Abstract
Background and Aims
Floral diversification driven by shifts between pollinators has been one of the key explanations for the radiation of angiosperms. According to the Grant–Stebbins model of pollinator-driven speciation, these shifts result in morphologically distinct ‘ecotypes’ which may eventually become recognizable as species. The current circumscription of the food-deceptive southern African orchid Eulophia parviflora encompasses a highly variable monophyletic species complex. In this study, two forms were identified within this complex that differ in distribution, floral morphology, scent chemistry and phenology, and a test was made of whether these differences represent adaptations for different pollinators.
Methods and Results
Multivariate analysis of floral and vegetative traits revealed that there are at least two discrete morphological forms in the species complex. Field observations revealed that each form is pollinated by a different insect species, and thus represent distinct ecotypes. The early-flowering coastal form which has long spurs and floral scent dominated by sesquiterpene compounds is pollinated exclusively by the long-tongued bee Amegilla fallax (Apidae, Anthophorinae), while the late-flowering inland form with short spurs and floral scent dominated by benzenoid compounds is pollinated exclusively by the beetle Cyrtothyrea marginalis (Cetoniinae; Scarabaeidae). Choice experiments in a Y-maze olfactometer showed that beetles are preferentially attracted to the scent of the short-spurred form. A spur-shortening experiment showed that long spurs are required for effective pollination of the bee-pollinated form. Although it was initially thought likely that divergence occurred across a geographical pollinator gradient, plants of the long-spurred form were effectively pollinated when transplanted to an inland locality outside the natural coastal range of this form. Thus, the underlying geographical basis for the evolution of ecotypes in the E. parviflora complex remains uncertain, although early flowering in the long-spurred form to exploit the emergence of naïve bees may restrict this form to coastal areas where there is no frost that would damage flower buds. Later flowering of the short-spurred form coincides closely with the emergence of the pollinating beetles following winter frosts.
Conclusions
This study identifies a shift between bee and beetle pollination as the main driver of floral divergence in an orchid species complex. Floral scent and spur length appear to be key traits in mediating this evolutionary transition.
Keywords: Grant–Stebbins model, pollinator-driven speciation, pollination ecotypes, scent, Eulophia, Orchidaceae, phenology, beetle pollination, Cetoniinae, bee pollination, Y-maze olfactometer, Amegilla
INTRODUCTION
Adaptation to pollinators is generally considered to be the primary reason for floral diversification in plants (Soltis et al., 2005). In a conceptual model first developed by Grant and Grant (1965) and Stebbins (1970), pollinator-driven diversification begins with adaptation by plants to their most effective pollinators in a local region. Given a geographical mosaic of pollinator availability, this can result in divergence of ‘pollination ecotypes’ within a species (Ambruster, 1985; Herrera et al., 2006). Ultimately, this process could result in speciation if allopatric forms become sufficiently morphologically distinct, or when forms become reproductively isolated enough to coexist without genetic dissolution through hybridization (Johnson, 2006). The latter aspect of the model is especially appealing to adherents of the biological species concept because adaptive shifts between pollinators can have pleiotropic consequence for reproductive isolation (Fulton and Hodges, 1999; Bradshaw and Schemske, 2003). However, by selective modification of some of the most conspicuous traits of plants, pollinators are also responsible for the evolution of many of the characters that are conventionally used to diagnose species (Grant, 1949; Johnson, 1996). The Grant–Stebbins model, as it was termed by Johnson (2006), is well supported by microevolutionary studies of selection imposed by pollinators (cf. Conner, 2006; Morgan, 2006), as well as macroevolutionary studies that show links between shifts in pollination system and cladogenesis (Givnish and Sytsma, 1997; Johnson et al., 1998; Soltis et al., 2005; Larsen et al., 2008; Forest et al., 2014). However, an intermediate stage, the evolution of local pollination ecotypes within species, remains very poorly documented (Herrera et al., 2006, Johnson, 2006).
The most basic evidence for pollination ecotypes consists of correlations between floral forms and particular pollinators. The most frequently documented of these ‘trait–environment’ correlations involve ecotypes with differing flower tube length and pollinators of correspondingly variable tongue length (Robertson and Wyatt, 1990; Johnson, 1997; Johnson and Steiner, 1997; Boyd, 2004; Anderson et al., 2010; Nattero et al., 2010; Boberg et al., 2014; Newman et al., 2014; van der Niet et al., 2014). Flowering phenology is another trait that has been investigated with respect to pollinator shifts among forms within a species (Herrera et al., 2002). There is also some evidence that intraspecific variation in floral scent chemistry can be associated with different pollinators (Pellmyr, 1986; Johnson et al., 2005b; Schlumpberger and Raguso, 2008; Peakall and Whithead, 2014), but this remains poorly documented. It should also be noted that several studies have not found clear evidence that the distribution of floral forms corresponds to a geographical mosaic of pollinators (Robertson and Wyatt, 1990; Herrera et al., 2002, 2006). In some recent studies, geographical variation in floral traits has been attributed to geographical variation in the behaviour of a single pollinator (Ellis and Johnson, 2009; Newman et al., 2012).
Very few of the above-mentioned studies of pollination ecotypes include evidence that traits that characterize putative ecotypes arose through selection by pollinators. Such evidence can be derived from experiments where pollinators of one of the ecotypes are presented with an array of all the ecotypes to determine foraging preferences or pollination effectiveness (Ellis and Johnson, 2009; Newman et al., 2012). Arrays can be assembled under laboratory conditions and presented to captive insects (Galen, 1989; der Jager and Ellis, 2014) or arranged in the field by means of transplant experiments (Robertson and Wyatt, 1990; Newman et al., 2012; Sun et al., 2014) or by manipulating flowers within a population (Johnson and Steiner, 1997).
While investigating the pollination biology of Eulophia parviflora, we encountered two forms that appeared to differ in floral morphology, floral fragrance and flowering time. We hypothesized that these differences reflect adaptations to different pollinators, and thus constitute pollination ecotypes. To test this hypothesis, we tested the following predictions that emerge from it: (1) morphology, scent chemistry and flowering times would be quantitatively different between the forms; (2) suites of floral traits of each form would show integration into syndromes and be geographically structured; (3) pollinators would differ between the two forms; (4) pollinators would discriminate between the two forms when offered a choice; and (5) floral traits that differ among forms would influence the effectiveness of pollinators.
MATERIALS AND METHODS
Study species
Eulophia is a large genus of terrestrial orchid found predominantly in Africa. They have sympodal growth with sub-terranean tubers that produce a new vegetative shoot and an inflorescence from the base of the vegetative shoot each year. All taxa of Eulophia that we have examined, including the two forms described here, are deceptive and do not reward their pollinators (Peter and Johnson, 2013), although there is evidence that two pantropical species may reward their pollinators (Singer and Cocucci, 1997; Jürgens et al., 2009).
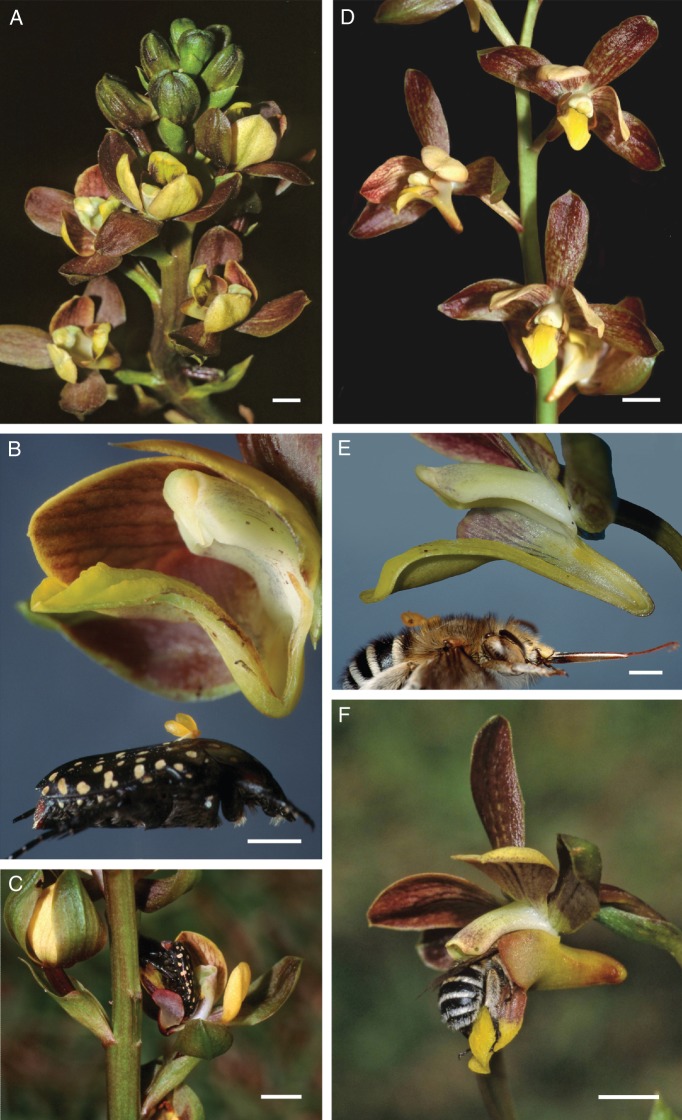
Eulophia parviflora (Fig. 1), described by Hall (1965, p. 149) as a ‘rather variable species’, occurs in grasslands of the eastern parts of South Africa (Fig. 2). While investigating the pollination biology of this species complex, we recognized two distinct forms. One form has tall dense inflorescences with large numbers of non-resupinate flowers and short spurs (Fig. 1A). This form, ‘the short-spurred form’, flowers later in the season, and has relatively well developed vegetative shoots and a distinct sweet cherry scent. In contrast, the early flowering ‘long-spurred form’ has shorter inflorescences made up of many fewer resupinating flowers with long spurs (Fig. 1D) and an attractive sweet, floral scent. At anthesis, the vegetative shoots rarely have emerged from the ground. Recent phylogenetic analyses of Eulophia revealed that both forms currently recognized as E. parviflora constitute a clade (Martos et al., 2014).
