollen grains. (C) Pedicularis dichotoma, n = 15 bumble-bees, 1606 pollen grains.
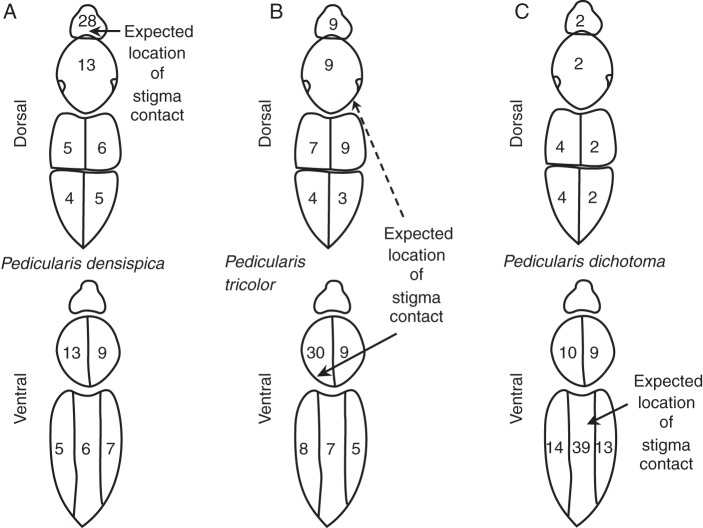
Fig. 6.
Distribution of all (dyed + undyed) pollen on bumble bees as a percentage in each sector of total pollen grains. (A) Pedicularis densispica, n = 16 bumble-bees, 73 354 pollen grains. (B) Pedicularis tricolor, n = 23 bumble-bees, 62 069 pollen grains. (C) Pedicularis dichotoma, n = 15 bumble-bees, 37 508 pollen grains.
In P. tricolor, assuming ventral stigma contact, 22·8 % of the dyed pollen on 23 bees visiting treatment flowers was on the wrong side (Fig. 5B). Of all counted pollen on the bees (almost all of which was expected to have been from P. tricolor), 40·6 % of the pollen was on the wrong side (Fig. 6B). Assuming dorsal stigma contact, 77·2 and 59·4 % of the dyed and total pollen, respectively, was on the wrong side.
In P. dichotoma, a species with ventral stigma contact, 2·2 % of the dyed pollen on 15 bees visiting treatment flowers was on the ‘wrong’ side (Fig. 5C). Of all counted pollen on the bees (almost all of which was expected to have been from P. dichotoma), 16·4 % of the pollen was on the wrong side (Fig. 6C). Thus all three species show a large degree of qualitative realized inaccuracy in where pollen is placed.
To gain a more detailed insight into the precision of pollen placement, quantitative inaccuracy was calculated from the dyed pollen distribution data from the ‘correct’ side of the body of bees visiting P. densispica. Mean2-scaled realized adaptive inaccuracy of P. densispica pollen placement on the dorsal side of Bombus spp. was 0·258, of which most was caused by imprecision in final pollen location on bees (Table 1). The scaled inaccuracy was about half (48 %) that of an equiprobable distribution, suggesting some adaptive improvement over ‘random’ pollen placement (the observed pollen distribution deviated significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·465, n = 17 650, P < 0·001).
Table 1.
Realized inaccuracy values calculated from locations of pollen deposition on 12, 23 and 15 bumble-bees visiting Pedicularis densispica, P. tricolor and P. dichotoma, respectively (pollen deposition inaccuracy) and stigma contact tallied from photographs of fluorescent powder on bees and bees on flowers
| Pedicularis species | Fitness indicator measured | Mean2-scaled inaccuracy | Proportion of inaccuracy due to deviation from optimum (%) | Proportion of inaccuracy due to imprecision (%) |
|---|---|---|---|---|
| P. densispica | Dyed pollen | 0·258 | 17·9 | 82·1 |
| Total pollen | 0·367 | 31·9 | 68·1 | |
| Stigma contact position | 0·156 | 48·0 | 52·0 | |
| P. tricolor | Dyed pollen | – | ||
| Total pollen | 0·159 | 35·2 | 64·8 | |
| Stigma contact position | 0·153 | 62·3 | 37·7 | |
| P. dichotoma | Dyed pollen | – | ||
| Total pollen | 0·068 | 16·9 | 83·1 | |
| Stigma contact position | 0·016 | 57·2 | 42·8 |
Looking at all pollen on the bee's bodies (larger sample size, with small but non-zero risk of including foreign pollen), we calculated a similar mean-scaled inaccuracy (0·367), of which most was due to imprecision (Table 1). The scaled inaccuracy was 68 % of that of an equiprobable distribution, suggesting limited adaptive improvement over ‘random’ pollen placement (but the observed pollen distribution did deviate significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·209, n = 73 354, P < 0·001).
