Abstract
Background and Aims
Reproductive character displacement (RCD) is often an important signature of reinforcement when partially cross-compatible taxa meet in secondary sympatry. In this study, floral evolution is examined during the Holocene range expansion of Clarkia xantiana subsp. parviflora from eastern Pleistocene refugia to a western zone of sympatry with its sister taxon, subsp. xantiana. Floral divergence between the two taxa is greater in sympatry than allopatry. The goal was to test an alternative hypothesis to reinforcement – that floral divergence of sympatric genotypes is simply a by-product of adaptation to pollination environments that differ between the allopatric and sympatric portions of the subspecies' range.
Methods
Floral trait data from two common garden studies were used to examine floral divergence between sympatric and allopatric regions and among phylogeographically defined lineages. In natural populations of C. x. parviflora, the magnitude of pollen limitation and reproductive assurance were quantified across its west-to-east range. Potted sympatric and allopatric genotypes were also reciprocally translocated between geographical regions to distinguish between the effects of floral phenotype versus contrasting pollinator environments on reproductive ecology.
Key Results
Sympatric populations are considerably smaller flowered with reduced herkogamy. Pollen limitation and the reproductive assurance value of selfing are greater in sympatric than in allopatric populations. Most significantly, reciprocal translocation experiments showed these differences in reproductive ecology cannot be attributed to contrasting pollinator environments between the sympatric and allopatric regions, but instead reflect the effects of flower size on pollinator attraction.
Conclusions
Floral evolution occurred during the westward range expansion of parviflora, particularly in the zone of sympatry with xantiana. No evidence was found that strongly reduced flower size in sympatric parviflora (and RCD between parviflora and xantiana) is due to adaptation to limited pollinator availability. Rather, floral divergence appears to have been driven by other factors, such as interactions with congenerics in secondary sympatry.
Keywords: Breeding system evolution, phylogeography, plant–pollinator interactions, pollen limitation, pollination, reproductive assurance, reproductive character displacement, secondary contact, speciation
INTRODUCTION
Pollinator-mediated floral divergence is considered to be a primary driver of speciation in plants (Coyne and Orr, 2004; Johnson, 2006). Simple shifts in flower colour, orientation, scent and form have all been implicated in the evolution of strong reproductive isolation (Fulton and Hodges, 1999; Bradshaw and Schemske, 2003; Ippolito et al., 2004; Kay, 2006; Schiestl and Schluter, 2009; Hopkins and Rausher, 2012; Peakall and Whitehead, 2014; Peter and Johnson, 2014; van der Niet et al., 2014). These traits confer reproductive isolation primarily because they result in the differential attraction or deterrence of (suites of) pollinators. Although less commonly studied, mating system transitions may also facilitate the evolution of reproductive isolation (e.g. Martin and Willis, 2007). Mating systems are well known to be among the most labile traits of plants, with repeated transitions from outcrossing to selfing in many taxonomic groups (Stebbins, 1974; Jain, 1976; Wyatt, 1988; Goldberg and Igic, 2012). Moreover, mating system transitions are often associated with the recent divergence of sister species and subspecies (Foxe et al., 2009; Busch et al., 2011; Pettengill and Moeller, 2012a) suggesting a potential role in the evolution of reproductive isolation.
Pollen limitation (PL), which occurs when plants receive less pollen than is necessary for full reproductive success, is often considered to be a driving force in the evolution of mating systems in plants (Stebbins, 1970; Lloyd, 1992; Schoen et al., 1996; Ashman et al., 2004). PL can be strong when effective pollinators are scarce or when plants occur in small populations or at low density, reducing the probability that mates encounter one another (Lamont et al., 1993; Groom, 1998; Hackney and McGraw, 2001; Moeller, 2004). One possible outcome of chronic PL is the evolution of traits that promote autonomous selfing and thereby provide reproductive assurance (Pannell and Barrett, 1998; Morgan and Wilson, 2005; Eckert et al., 2006; Dornier et al., 2008). Experimental field studies have supported the reproductive assurance hypothesis by showing that selfing phenotypes are adaptive under strong PL (e.g. Kalisz et al., 2004; Moeller and Geber, 2005; but see Herlihy and Eckert, 2002) and that intraspecific geographical differentiation in mating systems is related to the reliability of plant–pollinator interactions and the reproductive assurance value of selfing (Fausto et al., 2001; Moeller, 2006; Brys et al., 2013).