Fig. 1.
(A–F) Floral morphology and pollinators of Eulophia parviflora. (A) Short-spurred morph. (B) Dissected flower of the short-spurred form showing the short wide spur that accommodates the short blunt head of pollinating Cyrtothyrea marginalis beetles. (C) Cyrtothyrea marginalis visiting a flower of the short-spurred form. (D) Long-spurred form of Eulophia parviflora. (E) Dissected flower of the long-spurred form showing the relatively long and slender spur that accommodates the long proboscides of Amegilla fallax. (F) Amegilla fallax visiting a flower of the long-spurred form. Scale bars = 5 mm, except B and E = 2 mm.
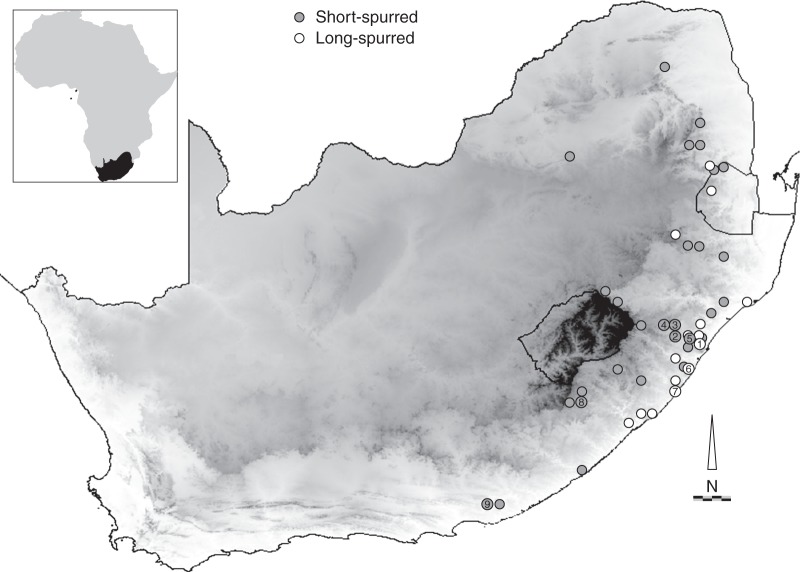
Fig. 2.
Distribution of the short- and long-spurred forms (as indicated in the key) of Eulophia parviflora in South Africa. Study sites in KwaZulu-Natal include (1) Kloof and Hillcrest [1a, Krantzkloof Nature Reserve, Bridal Road (top and bottom sites); 1b and 1c, Stockville valley (top and bottom respectively); 1d, Galloway Road]; (2) Victoria Country Club and Worlds View on the outskirts of Pietermaritzburg; (3) Umgeni Valley Nature Reserve near Howick; and (4) road verge near Balgowan in the KwaZulu-Natal midlands. Additional observations were made at (5) Drummond; (6) Vernon Crookes nature reserve; and (7) Umtamvuna nature reserve. In the Eastern Cape Province, sites include (8) the outskirts of the town of Maclear and (9) the Rietberg, near Grahamstown. Darker shades of grey represent increasing altitude. Scale bar = 100 km.
Both forms of E. parviflora have a pair of solid pollinia forming a single pollinarium which is removed as a unit by pollinators. The pollinarium undergoes a bending movement similar to that of E. streptopetala (described by Peter and Johnson, 2013) which is thought to limit geitonogamous selfing (Peter and Johnson, 2006b). The pollinaria of these two forms are easily distinguished from those of co-ocurring congeners (E. clitellifera, E. clavicornis, E. ovalis, E. ensata and E. foliosa), all of which have obviously smaller pollinaria.
Study sites
Populations of both forms of E. parviflora were observed at various sites in KwaZulu-Natal and the Eastern Cape (Fig. 2) between September 2000 and October 2002. Additional observations of pollinators visiting the short-spurred form were made at Krantzkloof in September 2004, Grahamstown in 2006 and Pietermaritzburg in 2012, and of the long-spurred form in August 2013.
Morphometric analysis
Floral and vegetative characters
To determine if there are morphological discontinuities between the forms, a total of 69 characters were measured for 52 specimens from ten populations and 47 specimens from eight populations of the short- and long-spurred forms, respectively (Supplementary Data Table S1). Missing data accounted for 1·5 % of the characters scored. The majority (61) of these measurements are quantitative characters, with the balance being ratios which describe the shape of floral parts such as petals (Supplementary Data Table S1). Morphological measurements were recorded from living plants or specimens in 70 % ethanol.
Morphological data were analysed using PCA in STATISTICA (StatSoft, 2012). Quantitative data were first log-transformed (Jolicoeur, 1963; Humphries et al., 1981) and a correlation matrix calculated. The first two principle components were plotted against each other. Eigenvalues are given in Supplementary Data Table S2.
Colour analysis
To determine if the two forms differ in flower colour, spectral reflectance of various flower parts (lateral petals and labella) was measured with an Ocean Optics S2000 spectrophotometer. An Ocean Optics Mini-D2T (tungsten–deuterium–halogen) light source was used to illuminate the sample. The reflection probe (UV/VIS 400 micron) was orientated at 45 ° to the surface of the floral part being measured.
Spectra were summarized using both the Endler segment classification method (Endler, 1990) and the Chittka bee vision model (Chittka, 1992). Given the very different pollinators, including a beetle with unknown visual sensitivity and the presence of red coloration on the adaxial surface of the lateral petals, colours to which some beetles are known to respond, we selected the Endler (1990) method as being independent of the visual physiology of the insects concerned. We also use the Chittka (1992) model to summarize these colours in a bee vision model (Chittka and Kevan, 2005).
The Endler (1990) segment classification takes the integral of light reflected from floral parts and the light incident on the sample (the D65 norm-function in this case) for each of four equal segments between 300 and 700 nm. These values are divided by the integral for the entire spectrum of interest (300–700 nm) to separate colour from brightness and subtracted from one another to determine values for colour ‘opponents’. The value for the medium–short wavelength segment is subtracted from the long wavelength to give a long–medium (LM) opponent, and the short wavelength segment is subtracted from medium–long segment to determine the medium–short (MS) opponent. Colour measurements were recorded from freshly harvested flowers.
Distribution, flowering phenology and pollinator flight times
Distribution and flowering phenology data were collected from specimens from a variety of herbaria including NU, NH, PRE, BOL, GRA and K. In addition, our field observations were included as equivalent to these herbarium records (each observation representing an opportunity where a single herbarium specimen could have been collected). Localities accurate to at least one minute of latitude and longitude were used to extract start and end dates for frost periods from the climatic surfaces of Schulze et al. (1997).
Flight times of the pollinating insects were determined from specimens in AMGS, TMSA and SANC, as well as from Eardley's (1994) revision of Amegilla. Distribution data for the two pollinator species are given by Eardley (1994) and Holm and Marais (1992).
In all cases, dates were numbered consecutively, with 1 July being the first, and 30 June the 365th day of the season to span the austral summer.
Insect pollinators
A total of 109 and 63 h were spent in the field studying pollinators of the long- and short-spurred forms, respectively. Observations at each site ranged between 1 h at the Maclear site and a total of about 67 h at the two Stockville Valley sites. At all sites, besides Maclear, observations were made on two or more days. All insects found on the inflorescences and other possible pollinators (primarily Hymenoptera, Coleoptera and Diptera) visiting other plants in the vicinity were collected and inspected for pollinaria or viscidia. Where pollinators were observed visiting flowers, the duration of the visits to individual inflorescences was recorded. Voucher specimens are lodged in the Albany Museum and the personal collection of the first author.