Stigmas were more accurate than stamens as a result of higher precision in contacting pollinators. The mean2-scaled adaptive inaccuracy of P. densispica stigma contact (on the dorsal side of Bombus spp.) was 0·156, of which about half was due to imprecision (Table 1). The scaled inaccuracy was about one-third (36·3 %) that of an equiprobable distribution, suggesting considerable adaptive improvement over ‘random’ (the observed stigma contact distribution deviated significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·645, n = 19, P < 0·001).
Inaccuracy for P. tricolor pollen placement was estimated only for ventrally deposited pollen. The data were too sparse to use the dyed pollen for these calculations, so the total pollen distribution was used. The mean2-scaled inaccuracy was 0·159, of which about two-thirds was due to imprecision (Table 1). The scaled inaccuracy was nearly identical to that of an equiprobable distribution, suggesting very little adaptive improvement over ‘random’ pollen placement (but the observed pollen distribution did deviate significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·175, n = 62 069, P < 0·001).
Stigmas were a little more accurate and considerably more precise in contacting pollinators than stamens. The mean2-scaled adaptive inaccuracy of P. tricolor stigma contact (on the ventral side of Bombus spp.) was 0·153, of which only one-third was due to imprecision (Table 1). The scaled inaccuracy was about one-third (36·2 %) that of an equiprobable distribution, suggesting substantial adaptive improvement over ‘random’ (the observed stigma contact distribution deviated significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·417, n = 12, P = 0·02).
Pedicularis dichotoma tended to place pollen on the underside of the bees' bodies. As for P. tricolor, only total pollen deposits were analysed for reasons of small numbers of dyed pollen. Mean2-scaled adaptive inaccuracy of total pollen placement on the ventral side of Bombus spp. was 0·068, of which well over three-quarters was due to imprecision (Table 1). The scaled inaccuracy was about half (43·2 %) that of an equiprobable distribution (the observed pollen distribution deviated significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·339, n = 37, P < 0·001). These values indicate fairly high overall accuracy but fairly poor precision in pollen placement.
Pedicularis dichotoma stigmas were much more accurate than the stamens as a result of greater precision in contacting pollinators. The mean2-scaled adaptive inaccuracy of P. densispica stigma contact (on the dorsal side of Bombus spp.) was 0·016, of which under half was due to imprecision (Table 1). The scaled inaccuracy was only 3·9 % of that of an equiprobable distribution, suggesting adaptive evolution of high stigmatic accuracy (the observed stigma position distribution deviated significantly from the uniform distribution, Kolmogorov–Smirnov t = 0·282, n = 34, P < 0·01).
Insights into speciation dynamics
The three species studied above are reasonably representative morphologically of the three floral types of Pedicularis. Indeed, other species we have observed in each of these types show similar qualitative patterns of imprecise pollen location on pollinators (Huang and Shi, 2013; S.-Q. Huang and W.S. Armbruster, unpubl. obs.; e.g. P. longiflora in Fig. 2). This suggests that mechanical isolation in Himalayan Pedicularis is unlikely to be sufficiently effective to contribute to speciation either in initial divergence or upon secondary contact. However, the ‘improvements’ over even distributions of pollen on pollinators are likely to be adaptive and reflect selection for adaptive accuracy. This is evident upon examination of the distribution of pollen in Figs 5 and 6. The sectors on pollinators' bodies most populated with pollen (both dyed and total) in all cases are the sectors of stigma contact for each species, hence close to the optimal location on the pollinator. Thus the modal pollen location is essentially optimal, despite some deviation of the mean from the optimum and the low precision. This appears to show the signature of natural selection promoting mate choice (getting most pollen to the right stigmas and mostly conspecific pollen onto the stigmas). However, the low precision means that differential pollen placement is unlikely to generate reproductive isolation.