Self-fertilization may also evolve because of interactions between pollinator-sharing taxa where their geographical ranges overlap. In particular, mating system differences in sympatry may reduce pollen transfer between taxa (Martin and Willis, 2007; Grossenbacher and Whittall, 2011). For example, Arenaria uniflora has selfing populations only where it overlaps with a congener, A. glabra, and experimental work showed that interference competition (heterospecific pollen transfer) is the likely mechanism that drove this mating system transition (Fishman and Wyatt, 1999). In cases where unfit hybrids form between partially isolated taxa with divergent mating systems, the process of reinforcement may drive mating system evolution and reproductive character displacement. Reinforcement is a well-known model of speciation that involves allopatric divergence and secondary contact between taxa with partial, but incomplete, postzygotic isolation (Blair, 1955; Dobzhansky, 1937, 1940). Under reinforcement, natural selection strengthens premating isolation by favouring traits that minimize the wastage of gametes on the formation of unfit hybrids (Servedio and Noor, 2003). Evidence of reinforcement in plants has primarily come from systems where a shift in pollinator associations has occurred in sympatry (Levin and Schaal, 1970; Kay and Schemske, 2008; Hopkins and Rausher, 2012; Hopkins, 2013). It remains unclear whether reproductive character displacement in mating systems may occur as a result of reinforcement selection.
Of the research on reinforcement in plants, we are not aware of studies that have established the geographical and historical context of taxon divergence. Novel floral forms could evolve in allopatry (followed by range expansion to secondary sympatry) or in the face of gene flow among interconnected populations (Harrison, 2012). By the same token, the exaggeration of floral differences in sympatry (reproductive character displacement) could arise coincident with or prior to secondary contact. For example, floral differentiation during range expansion to the zone of secondary sympatry could result in the exaggeration of differences in sympatry relative to allopatry, inconsistent with expectations of reinforcement selection. These alternative evolutionary histories have important implications for understanding the causes of the initial phases of floral and mating system divergence. To address these questions, field studies aimed at elucidating ecological processes can be coupled with historical reconstructions of population history.
It has been argued that a comprehensive test of the reinforcement hypothesis must include an assessment of alternative hypotheses (Butlin, 1989; Howard, 1993). Reproductive character displacement may occur due to reinforcement, where selection favours traits that minimize the formation of unfit hybrids, but reproductive character displacement could also occur as a by-product of adaptation to other aspects of the environment. For example, floral phenotype could evolve as a correlated evolutionary response to adaptation to novel soil environments, antagonistic biotic pressures or pollinator communities (Warren and Mackenzie, 2001; Strauss and Whittall, 2006; Schemske and Bierzychudek, 2007; Rausher, 2008; Boberg et al., 2014; Cosacov et al., 2014; de Jager and Ellis, 2014; Gómez et al., 2014; Newman et al., 2014; Sun et al., 2014) where taxa occur in sympatry rather than as a mechanism to minimize hybrid formation. Divergence in floral form and/or mating system and the strengthening of assortative mating between taxa could simply be a by-product of adaptation to a pollinator-poor environment in sympatry rather than reinforcement selection. Although in this example natural selection plays an important role in the evolution of reproductive isolation, floral divergence was not caused by interactions among congenerics. Tests of alternative explanations to reinforcement to explain reproductive character displacement (RCD) are rare in plants (Hopkins and Rausher, 2012).
Here, we describe a series of studies aimed at dissecting the causes of floral divergence among populations of Clarkia xantiana subsp. parviflora. Specifically, we use field experiments along with the results of phylogeographical analyses to test an alternative hypothesis to reinforcement for floral divergence in secondary sympatry between C. x. parvifora and its sister taxon, C. x. xantiana (hereafter parviflora and xantiana). Subspecies parviflora is a partially selfing annual plant that diverged from its primarily outcrossing progenitor, xantiana, approx. 30 000–65 000 years ago (Pettengill and Moeller, 2012a). The two taxa are partially cross-compatible (R. D. Briscoe Runquist and D. A. Moeller, unpublished data) and xantiana has considerably larger flowers than parviflora and mating system traits that largely prevent autonomous self-fertilization from occurring (Runions and Geber, 2000). Both taxa exhibit reproductive character displacement, with xantiana flowers larger in sympatry than allopatry (unpublished data) and parviflora flowers smaller in sympatry than allopatry (see below). Molecular population genetic studies have suggested that the taxa diverged with an allopatric phase and that allopatric populations of parviflora are ancestral to sympatric ones (Pettengill and Moeller, 2012b). Secondary sympatry occurred following Holocene range expansion from eastern Pleistocene refugia to the current western range limit, where xantiana also occurs (Pettengill and Moeller, 2012b).