Pollinarium reconfiguration
Pollinaria in most species of Eulophia undergo a reconfiguration following removal from the flower. This effectively prevents self-pollination until the reconfiguration is completed, and there is evidence from surveys of a variety of orchids that the timing of this movement is adapted to exceed the average visit times of specific pollinators (Peter and Johnson, 2006b). Given the hypothesis that these two forms are pollination ecotypes and pollinated by different pollinators, we expected the pollinarium reconfiguration times of the two forms to differ. We therefore recorded reconfiguration times of one pollinarium per inflorescence for 49 and 81 plants of the short- and long-spurred forms, respectively. Bending times for both forms could not be normalized and were hence compared using the Mann–Whitney U-test.
Scent analysis
The scents of the two forms differ to the human nose. We therefore analysed the composition of headspace samples collected from the two forms. Initially, scent samples of three inflorescences of each form were collected in the field (sites of all scent collections are given in Supplementary Data Table S4). Each inflorescence was enclosed in a glass bell jar and headspace air was drawn through a filter containing 3 mg of Porapak for approx. 6 h (at approx. 2 L h−1). Scent compounds were eluted in a 5:1 hexane:acetone mixture and their relative abundances were determined using gas chromatography–mass spectometry (GC-MS) according to the method of Kaiser and Tollsten (1995) on Carbowax GC-MS columns.
In subsequent analyses, the scent of six short- and 12 long-spurred inflorescences from different sites was concentrated using polyacetate bags and trapped over approx. 30 min on filters filled with 1 mg of tenax® and 1 mg of carbotrap® activated charcoal. The samples were thermally desorbed and analysed on a Varian CP-3800 gas chromatograph (Varian, Palo Alto, CA, USA) with a 30 m × 0·25 mm internal diameter (film thickness 0·25 µm) Varian VF-5ms (DB5 equivalent) column coupled to a Varian 1200 quadrupole mass spectrometer.
The relative amount of each compound was expressed as a percentage of the total ion count for all compounds not also present in control samples. A similarity matrix of these square-root transformed values was calculated using the Bray–Curtis method and non-metric multidimensional scaling (NMDS) used to visualize the data using Primer 6.1.6 (Clarke and Gorley, 2006). These data were compared using two-way ANOSIM in Primer 6.1.6 with morph and column type treated as explanatory factors.
To locate the site of scent emission, freshly harvested flowers were immersed in neutral red dye (Dafni et al., 2005). Because both forms of E. parviflora are deceptive and no nectar was found in any flowers, stained parts of the flower are likely to indicate the position of osmophores, rather than nectaries.
Scent choice experiments
To determine the response of pollinators to the scent of the two forms, we constructed a ‘Y-shaped’ olfactometer with small computer fans blowing ambient air through stainless steel scent chambers into each of two arms of the perspex olfactometer. Each arm was 150 mm long with a common base arm of 150 mm. The clear perpex tubing was 60 mm in diameter (Supplementary Data Fig. S1). Pollinators were introduced to the olfactometer and their choices recorded. Although this experiment was attempted using bees and beetles, bees did not move within the olfactometer. Insects for these experiments were collected from sites where the plants were not present to avoid prior conditioning, but were within the general range of the plants. Insects were kept without food in a cool dark cage for 24 h before the experiment. Experimental trials were conducted mid morning to avoid the hottest part of the day when the activity of these insects is lower. Beetles were used no more than twice in an experiment.
Choice experiments were conducted in a greenhouse made with opaque fibreglass sheets to diffuse light. The alignment of the olfactometer in relation to the sun was critical even within the greenhouse. When the axis of the olfactometer was aligned directly at the sun, the beetles moved randomly through the olfactometer (of 43 beetles, 21 selected one arm and 22 the other arm; χ2 = 0·02, P = 0·879; Fig. 7A).
Fig. 7.
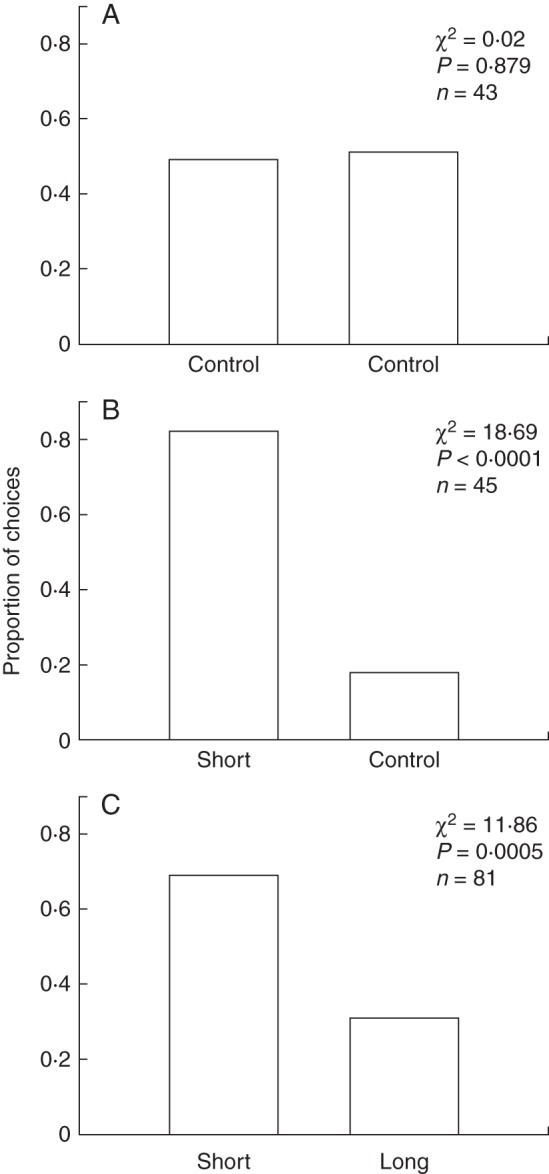
Choices made by C. marginalis beetles in a Y-maze olfactometer. (A) Olfactometer with empty arms aligned correctly to the sun. (B) Olfactometer with one arm containing flowers of the short-spurred form of Eulophia parviflora. (C) Olfactometer with one arm containing flowers of the short-spurred form of Eulophia parviflora and the other containing flowers of the long-spurred form.
Once the olfactometer was correctly calibrated with beetles not showing a preference for either arm of the olfactometer in the absence of odour cues, fresh flowers producing scent were introduced to one of the two arms (selected randomly), flow rates were re-balanced, beetles were introduced and their choices were recorded. We tested two combinations of scents: the scent of the short-spurred form of the orchid against a blank control with no odour; and the scent of the short-spurred flowers against the scent of the long-spurred form. Beetle choices between presented scents or between scent and a blank control were compared using a χ2 goodness of fit test.
Visitation rates and pollen transfer efficiency
Between 28 and 268 flowers from between four and 98 inflorescences (Supplementary Data Table S5) from different populations were sampled and scored for pollinaria removal and pollinia deposition. We also determined the number of flowers that showed any sign of visitation as well as flowers that had their anther caps disturbed, which represents a failed visit. Finally, we calculated the pollination transfer efficiency as the percentage of removed pollinia (removed pollinaria multiplied by two) that are deposited on the stigmas (Johnson et al., 2005a). The averages for each population, pooled where necessary across years, were compared using Mann–Whitney U-test.
Translocation experiment
A translocation experiment was conducted to test the prediction that the pollination success of an ecotype would be higher in its native range than in the range of the other ecotype. This experiment was only possible for the long-spurred form which is restricted to the coastal zone (the short-spurred form has a wide natural distribution; Fig. 2).
A large population of the long-spurred form flowered in a firebreak shortly before this area was scheduled to be burnt. We therefore harvested these inflorescences before they were burnt without disturbing the below-ground tuber stock and used them for a translocation experiment. Inflorescences were inspected, and flowers showing signs of either pollinarium removal or pollinia deposition were removed. The inflorescences were then positioned at a natural height in the grass canopy with their cut ends immersed in water in glass pill vials. Inflorescences were assigned randomly to two sites, one within the natural distribution of the long-spurred form, the second about 60 km outside the natural distribution of the long-spurred form, but in amongst a population of the short-spurred form. Inflorescences were left for 7 d before the flowers were inspected for signs of pollen removal and deposition. Data were compared between sites using a generalized linear model (GLM) with a binomial error distribution and a logit link function, implemented in SPSS 19 (IBM Corp.). Significance was assessed using likelihood ratios.