Another expectation of the Grant Model of mechanical isolation is that speciation occurs by initial reproductive isolation in close proximity (sympatry or parapatry) or that speciation is completed by reinforcement of reproductive isolation (by floral divergence) upon secondary sympatry. If these processes have operated in Himalayan Pedicularis, we expect to find sister species often co-occurring, or at least occurring in geographically close regions or nearby habitats. Instead, we almost always observe very dissimilar species (presumably unrelated) co-occuring. The nine species at our study site (one nectar-producing, short-tubed, beakless species, P. densispica; four nectarless, short-tubed, beaked species, P. dichotoma, P. monbeigiana, P. confertifolia and P. rhinanthoides; and four nectarless, long-tubed, beaked species, P. tricolor, P. longiflora, P. siphonantha and P. cephalantha) represent an evenly distributed sample of the most recently published phylogeny, with no two sympatric species being sisters or drawn from the same terminal clade (see fig. 3 in Eaton et al., 2012). Eaton et al. (2012, p. S188), used a quantitative analysis and came to the same conclusion about species of Pedicularis in the broader Sino-Himalayan region: ‘ … indices of phylogenetic community structure did not deviate significantly from zero, … meaning that patterns of relatedness within sites cannot be distinguished from random assembly.’
Fig. 3.
Study species of Pedicularis. (A) P. densispica Franchet ex Maximowicz, (B) P. tricolor Handel-Mazzetti, and (C) P. dichotoma Bonati.
Fig. 4.
Bumble-bee pollinators obtaining nectar or pollen from Pedicularis. (A) Bombus friseanus Skorikov obtaining nectar from P. densispica. Note pollen deposited ‘correctly’ on top of the head. (B) Bombus festivus Smith collecting pollen from P. cephalantha Franchet ex Maximowicz. (C) Bombus friseanus collecting pollen from P. longiflora Rudolph. Note the imprecise distribution of pollen grains across the ventral side of the thorax and abdomen.
Taken together, these observations suggest that sympatric or parapatric speciation by adopting new pollinators or divergent mechanical pollination mechanisms (e.g. different sites of pollen placement) has not played a major role in diversification. Although the three species intensively studied here do not overlap in position of stigma contact or peak pollen deposition, this fact alone does not generate reproductive isolation because of the large imprecision (variance), especially in pollen placement.
DISCUSSION
Pollen placement on pollinators was surprisingly imprecise in the three species studied in detail, although the sector of maximum pollen deposition corresponded to the site of expected stigma contact in all cases (Figs 5 and 6). Interestingly, in these three species of Pedicularis, flowers are more precise and accurate in female function (collecting pollen from pollinators) than in male function (placing pollen on pollinators). This is probably a result of the tendency for the comparatively dry pollen of buzz-pollinated species to move considerable distances during sonication and grooming. Our interpretation that pollen deposition is imprecise was drawn from the patterns of distribution of both the total pollen and the stained pollen. There was a risk of underestimating realized male precision from total pollen counts because some heterospecific pollen might have been included in the total loads. However, we came to qualitatively similar conclusions about male imprecision from the stained pollen, the origin of which was known to be conspecific.
That the tips of the galeae of the flowers of sympatric species of Pedicularis usually contact pollinators in different locations might lead us to expect (as did Grant, e.g. 1994a, b) that the pollen of sympatric species sharing pollinators does not move between species. This inference is partly correct in that the stigmas usually collected pollen from different places on bees, with different sympatric species commonly having different ‘private safe sites’. However, the pollen collected is likely to be a mixture of species if the same bee has moved between species (which they do, although at a lower rate than within species). This is because pollen grains, especially in buzz-pollinated Pedicularis species (and most species are buzz pollinated), tend to be distributed over large areas of the bees' bodies, at least in low numbers, regardless of where the tip of the galea contacts the bee (Figs 5 and 6).
This wide dispersion of pollen clearly precludes pre-pollination reproductive isolation. Yet numerous sympatric species (up to ten co-flowering) share pollinators without hybridizing. Lack of hybridization must reflect post-pollination reproductive isolation, as Stebbins (1974) argued. It appears that reproductive isolation is instead effected largely through pollen tube discrimination in the style (Mao, 2010; S.-Q. Huang and Y.Y. Mao, unpub. data). This is probably an adaptation for avoiding interspecific fertilization and loss of ovules to abortion in sympatry (see Grant, 1966 for a similar example in Californian Gilia, Polemoniaceae). Interestingly, avoiding interspecific fertilization and ovule wastage may explain the evolution of long styles and, indirectly, long floral tubes in Himalayan Pedicularis (Mao, 2010; S.-Q. Huang and Y.Y. Mao, unpubl. data).