We first examined whether floral traits important for plant–pollinator interactions and mating systems differed between sympatric and allopatric populations. We also examined the sequence of floral evolution during the process of Holocene range expansion by examining floral differentiation in relation to phylogeographical clusters that represent lineage differentiation during westward range expansion. Second, we conducted floral manipulations across the geographical range of parviflora to compare the magnitude of PL and the reproductive assurance value of selfing between the allopatric and sympatric portions of the range. Finally, we conducted a reciprocal translocation experiment between allopatric and sympatric populations to dissect whether differences in PL and reproductive assurance between regions are caused by adaptation to contrasting pollinator environments or for an alternative reason. If contrasting pollinator environments are a driving force of mating system and floral evolution in parviflora, we would expect both allopatric and sympatric genotypes to experience more pollinator-mediated PL and reproductive assurance in sympatry. Importantly, this translocation experiment distinguishes between the effects of genotype (floral form) and environment, which are often confounded in intraspecific geographical studies of floral differentiation.
MATERIALS AND METHODS
Study system
Clarkia xantiana subsp. parviflora (Eastw.) Harlan Lewis & P. H. Raven (hereafter parviflora) is an annual plant endemic to the southern Sierra Nevada foothills of Kern and Tulare counties of California (Eckhart and Geber, 1999). Populations occur mainly in open xeric habitats or in pinyon pine–juniper woodlands. Populations are distributed patchily across the landscape, typically occurring in colonies of hundreds to thousands of individuals. Significant distances (e.g. kilometres) often separate colonies and migration between them is limited; population genetic studies indicate high levels of population structure with little contemporary gene flow (Pettengill and Moeller, 2012b). There is no known active seed dispersal mechanism. Seeds can remain dormant for at least several years (Eckhart et al., 2011), which may provide an important mechanism for population persistence in the stochastic environment in which it occurs (Eckhart et al., 2011). Plants germinate with the onset of winter rains, grow through the winter, and flower in the spring between late April and early June (Eckhart and Geber, 1999).
Clarkia xantiana subsp. parviflora and its progenitor, subsp. xantiana, are largely parapatric with a narrow area of sympatry (∼5–10 km) located in the environs of the North Fork of the Kern River and Lake Isabella (Fig. 1). In this zone of sympatry, populations co-occur within the same sites and can be found within metres of one another. In these populations, putative hybrid individuals are occasionally encountered (R. D. Briscoe Runquist and D. A. Moeller, personal observation). Populations of parviflora extend east from the zone of sympatry to the eastern slope of the Sierra Nevada Mountains in a larger zone of allopatry.
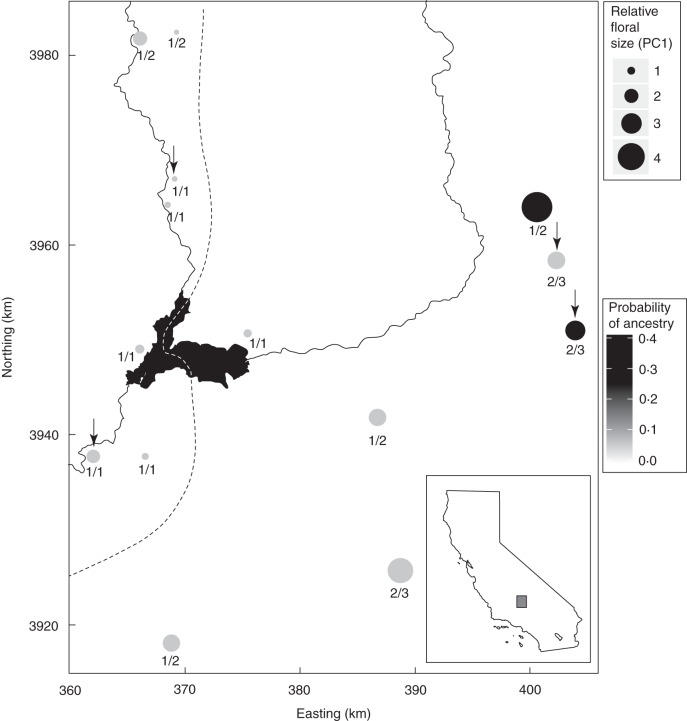
Fig. 1.