Spur-shortening experiment
To investigate the role of floral morphology in pollinator effectiveness, we shortened the length of the spurs of the long-spurred form at the Stockville Valley site by approx. 50 %. Because of the fleshy nature of the spurs of these flowers, finger pressure was used to press the spur flat and then a small plastic clamp was used to hold the spur closed. For pairs of adjacent plants, we assigned one plant to the shortening treatment and left the other unmanipulated to serve as a control. Flowers on both treatment and control plants were first examined for any signs of visitation, and visited flowers were removed before the treatment was performed. Flowers were left for 5 d and then inspected for pollen removal or deposition. The proportion of flowers with pollinia removed was compared between treatments using a GLM with a binomial error distribution and a logit link function.
Self-compatibility and pollinator dependence
To test the degree of self-compatibility and potential for autonomous self-pollination which is common in Eulophia (Williamson, 1984; Peter and Johnson, 2009a), inflorescences of both morphs were bagged to exclude pollinators and then flowers were self-pollinated, cross-pollinated or left unmanipulated. Breeding system experiments were conducted at the Victoria Country Club and Stockville Valley sites on the short- and long-spurred forms, respectively. In addition, reciprocal crosses were made between the two forms to establish their genetic compatibility. Following the treatments, bagged flowers were allowed to delevop to mature capsules and harvested shortly before dehiscence. Capsules and their seed contents were weighed and then the seeds from a single capsule were homogenized and four random samples of 50 seeds were scored for the presence or absence of embryos. Combined capsule and seed weights and the percentage of fertile seeds produced were compared using a Kruskal–Wallis test as the data did not conform to known distributions.
RESULTS
Morphometric analysis
Multivariate analysis of floral and vegetative characters revealed two distinct clusters in the phenotype space, associated with plants that possess either long or short spurs (Fig. 3). Most characters measured differed significantly between the two forms (Supplementary Data Table S2). Spur length shows a clear bimodal distribution with no overlap between the two forms (Fig. 4; Supplementary Data Table S2).
Fig. 3.
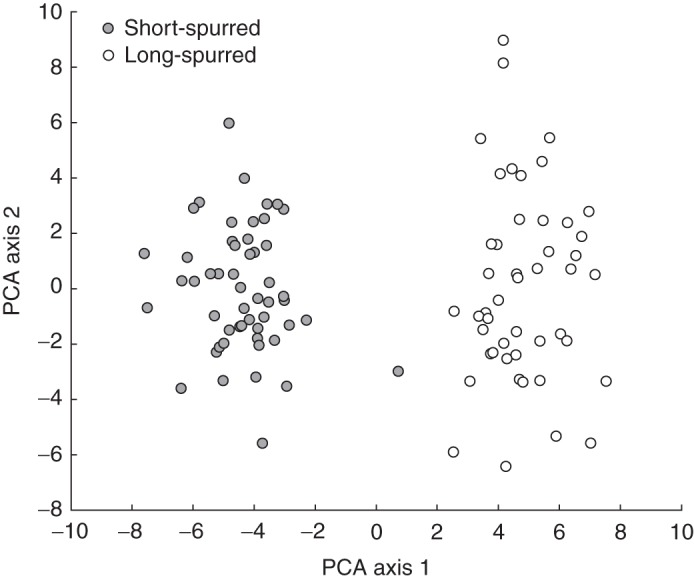
Principle component analysis of the morphological characters of the long- (n = 47) and short- (n = 52) spurred forms of E. parviflora.
Fig. 4.
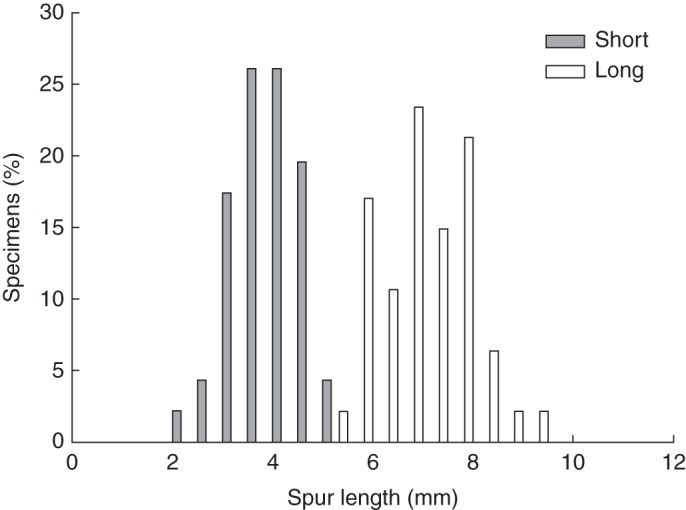
The bimodal distribution of spur length in Eulophia parviflora corresponding to the long- (n = 47) and short-spurred (n = 52) forms.
Colour
The colours of the two forms are nearly identical and overlap extensively in both analyses (Supplementary Data Fig. S2). The adaxial surface of the lateral petals is the most variable colour, ranging from light creamy-yellow to dark brick-red with many combinations of mottling of these two colours, explaining the variation in measured points (Supplementary Data Fig. S2A). This variation is not specific to either form.
Distribution, flowering phenology and flight times
The long-spurred form is found primarily at lower altitudes along the coast of KwaZulu-Natal, while the short-spurred form is found at higher altitudes through the Eastern Cape, KwaZulu-Natal and Mpumalanga Provinces (Fig. 2). There is a zone of overlap of the two forms near Durban, where the short-spurred form is found at lower altitude (Fig. 2, site 1) and in the north-east part of the range where the long-spurred form which otherwise has a coastal distribution is found at higher altitude in the mountains of Mpumalanga and Swaziland.
The long-spurred form flowers significantly earlier than the short spurred-form in the central part of the distribution (t84 = 10·0, P < 0·0001; Fig. 5). There is some overlap in the flowering phenology of the two forms, particularly when the long-spurred form flowers later in the season in response to late fires.
Fig. 5.
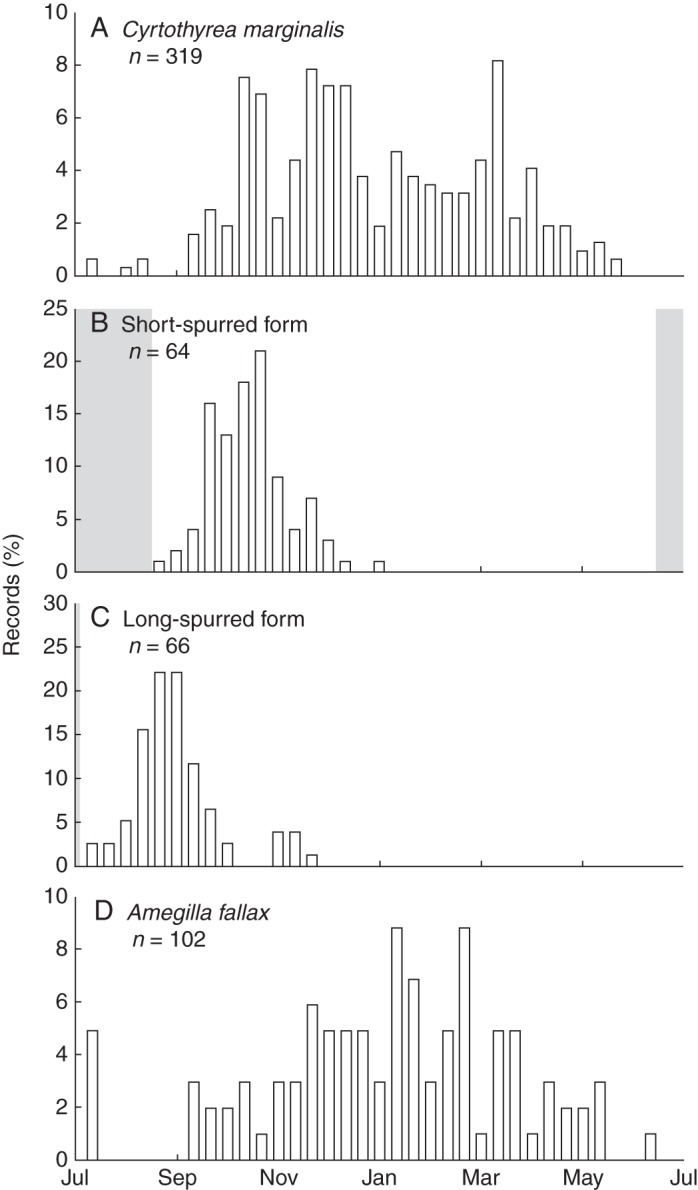
Flowering phenology of the short- (B) and long-spurred (C) forms of Eulophia parviflora in relation to the flight periods of C. marginalis (A) and A. fallax (D), their respective pollinators. The average frost periods for sites where each form is found is indicated in grey.