If floral specialization in Pedicularis is not effective in segregating pollen flow and generating reproductive isolation between related sympatric species, what has driven floral specialization in Pedicularis? As sloppy as pollen placement is, the sector with the highest amount of pollen in all cases was the optimal sector (i.e. where the stigma contacts the bee). This presumably reflects the fact that selection has favoured any improvement over an even distribution of pollen across the pollinators.
The following evolutionary scenario may hold in Pedicularis. A variety of processes of allopatric divergence, such as local adaptation to unusual parent material and soil (see van der Niet et al., 2006) or local pollinators (see Johnson, 2006, 2010), may have led to ecotypic differentiation and speciation. Given the accumulation of regional species diversity, congeners commonly occur in sympatry and either: (1) experience competitive exclusion (ecological species sorting) such that only species pre-adapted to coexistence co-occur; or (2) have undergone floral specialization and character displacement as an evolutionary response to sympatry. Because floral specialization has allowed stigma contact and peak pollen placement on parts of bees' bodies different from those used by sympatric congeners, Pedicularis species achieve targeted pollination and experience higher fitness that would be the case if they did not diverge, even though the partitioning is not clean enough to prevent all interspecific pollination completely. Thus floral specialization may be a consequence of species diversity (at least locally), not a cause of it (see Armbruster and Muchhala, 2009).
There is additional support for the scenario that ecological sorting and/or character displacement have operated in Pedicularis in our study region. Eaton et al. (2012) found that members of sympatric assemblages of co-flowering Pedicularis species in the eastern Himalayan region tended to differ more from each other in floral characters than expected by chance.
Is this a unique situation, a product of extreme diversity in a small area and special post-pollination isolating mechanisms not available to other plants? The answer appears to be ‘no’. Although orchids seem to be particularly good at achieving pre-pollination isolation in sympatry despite sharing pollinators (Dressler, 1968; but see Cozzolino et al., 2005; Cozzolino and Scopece, 2008), other plants with free, granular pollen do not seem to do so, e.g. Stylidium (Stylidiaceae), Collinsia (Plantaginaceae) and Burmeistera (Campanulaceae; Armbruster and Muchhala, 2009). As in Pedicularis, pollen flow is not perfectly segregated despite considerable floral specialization and high precision in female function (Armbruster et al., 1994, 2002; Muchhala and Thomson, 2012).
The working assumption in this analysis has been that, for reasons of pollination efficiency and avoidance of interspecific pollination, greater precision in pollen placement is adaptive. However, other scenarios are possible. For example, inaccurate pollen deposition could reduce the grooming of pollen deposited near the optimal site. This might be a transitional state en route to heteranthery, where pollen is differentiated into ‘feeder’ pollen and fertile pollen. There is no evidence yet that this has happened in Pedicularis, but it is a possibility that needs to be considered as more species are studied.
Conclusions
In summary, Pedicularis flowers are comparatively accurate, particularly in female function, but, because pollen grains are loose, pollen becomes broadly distributed on pollinators (low realized male precision). Due to this broad distribution of pollen, reproductive isolation in sympatry is unlikely to be achieved by pre-pollination mechanisms, although similarly high optimality might lead to reproductive isolation in other plants with pollen fused into pollinia, such as orchids or asclepioids. Although not by itself generating reproductive isolation or preventing hybridization in these species, high floral optimality should increase mating success and is likely to be adaptive. Thus it appears that, although pollinators have helped drive the high diversity of floral morphology in Pedicularis, they may have directly contributed very little to speciation in this genus.
ACKNOWLEDGEMENTS
We thank Q. Fang and M. Xie for help in the field, and Z.-D. Fang, Z.-L. Ma and X. Hai from Shangri-La Alpine Botanical Garden for their logistical support. This research was supported by the UK Royal Society (grants to W.S.A.) and the NSF of China (Grants NSFC nos 31030016 and 31270281 to S.Q.H).