In both forms, flowering of the orchids corresponds to the emergence of their respective pollinators (described below), although the flight period of the pollinators extends much later than flowering of the orchids (Fig. 5A, D). While there are a few unusual flowering records, flowering of both forms typically commences 30 d after the last day of frost in their respective distributions (Fig. 5B, C). Frost is rare within the range of the long-spurred form, and restricted to only a few days at the height of winter. Frost is more widespread at the sites occupied by the short-spurred form, extending from the middle of June to early August.
Insect pollinators
We collected or inspected a total of 589 Cyrtothyrea marginalis (Cetoniinae; Scarabaeidae) beetles visiting either the short-spurred form of E. parviflora or other species (primarily Asteraceae, but also Iridaceae and Hyacinthaceae) in the vicinity of the orchids. A total of 61 of the beetles of both sexes that we observed or collected at six sites bore pollinaria or viscidia of this form of the orchid (Fig. 2; Supplementary Data Table S3). The short open spur of this form accommodates the blunt anterior of the beetles (Figs. 1B, C).
In contrast, the long-spurred form appears to be pollinated by the solitary bee Amegilla fallax (Apidae; Anthophorinae) at the sites we examined. We collected 81 of these bees of both sexes at two sites near Durban (Fig. 2; Supplementary Data Table S3). Of these, 25 (to include most recent observations as listed in the revised supplementary table S3) bees bore pollinaria or viscidia of the long-spurred form. The majority of bees were collected while foraging on co-occuring nectar plants, but we did observe two direct visits to inflorescences. These bees have relatively long proboscides which are matched by the long, slender spurs of the long-spurred form (Fig. 1E, F).
Visit times and pollinarium reconfiguration
We timed five visits of C. marginalis beetles to inflorescences of the short-spurred form. During these visits, beetles alighted and usually clambered around the inflorescence, entering two or three flowers and depositing or extracting pollinia (Supplementary Data Video). Visits by the beetles to the inflorescences lasted 69 s on average (n = 5, range: 20–110 s). This is shorter than the average pollinarium reconfiguration time of 119 s (Supplementary Data Fig. S3).
Most of the fast moving A. fallax bees were collected while foraging for nectar on a variety of other food plants. We did, however, observe and record the duration of two visits (of 25 and 22 s) to inflorescences of the long-spurred form. As in the case of the short-spurred form, visit times by Amegilla bees to the long-spurred inflorescences (mean 23·5 s) were shorter than the mean pollinarium reconfiguration time of 28 s (Supplementary Data Fig. S3). The pollinarium bending time of the long-spurred form is significantly shorter than that of the short-spurred form (U = 0, z = –9·53, P < 0·0001).
Scent analysis
Non-metric multidimensional scaling analysis revealed that there are discrete clusters in scent phenotype space that correspond to each morph (Fig 6). Although the phase of the GC column used in the analyses has a slight effect on the position of these clusters in the scent phenotype space (Fig. 6), there were highly significant differencences in scent composition between the two morphs that were independent of column phase (two-way ANOSIM: R = 0·956, P < 0·0001).
Fig. 6.
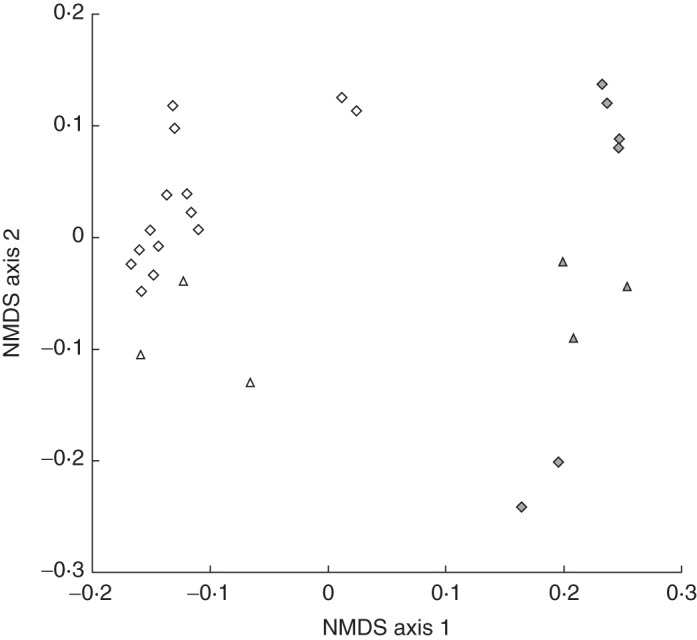
Non-metric multidimensional scaling representation of the scent composition of the long-spurred (open symbols) and short-spurred (solid symbols) forms of Eulophia parviflora as detemined using GC-MS with different columns: Carbowax (triangles) and DB5 (diamonds). The scent chemistry of the two forms is significantly different (ANOSIM, R = 0·956, P < 0·0001).
The scent of the short-spurred form is dominated by aromatic benzenoid compounds such as benzaldehyde, 4-methoxy-benzaldehyde, benzyl alcohol, benzyl benzoate and methyl benzoate, as well as the monoterpenes linalool, trans-geraniol and geraniol (Supplementary Data Table S4). In contrast, the scent of the long-spurred form is dominated by various derivatives of the sesquiterpene farnesene, such as farnasal, 2,3-dihydrofarnesal, farnasol and 2,3-dihydrofarnesol, and the irregular terpene 6-methyl-5-hepten-2-one (Supplementary Data Table S4).
Only the central, rugose ridges of the central lobe of the labellum were stained by neutral red (excluding the stigmatic cavities and pollinia), indicating this as the main site of scent production in these deceptive orchids.
Scent choice experiments
Scent preference experiments with C. marginalis beetles in the Y-olfactometer revealed that these insects responded positively to the scent of flowers of the short-spurred form. Significantly more beetles (37 of 45) chose the arm of the olfactometer containing the flowers of the short-spurred form over the arm without any scent (χ2 = 18·69, P < 0·0001; Fig. 7B). The beetles showed a preference for the scent of the short-spurred form over that of the long-spurred form, with significantly more beetles (56 of 81) choosing the arm with flowers of the short-spurred form over the arm containing the scent of the long-spurred form (χ2 = 11·86, P = 0·0005; Fig. 7C).
Visitation rates and pollen transfer efficiency
Rates of pollinaria removal and deposition as well as overall visitation rates are significantly higher in the beetle-pollinated short-spurred form compared with the bee-pollinated long-spurred form (Supplementary Data Table S5). This translates into very high pollen transfer efficiencies in the short-spurred form, with nearly 25 % of all removed pollinia being subsequently deposited on stigmas. In contrast, only 6 % of removed pollinia were deposited on stigmas of the long-spurred form. Pollination failure (visits that remove the anther cap of the flower, but not the pollinarium) was slightly higher in the beetle-pollinated form.
Translocation experiment
There were no significant differences in mean (± s.e.) rates of pollinaria removal (translocated within the natural range, 0·14 ± 0·04; translocated out of the natural range, 0·19 ± 0·04; χ2 = 0·886, P = 0·346) and pollinia deposition (translocated within the natural range, 0·01 ± 0·01; translocated out of the natural range, 0·02 ± 0·01; χ2 = 0·276, P = 0·599) for 21 inflorescences of the long-spurred form translocated within a natural population and 16 inflorescences translocated 60 km outside the natural distribution range.
Spur shortening experiment
Experimental shortening of the spurs of the long-spurred form significantly reduced the mean (± s.e.) proportion of flowers with pollinaria removed on inflorescences from 0·35 ± 0·054 to 0·06 ± 0·027 (χ2 = 21·926, P < 0·0001). Pollen deposition on flowers in both treatment groups was too infrequent for statistical analysis.
Breeding system
Both forms of E. parviflora showed evidence of self-compatibility, with capsules produced by the self-treatment comparable in weight with those produced by cross-pollination. In both forms, however, the quality of seed produced in the self-pollinated capsules was significantly lower than that produced by outcrossing (Table 1). In the short-spurred plants, selfed capsules produce 50 % fewer fertile seeds (as indicated by the presence of an embryo) while in the long-spurred form, selfed capsules produced 75 % fewer fertile seed (Table 1). Hand-pollinated flowers usually resulted in fruit production, but none of the bagged and unmanipulated flowers, in both forms, set fruit, indicating that the plants are not autogamous. The two forms appear to be interfertile, with reciprocal crosses leading to fruits and seeds of quality comparable with those produced by cross-pollination within each form (Table 1).
Table 1.