LITERATURE CITED
- Armbruster WS. Evolutionary and ecological perspectives on specialization: from the arctic to the tropics. In: Waser N, Ollerton J, editors. Plant–pollinator interactions: From specialization to generalization. Chicago, IL: University of Chicago Press; 2006. pp. 260–282. [Google Scholar]
- Armbruster WS, Muchhala N. Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology. 2009;23:159–179. [Google Scholar]
- Armbruster WS, Herzig AL, Clausen TP. Pollination of two sympatric species of Dalechampia (Euphorbiaceae) in Suriname by male euglossine bees. American Journal of Botany. 1992;79:1374–1381. [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. Character displacement generates assemblage structure of Western Australian triggerplants (Stylidium) Ecology. 1994;75:315–329. [Google Scholar]
- Armbruster WS, Pélabon C, Hansen TF, Mulder CPH. Floral integration and modularity: distinguishing complex adaptations from genetic constraints. In: Pigliucci M, Preston KA, editors. The evolutionary biology of complex phenotypes. Oxford: Oxford University Press; 2004. pp. 23–49. [Google Scholar]
- Armbruster WS, Hansen TF, Pélabon C, Pérez-Barrales R, Maad J. The adaptive accuracy of flowers: measurement and microevolutionary patterns. Annals of Botany. 2009;103:1529–1545. doi: 10.1093/aob/mcp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HG. Reproductive methods as factors in speciation in flowering plants. Cold Spring Harbor Symposia on Quantitative Biology. 1960;24:177–191. doi: 10.1101/sqb.1959.024.01.019. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Coyne J, Orr HA. Speciation. Sunderland, MA: Sinauer Associates Ltd; 2004. [Google Scholar]
- Cozzolino S, Scopece G. Deceptive orchids: the promise of sex or food and its consequences for reproductive isolation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3037–3046. doi: 10.1098/rstb.2008.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino S, Schiestl FP, Muller A, De Castro O, Nardella AM, Widmer A. Evidence for pollinator sharing in Mediterranean nectar-mimic orchids: absence of premating barriers? Proceedings of the Royal Society B: Biological Sciences. 2005;272:1271–1278. doi: 10.1098/rspb.2005.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The origin of species. London: John Murray; 1859. [Google Scholar]
- Darwin C. The effects of cross- and self-fertilization in the vegetable kingdom. London: John Murray; 1876. [Google Scholar]
- Darwin C. The various contrivances by which orchids are fertilised by insects. Chicago: University of Chicago Press; 1877. Republished 1984. [Google Scholar]
- Dodd ME, Silvertown J, Chase MW. Phylogenetic analysis of trait evolution and species diversity variation among angiosperm families. Evolution. 2000;53:732–744. doi: 10.1111/j.1558-5646.1999.tb05367.x. [DOI] [PubMed] [Google Scholar]
- Dressler RL. Pollination by euglossine bees. Evolution. 1968;22:202–210. doi: 10.1111/j.1558-5646.1968.tb03463.x. [DOI] [PubMed] [Google Scholar]
- Eaton DAR, Fenster CB, Hereford J, Huang S-Q, Ree RH. Floral diversity and community structure in Pedicularis (Orobanchaceae) Ecology. 2012;93:S182–S194. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. London: Pergamon; 1971. [Google Scholar]
- Grant V. Pollination systems as isolating mechanisms in flowering plants. Evolution. 1949;3:82–97. doi: 10.1111/j.1558-5646.1949.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Grant V. The selective origin of incompatibility barriers in the plant genus Gilia. American Naturalist. 1966;100:99–118. [Google Scholar]
- Grant V. Plant speciation. New York: Columbia University Press; 1971. [Google Scholar]
- Grant V. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences, USA. 1994a;91:3–10. doi: 10.1073/pnas.91.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. Mechanical and ethological isolation between Pedicularis groenlandica and P. attollens (Scrophulariaceae) Biologisches Zentralblatt. 1994b;113:43–51. [Google Scholar]
- Hansen TF, Carter AJR, Pelabon C. On adaptive accuracy and precision in natural populations. American Naturalist. 2006;168:168–181. doi: 10.1086/505768. [DOI] [PubMed] [Google Scholar]
- Hentrich H, Kaiser R, Gottsberger G. Floral biology and reproductive isolation by floral scent in three sympatric aroid species in French Guiana. Plant Biology. 2010;12:587–596. doi: 10.1111/j.1438-8677.2009.00256.x. [DOI] [PubMed] [Google Scholar]
- Huang SQ, Guo YH. Measuring pollen flow in entomophilous plants by pollen grain dyeing. Acta Botanica Sinica. 1999;41:788–790. [Google Scholar]