Results of experiments to determine the breeding systems of the two forms of Eulophia parviflora
| Form | Mesurement of fecundity | Treatment |
Test statistic | |||
|---|---|---|---|---|---|---|
| Bagged and unmanipulated | Self-pollinated | Cross-pollinated | Intermorph cross | |||
| Short-spurred | Percentage fruit set (n) | 0 (n = 18) | 66 (n = 29) | 70 (n = 26) | 85 (n = 7) | |
| Mean (± s.e.) capsule and seed mass in g* (n) | – | 0·48 ± 0·08 (n = 19) | 0·56 ± 0·09 (n = 18) | 0·33 ± 0·05 (n = 6) | H = 1·731, P = 0·421 | |
| Mean (± s.e.) percentage of seeds with embryos* (n) | – | 44·7 ± 6·5a (n = 19) | 83·8 ± 4·1b (n = 18) | 86·3 ± 2·5b (n = 6) | H = 19·88, P < 0·0001 | |
| Long-spurred | Percentage fruit set (n) | 0 (n = 32) | Not recorded | Not recorded | Not recorded | |
| Mean (± se) capsule and seed mass in grams* (n) | – | 0·22 ± 0·02 (n = 9) | 0·23 ± 0·03 (n = 8) | 0·29 ± 0·05 (n = 7) | H = 1·70, P = 0·428 | |
| Mean (± se) percentage of seeds with embryos* (n) | – | 20·2 ± 5·3a (n = 9) | 86·6 ± 1·6b (n = 8) | 84·5 ± 1·6b (n = 7) | H = 16·31, P = 0·0003 | |
*Mass and seed set data were compared using Kruskall–Wallis test.
Homogenous groups were identified using Mann–Whitney post-hoc pairwise test and indicated with letters.
DISCUSSION
The results of this study are consistent with the specific predictions generated by the hypothesis that floral divergence between the two forms of Eulophia parviflora reflects an evolutionary shift between different pollinators. Specifically, these analyses show that there are at least two forms in the complex, differing in flower shape (Fig. 3), scent (Supplementary Data Table S4), phenology (Fig. 5) and pollinarium reconfiguration time (Supplementary Data Fig. S3). Our observations at a wide range of sites suggest that each of these forms is pollinated by a specific insect species (a bee and beetle species, respectively). Experiments (spur manipulation, scent choices), phenological matching of flowering and pollinator emergence, and correlations between pollinator visitation times and pollinarium reconfiguration suggest a functional role for the traits that characterize these forms. Coexistence of the two forms at some sites near Durban substitutes for a common garden experiment in establishing that the differences between the two forms have a genetic basis and are not the result of phenotypic plasticity.
This study is unusual in that a whole suite of traits was considered, unlike many previous studies which focused on pollinator-driven evolution of single traits, such as spur length (Robertson and Wyatt, 1990; Johnson, 1997; Johnson and Steiner, 1997; Boyd, 2004), colour (Newman et al., 2012), scent chemistry (Pellmyr, 1986; Johnson et al., 2005b) and flowering phenology (Herrera et al., 2002), although see studies in this special issue (e.g. Sun et al., 2014; van der Niet et al., 2014). Interestingly, we found no quantitative difference between the flower colours of the two forms of E. parviflora (Supplementary Data Fig. S2), which suggests this trait was not important for mediating the transition between bee and beetle pollination systems.
Olfactometer experiments showed that beetles were strongly attracted to the fruity, cherry-like scent of the short-spurred form, and that they preferred the scent emitted by flowers of this form over the scent emitted by a similar number of flowers of the long-spurred form (Fig. 6C). A number of the constituents of the scent of this form have been shown to be attractive to various beetles including other cetoniid beetles. These include geraniol (Klein and Edwards, 1989; Cherry and Klein, 1992; Imai et al., 1998; Toth et al., 2003), benzaldehyde (Leal et al., 1994), benzyl alcohol (Leal et al., 1994), anis aldehyde (Imai et al., 2002), (E) ocimene, methyl benzoate (Leal et al., 1996; Jürgens et al., 2000; Hammack and Petroski, 2004; Steenhuisen et al., 2013), benzyl benzoate (Leal et al., 1994) and linalool (Donaldson et al., 1990; Imai et al., 1998). Further experiments are required to unravel the relative attractivness of the different compounds making up the scent of the short-spurred form.
There are relatively few reports of beetle pollination in orchids, and in most of these cases the flowers are reported to be scented and pale green or brown in colour (Nilsson, 1981; Singer and Cocucci, 1997, Peter and Johnson, 2006a; Johnson et al., 2007; Pedersen et al., 2013). The pollination system of the short-spurred form of E. parviflora has similarities to that of Eulophia (Pteroglossaspis) ruwenzoriensis which is also pollinated by cetoniid beetles and has dense inflorescences of scented flowers and petals with dark adaxial surfaces (Singer and Cocucci, 1997). However, E. ruwenzoriensis has drab pale pink and green inflorescences; is rewarding, producing ‘jelly-like’ nectar; and has a yeast-like scent. The short-spurred beetle-pollinated form is also similar to Ceratandra grandiflora, a yellow-flowered orchid with dense inflorescences. Monkey beetles aggregate on the deceptive inflorescences of C. grandiflora which serve as mating rendezvous sites (Steiner, 1998). Crowded inflorescences (Character 14, Supplementary Data Table S1) appear to be an important characteristic of many beetle-pollinated orchids (Singer and Cocucci, 1997; Steiner, 1998; Peter and Johnson, 2006a, 2009b) and are consistent with the general syndrome of beetle pollination (van der Pijl, 1961; Faegri and van der Pijl, 1966; Bernhardt, 2000), but see Pedersen et al. (2013). Crowded inflorescences appear to be an efficient way of producing large attractive displays from small flowers, and also allow beetles to clamber from flower to flower without flying (Supplementary Data Video).
The only bee species found to carry pollinaria of this morphotype at the sites we examined was A. fallax (both sexes), but we cannot exclude the possibility that other medium- to small-sized solitary bees contribute to pollination. The two bees observed visiting flowers exhibited a typical zig-zag, scent-orientating flight pattern when approaching inflorescences, suggesting that scent is an important component of the attractiveness of the flowers. Three of these approaches ended with the bees briefly hovering close to the inflorescence before flying away, while two approaches ended with the bees landing and probing a flower. The scent of the long-spurred form is strongly dominated by various derivatives of the sesquiterpene farnesene (Supplementary Data Table S4), compounds known from a number of floral scents including various orchid species (Knudsen et al., 2006). The isomers of farnesol appear to be important components of the female-attracting pheromone in Xylocopa carpenter bees (Williams et al., 1987; Minckley et al., 1991) and Bombus pratorum (Bergman and Bergström, 1997). Xylocopa bees may have a ‘dispersed lek system’, with males competing over large areas by scent-marking territories to attract female bees for mating (Andersen et al., 1988; Minckley et al., 1991). Male Bombus bees have a similar scent mark behaviour to attract virgin queens (Bergman and Bergström, 1997). It is possible, therefore, that farnesene derivatives play a role in the biology of A. fallax, either to mark communal roost positions or to co-ordinate the mating rendezvous. If this is the case, this may represent a novel aspect of pollinator deception that has not been documented previously. Johnson et al. (2005b) speculated that the attraction of both male and female Tetraloniella (Anthophorinae) bees, known to have a strong lekking behaviour (C. Eardley, pers. comm.), to the deceptive flowers of Disa spathulata may be due to mimicry of scent blends used by bees to mark nest sites or leks. However, the biological role of floral scent compounds in attraction of bees to these orchids has still to be confirmed using behavioural assays with single compounds and blends.
Long-spurred forms in the E. parviflora complex are found mainly at low altitude along the coast, while the short-spurred form is found mainly at high altitudes (Fig. 2). The ultimate basis for the evolution of these geographical forms within the E. parviflora complex remains uncertain. As both pollinator species have wide distributions in southern Africa (Holm and Marais, 1992; Eardley, 1994), there is not the underlying geographical gradient in pollinator availability that would be expected from the Grant–Stebbins model. The wide distribution of the pollinators probably explains why the prediction that forms would have higher pollination success in their own distribution range was not upheld by the translocation experiment.
A possible alternative explanation for the distribution of the two forms is suggested by comparison of flowering and phenology times of respective forms and their pollinators as well as local frost periods (Fig. 5). The flowering of the inland short-spurred form peaks in October, coinciding with the emergence of the Cyrtothyrea marginalis beetles. For most sites, this is at least a month after the end of the winter frosts. In contrast, the long-spurred form begins flowering at the end of the austral winter in July, coinciding with the very early emergence of the A. fallax bees. This early flowering coinciding with newly emerged bees is possible at the coast because of the lack of frost, but may not be viable at colder inland localities.