- Huang S-Q, Shi X-Q. Floral isolation in Pedicularis: how do congeners with shared pollinators minimize reproductive interference? New Phytologist. 2013;199:858–865. doi: 10.1111/nph.12327. [DOI] [PubMed] [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 295–310. [Google Scholar]
- Johnson SD. The pollination niche and its role in the diversification and maintenance of the southern African flora. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365:499–516. doi: 10.1098/rstb.2009.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay KM, Sargent RD. The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annual Review of Ecology, Evolution, and Systematics. 2009;40:637–656. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Niwot, CO: University Press of Colorado; 1993. [Google Scholar]
- Lennartsson T. Seasonal differentiation – a conservative reproductive barrier in two grassland Gentianella (Gentianaceae) species. Plant Systematics and Evolution. 1997;208:45–69. [Google Scholar]
- Li H. Evolution in the flowers of Pedicularis. Evolution. 1951;5:158–164. [Google Scholar]
- Macior LW. The pollination dynamics of sympatric species of Pedicularis (Scrophulariaceae) American Journal of Botany. 1983;70:844–853. [Google Scholar]
- Mao Y-Y. Evolutionary adaptation of long styles in Pedicularis flowers. Wuhan University, Wuhan: China; 2010. PhD thesis. [Google Scholar]
- Muchhala N, Thomson JD. Interspecific competition in pollination systems: costs to male fitness via pollen misplacement. Functional Ecology. 2012;26:476–482. [Google Scholar]
- Muchhala N, Brown Z, Armbruster WS, Potts MD. Competition drives specialization in pollination systems through costs to male fitness. American Naturalist. 2010;176:732–743. doi: 10.1086/657049. [DOI] [PubMed] [Google Scholar]
- Newman E, Manning J, Anderson B. Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany. 2014;113 doi: 10.1093/aob/mct203. 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Niet TS, Johnson SD, Linder HP. Macroevolutionary data suggest a role for reinforcement in pollination system shifts. Evolution. 2006;60:1596–1601. doi: 10.1554/05-705.1. [DOI] [PubMed] [Google Scholar]
- Nosil P. Ecological speciation. Oxford: Oxford University Press; 2012. [Google Scholar]
- Nunney L. Population structure. In: Fox CW, Roff DA, Fairbairn DJ, editors. Evolutionary ecology. Concepts and case studies. Oxford: Oxford University Press; 2001. pp. 70–83. [Google Scholar]
- Peakall R, Whitehead MR. Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Annals of Botany. 2014;113 doi: 10.1093/aob/mct199. 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J, Bradshaw HD, Schemske DW. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae) Evolution. 2003;57:1520–1534. doi: 10.1111/j.0014-3820.2003.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Ree RH. Phylogeny and the evolution of floral diversity in Pedicularis (Orobanchaceae) International Journal of Plant Science. 2005;166:595–613. [Google Scholar]
- Roughgarden J. Theory of population genetics and evolutionary ecology: an introduction. New York: MacMillan; 1979. [Google Scholar]
- Sargent RD. Floral symmetry affects speciation rates in angiosperms. Proceedings of the Royal Society B: Biological Sciences. 2004;271:603–608. doi: 10.1098/rspb.2003.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences, USA. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M. Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Systematics and Evolution. 2002;234:111–119. [Google Scholar]
- Sprague EF. Pollination and evolution in Pedicularis (Scrophulariaceae) Aliso. 1962;5:181–209. [Google Scholar]
- Sprengel CK. Das entdeckte geheimniss der natur im bau und in der befruchtung der blumen. Berlin: Vieweg; 1793. [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York: Columbia University Press; 1950. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Stebbins GL. Plant species. Evolution above the species level. Cambridge, MA: Harvard University Press; 1974. [Google Scholar]
- Stiles FG. Ecology, flowering phenology, and hummingbird pollination of some Costa Rican Heliconia species. Ecology. 1975;56:285–301. [Google Scholar]
- Stone GN, Willmer P, Rowe JA. Partitioning of pollinators during flowering in an African Acacia community. Ecology. 1998;79:2808–2827. [Google Scholar]
- Waser NM. Flower constancy – definition, cause, and measurement. American Naturalist. 1986;127:593–603. [Google Scholar]
- Williams P, Tang Y, Yao J, Cameron S. The bumblebees of Sichuan (Hymenoptera: Apidae, Bombini) Systematics and Biodiversity. 2009;7:101–189. [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1951;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]