A recent species-level phylogeny for Eulophia (Martos et al., 2014) suggests that beetle pollination is derived in the short-spurred form in an otherwise bee-pollinated clade characterized by lax inflorescences with few resupinate flowers open at one time (Peter and Johnson, 2008, 2009c, 69). This apparent shift from bee to beetle pollination is associated with the evolution of shorter spurs and is at odds with the general evolutionary trend for shifts from shorter to longer floral tubes (Whittall and Hodges, 2007). It is noteworthy that pollination success of the beetle-pollinated form is almost always higher than that of the longer-spurred bee-pollinated form.
Finally, our investigations of the E. parviflora complex suggest that the short- and long-spurred forms could be considered closely related sister species rather than intraspecific variants. Although the crossing experiments suggest that the two forms are interfertile (at least capable of forming hybrid seeds with embryos), the two forms coexist at some sites, with differences in flowering time and pollinators serving as the main isolating barriers. This ability to ‘withstand the challenge of sympatry’ (Coyne and Orr, 2004) leads us to conclude that the forms arose as ecotypes and have reached the stage of being biological species with ethological and phenological isolating barriers. This is at odds with the current taxonomy, but would not be the first case where biological species have been overlooked by taxonomists working mainly from herbarium specimens.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Roman Kaiser for analysing the initial scent samples, and KZN Wildlife for access to their reserves. Funding was provided by the National Research Foundation (South Africa). Fred Gess (AMGS) and Connal Eardley both helped in the identification of pollinating insects. Ashley Kirk-Sprigg (AMGS), James Harrison (TMSA) and Beth Grobelaar (SANC) all kindly examined their C. marginalis specimens and provided data on flight times. Mark Robertson assisted in the analysis of frost periods at different collection sites. Andreas Jürgens and Lawrence Harder are thanked for their comments on the draft.
LITERATURE CITED
- Andersen JF, Buchmann SL, Weisleder D, Plattner RD, Minckley RL. Identification of thoracic gland constituents from male Xylocopa spp. latreille (Hymenoptera: Anthophoridae) from arizona. Journal of Chemical Ecology. 1988;14:1153–1162. doi: 10.1007/BF01019343. [DOI] [PubMed] [Google Scholar]
- Anderson B, Alexandersson R, Johnson SD. Evolution and coexistence of pollination ecotypes in an african gladiolus (Iridaceae) Evolution. 2010;64:960–972. doi: 10.1111/j.1558-5646.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. Patterns of character divergence and the evolution of reproductive ecotypes of Dalechampia scandens (Euphorbiaceae) Evolution. 1985;39:733–752. doi: 10.1111/j.1558-5646.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Bergman P, Bergström G. Scent marking, scent origin, and species specificity in male premating behavior of two Scandinavian bumblebees. Journal of Chemical Ecology. 1997;23:1235–1251. [Google Scholar]
- Bernhardt P. Convergent evolution and adaptive radiation of beetle-pollinated angiosperms. Plant Systematics and Evolution. 2000;222:293–320. [Google Scholar]
- Boberg E, Alexandersson R, Jonsson M, Maad J, Ågren J, Nilsson LA. Pollinator shifts and the evolution of spur length in the moth-pollinated orchid Platanthera bifolia. Annals of Botany. 2014;113:267–275. doi: 10.1093/aob/mct217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AE. Breeding system of Macromeria viridiflora (Boraginaceae) and geographic variation in pollinator assemblages. American Journal of Botany. 2004;91:1809–1813. doi: 10.3732/ajb.91.11.1809. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Jr, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Cherry RH, Klein MG. Attraction of adult Euphoria sepulchralis (Coleoptera: Scarabaeidae) to aromatic compounds. Florida Entomologist. 1992;75:383–385. [Google Scholar]
- Chittka L. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a general representation of colour opponency. Journal of Comparative Physiology A (Sensory, Neural, and Behavioral Physiology) 1992;170:533–543. doi: 10.1007/BF00199332. [DOI] [PubMed] [Google Scholar]
- Chittka L, Kevan PG. Flower colour as advertisement. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Cambridge, ON, Canada: Enviroquest Ltd; 2005. pp. 157–196. [Google Scholar]
- Clarke KR, Gorley RN. Primer v6: user manual/tutorial. Primer-E Ltd; 2006. [Google Scholar]
- Conner JK. Ecological genetics of floral evolution. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 260–277. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- Dafni A, Kevan PG, Husband BG. Practical pollination biology. Cambridge, ON, Canada: Enviroquest Ltd; 2005. [Google Scholar]
- Donaldson JMI, McGovern TP, Ladd TL., Jr. Floral attractants for Cetoniinae and Rutelinae (Coleoptera: Scarabaeidae) Journal of Economic Entomology. 1990;83:1298–1305. [Google Scholar]
- Eardley CD. The genus Amegilla Friese (Hymenoptera: Anthophoridae) in southern Africa. Entomology Memoir, Department of Agriculture, Republic of South Africa. 1994;91:1–68. [Google Scholar]
- Ellis AG, Johnson SD. The evolution of floral variation without pollinator shifts in Gorteria diffusa (asteraceae) American Journal of Botany. 2009;96:793–801. doi: 10.3732/ajb.0800222. [DOI] [PubMed] [Google Scholar]
- Endler JA. On the measurement and classification of color in studies of animal color patterns. Biological Journal of the Linnean Society. 1990;41:315–352. [Google Scholar]
- Faegri K, van der Pijl L. The principles of pollination ecology. Toronto: Pergamon Press; 1966. [Google Scholar]
- Forest F, Goldblatt P, Manning JC, et al. Pollinator shifts as triggers of speciation in painted petal irises (Lapeirousia: Iridaceae) Annals of Botany. 2014;113:357–371. doi: 10.1093/aob/mct248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society B: Biological Sciences. 1999;266:2247–2252. [Google Scholar]
- Galen C. Measuring pollinator-mediated selection on morphometric floral traits: bumblebees and the alpine sky pilot, Polemonium viscosum. Evolution. 1989;43:882–890. doi: 10.1111/j.1558-5646.1989.tb05185.x. [DOI] [PubMed] [Google Scholar]
- Givnish TJ., Sytsma KJ. Molecular evolution and adaptive radiation. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Grant V. Pollination systems as isolating mechanisms in flowering plants. Evolution. 1949;3:82–97. doi: 10.1111/j.1558-5646.1949.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Grant V, Grant KA. Flower pollination in the phlox family. New York: Columbia University Press; 1965. [Google Scholar]
- Hall AV. Studies of the South African species of Eulophia. Journal of South African Botany. 1965 Supplementary volume No. V. [Google Scholar]
- Hammack L, Petroski RJ. Field capture of northern and western corn rootworm beetles relative to attractant structure and volatility. Journal of Chemical Ecology. 2004;30:1809–1825. doi: 10.1023/b:joec.0000042403.88930.a7. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Cerda X, Garcia MB, et al. Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. Journal of Evolutionary Biology. 2002;15:108–121. [Google Scholar]
- Herrera CM, Castellanos MC, Medrano M. Geographical context of floral evolution: towards an improved research programme in floral diversification. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 278–294. [Google Scholar]
- Holm E, Marais E. Fruit Chafers of Southern Africa. Pretoria: Sigma Press; 1992. [Google Scholar]
- Humphries JM, Bookstein FL, Chernoff B, Smith GR, Elder RL, Poss SG. Multivariate discrimination by shape in relation to size. Systematic Zoology. 1981;30:291–308. [Google Scholar]
- Imai T, Maekawa M, Tsuchiya S. Attractiveness of p-anisaldehyde to the varied carpet beetle, Anthrenus verbasci (L.) (Coleoptera: Dermestidae) Applied Entomology and Zoology. 2002;37:505–508. [Google Scholar]
- Imai T, Maekawa M, Tsuchiya S, Fujimori T. Field attraction of Hoplia communis to 2-phenylethanol, a major volatile component from host flowers, Rosa spp. Journal of Chemical Ecology. 1998;24:1491–1497. [Google Scholar]
- de Jager ML, Ellis AG. Floral polymorphism and the fitness implications of attracting pollinating and florivorous insects. Annals of Botany. 2014;113:213–222. doi: 10.1093/aob/mct189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD. Pollination, adaptation and speciation models in the Cape flora of South Africa. Taxon. 1996;45:59–66. [Google Scholar]
- Johnson SD. Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society. 1997;123:225–235. [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 295–310. [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Linder HP, Steiner KE. Phylogeny and radiation of pollination systems in Disa (Orchidaceae) American Journal of Botany. 1998;85:402–411. [PubMed] [Google Scholar]
- Johnson SD, Neal PR, Harder LD. Pollen fates and the limits on male reproductive success in an orchid population. Biological Journal of the Linnean Society. 2005;86:175–190. [Google Scholar]
- Johnson SD, Steiner KE, Kaiser R. Deceptive pollination in two subspecies of Disa spathulata (Orchidaceae) differing in morphology and floral fragrance. Plant Systematics and Evolution. 2005;255:87–98. [Google Scholar]
- Johnson SD, Ellis A, Dotterl S. Specialization for pollination by beetles and wasps: the role of lollipop hairs and fragrance in Satyrium microrrhynchum (Orchidaceae) American Journal of Botany. 2007;94:47–55. doi: 10.3732/ajb.94.1.47. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Multivariate generalization of the allometry equations. Biometrics. 1963;19:497–499. [Google Scholar]
- Jürgens A, Webber AC, Gottsberger G. Floral scent compounds of Amazonian Annonaceae species pollinated by small beetles and thrips. Phytochemistry. 2000;55:551–558. doi: 10.1016/s0031-9422(00)00241-7. [DOI] [PubMed] [Google Scholar]
- Jürgens A, Bosch SR, Webber AC, Witt T, Frame D, Gottsberger G. Pollination biology of Eulophia alta (Orchidaceae) in Amazonia: effects of pollinator composition on reproductive success in different populations. Annals of Botany. 2009;104:897–912. doi: 10.1093/aob/mcp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R, Tollsten L. An introduction to the scents of cacti. Flavour and Fragrance Journal. 1995;10:153–164. [Google Scholar]
- Klein MG, Edwards DC. Captures of Popillia lewisi (Coleoptera; Scarabaeidae) and other scarabs on Okinawa with Japanese beetle lures. Jounral of Economic Entomology. 1989;82:101–103. [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Larsen MW, Peter C, Johnson SD, Olesen JM. Comparative biology of pollination systems in the African-Malagasy genus Brownleea (Brownleeinae: Orchidaceae) Botanical Journal of the Linnean Society. 2008;156:65–78. [Google Scholar]
- Leal WS, Hasegawa M, Sawada M, Ono M, Tada S. Scarab beetle Anomala albopilosa albopilosa utilizes a more complex sex pheromone system than a similar species A. cuprea. Journal of Chemical Ecology. 1996;22:2001–2010. doi: 10.1007/BF02040091. [DOI] [PubMed] [Google Scholar]
- Leal WS, Ono M, Hasegawa M, Sawada M. Kairomone from dandelion, Taraxacum officinale, attractant for scarab beetle Anomala octiescostata. Journal of Chemical Ecology. 1994;20:1697–1704. doi: 10.1007/BF02059891. [DOI] [PubMed] [Google Scholar]
- Martos F, Johnson SD, Peter CI, Bytebier B. A molecular phylogeny reveals paraphyly of the large genus Eulophia (Orchidaceae): a case for the reinstatement of Orthochilus. Taxon. 2014;63 in press doi:10.12705/631.6. [Google Scholar]
- Minckley RL, Buchmann SL, Wcislo WT. Bioassay evidence for sex attractant pheromone in the large carpenter bee, Xylocopa varipuncta (Anthophoridae: Hymenoptera) Journal of Zoology (London) 1991;224:285–291. [Google Scholar]
- Morgan MT. Selection on reproductive characters: conceptual foundations and their extension to pollinator interactions. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 25–40. [Google Scholar]
- Nattero J, Sérsic AN, Cocucci AA. Patterns of contemporary phenotypic selection and flower integration in the hummingbird-pollinated Nicotiana glauca between populations with different flower–pollinator combinations. Oikos. 2010;119:852–863. [Google Scholar]
- Newman E, Anderson B, Johnson SD. Flower colour adaptation in a mimetic orchid. Proceedings of the Royal Society B: Biological Sciences. 2012;279:2309–2313. doi: 10.1098/rspb.2011.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Manning J, Anderson B. Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany. 2014;113:373–384. doi: 10.1093/aob/mct203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Niet T, Pirie MD, Shuttleworth A, Johnson SD, Midgley JJ. Do pollinator distributions underlie the evolution of pollination ecotypes in the Cape shrub Erica plukenetii? Annals of Botany. 2014;113:301–315. doi: 10.1093/aob/mct193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LA. The pollination ecology of Listera ovata (Orchidaceae) 521. Nordic Journal of Botany. 1981;1:461–480. [Google Scholar]
- Peakall R, Whitehead MR. Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Annals of Botany. 2014;113:341–355. doi: 10.1093/aob/mct199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen HÆ, Watthana S, Kocyan A, Srimuang K. Pollination biology of Luisia curtisii (Orchidaceae): indications of a deceptive system operated by beetles. Plant Systematics and Evolution. 2013;299:177–185. [Google Scholar]
- Pellmyr O. Three pollination morphs in Cimicifuga simplex; incipient speciation due to inferiority in competition. Oecologia. 1986;68:304–307. doi: 10.1007/BF00384804. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Anther cap retention prevents self-pollination by elaterid beetles in the South African orchid Eulophia foliosa. Annals of Botany. 2006;97:345–355. doi: 10.1093/aob/mcj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Doing the twist: a test of Darwin's cross-pollination hypothesis for pollinium reconfiguration. Biology Letters. 2006;2:65–68. doi: 10.1098/rsbl.2005.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Mimics and magnets: the importance of color and ecological facilitation in floral deception. Ecology. 2008;89:209–221. doi: 10.1890/07-1098.1. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Autonomous self-pollination and pseudo-fruit set in South African species of Eulophia (Orchidaceae) South African Journal of Botany. 2009;75:791–797. [Google Scholar]
- Peter CI, Johnson SD. Pollination by flower chafer beetles in Eulophia ensata and Eulophia welwitschii (Orchidaceae) South African Journal of Botany. 2009;75:762–770. [Google Scholar]
- Peter CI, Johnson SD. Reproductive biology of Acrolophia cochlearis (Orchidaceae): estimating rates of cross-pollination in epidendroid orchids. Annals of Botany. 2009;104:573–581. doi: 10.1093/aob/mcn218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Generalized food deception: colour signals and efficient pollen transfer in bee-pollinated species of Eulophia (Orchidaceae) Botanical Journal of the Linnean Society. 2013;171:713–729. [Google Scholar]
- van der Pijl L. Ecological aspects of flower evolution. II. Zoophilous flower classes. Evolution. 1961;15:44–59. [Google Scholar]
- Robertson JL, Wyatt R. Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris. Evolution. 1990;44:121–133. doi: 10.1111/j.1558-5646.1990.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Schlumpberger BO, Raguso RA. Geographic variation in floral scent of Echinopsis ancistrophora (Cactaceae); evidence for constraints on hawkmoth attraction. Oikos. 2008;117:801–814. [Google Scholar]
- Schulze RE, Maharaj M, Lynch SD, Howe BJ, Melville-Thomas B. South African atlas of agrohydrology and climatology. 1997 Water Research Commission Report TT82/96. [Google Scholar]
- Singer RB, Cocucci AA. Pollination of Pteroglossaspis ruwenzoriensis (Rendle) Rolfe (Orchidaceae) by beetles in Argentina. Botanica Acta. 1997;110:338–342. [Google Scholar]
- Soltis DE, Soltis PS, Endress PK, Chase MW. Phylogeny and evolution of angiosperms. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- StatSoft I. STATISTICA (data analysis software system), version 11. 2012 www.statsoft.com . [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Steenhuisen S-L, Jurgens A, Johnson SD. Effects of volatile compounds emitted by Protea species (Proteaceae) on antennal electrophysiological responses and attraction of Cetoniine beetles. Journal of Chemical Ecology. 2013;39:438–446. doi: 10.1007/s10886-013-0259-2. [DOI] [PubMed] [Google Scholar]
- Steiner KE. The evolution of beetle pollination in a South African orchid. American Journal of Botany. 1998;85:1180–1193. [PubMed] [Google Scholar]
- Sun M, Gross K, Schiestl FP. Floral adaptation to local pollinator guilds in a terrestrial orchid. Annals of Botany. 2014;113:289–300. doi: 10.1093/aob/mct219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M, Klein MG, Imrei Z. Field screening for attractants of scarab (Coleoptera: Scarabaeidae) pests in Hungary. Acta Phytopathologica et Entomologica Hungarica. 2003;38:323–331. [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–709. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Williams HJ, Vinson SB, Frankie GW. Chemical content of the dorsal mesosomal gland of two Xylocopa species (Hymenoptera: Anthophoridae) from Costa Rica. Comparative Biochemistry and Physiology. 1987;86B:311–312. [Google Scholar]
- Williamson G. Observations of a mechanism by which self-pollination may occur in Eulophia (Orchidaceae) Journal of South African Botany. 1984;50:417–423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.