Map of populations used for the common garden study of floral variation and for phylogeographical analyses. The size of the population marker is proportional to flower size measured by petal PC1. The colour of the population marker indicates the probability of ancestry for each population estimated by Beast. The dashed line demarcates the eastern-most range limit of parviflora's sister taxon, C. xantiana subsp. xantiana. The parviflora populations to the west of this line are in the zone of sympatry and those to the east are allopatric. Numbers under each of the population marks indicate the cluster to which the population belongs from Instruct analyses of k = 2/k = 3 (Pettengill and Moeller, 2012a). Populations marked with an arrow were used in the reciprocal translocation experiment.
Previous phylogenetic analyses have shown that parviflora is a monophyletic group that is derived from within xantiana (Pettengill and Moeller, 2012a). Approximate Bayesian computation (ABC) models were subsequently used to test alternative divergence histories (e.g. primary vs. secondary contact); Pettengill and Moeller (2012a) found strong support that parviflora diverged from xantiana in allopatry followed by range expansion to a zone of secondary contact (Pettengill and Moeller, 2012b). Phylogeographical analyses using Beast (Lemey et al., 2009) further suggested that allopatric parviflora populations are ancestral to sympatric ones. Finally, patterns of population genetic structure assessed using Instruct (Gao et al., 2007) reflect a wave of westward migration from the eastern portion of the range, which palaeo-distribution models indicate was probably a refugium during the last glacial maximum (Pettengill and Moeller, 2012b).
Flowers of parviflora are self-compatible and self-pollination can occur autonomously but outcrossing is possible as well (Runions and Geber, 2000; Moeller, 2006). Dichogamy varies among populations and genotypes from adichogamy to a brief phase of protandry. In some genotypes, anthers begin to dehisce and stigmas begin to open prior to flower opening. Flowers of parviflora are smaller than that of xantiana. Crossing experiments have shown that the two taxa experience partial crossing failure and it is particularly apparent when parviflora pollen is used to sire xantiana seeds; reciprocal hybrids can be formed and those hybrids are viable and fertile in greenhouse environments (R. D. Briscoe Runquist and D. A. Moeller, unpublished data). Both Clarkia xantiana subspecies are pollinated by bees; however, bee visitors are less common in parviflora than xantiana populations (Fausto et al., 2001; Moeller, 2006). Solitary bee pollinators specialized on Clarkia are important to the reproduction of xantiana (Moeller, 2005), but absent from the allopatric range of parviflora (Moeller, 2006); specialists have been observed visiting parviflora in sympatry (Fausto et al., 2001; R. D. Briscoe Runquist and D. A. Moeller, personal observation). Flower colour is polymorphic in parviflora including pink and white morphs, with some populations containing both morphs and others fixed for a single morph (Eckhart and Geber, 1999).
Geographical variation in floral morphology
We used floral measurements of plants from 16 populations previously reported for a different analysis in Pettengill and Moeller (2012a; Supplementary Data Table S1). These populations span the east-to-west range of parviflora and include the same individuals used for molecular population genetic and phylogeographical analyses (Pettengill and Moeller, 2012a, b). Briefly, a single seed from five mothers from each population was planted in individual conetainers (Stuewe and Sons, Inc., Tangent, OR, USA). Plants were initially started in environmental growth chambers and then moved to the greenhouse and completely randomized across greenhouse benches. Floral traits were measured on the first two flowers that opened on each plant at stigma receptivity and averaged for further analysis. We measured petal length, petal width, herkogamy and dichogamy. Herkogamy was measured as the distance between the receptive stigma and the closest anther. Dichogamy was measured as the time between long-anther dehiscence and stigma receptivity. We also allowed plants to set fruits through autonomous selfing to quantify autofertility.
We conducted a second greenhouse common garden experiment to verify patterns of floral divergence observed between sympatric and allopatric regions (Supplementary Data Table S2). Here, we more intensively sampled individuals (n = 35 per population) from four sympatric and four allopatric populations chosen to span the east-to-west range of parviflora. We used the same methods for floral measurements and analysis for this study as in the common garden study described above.
First, we used a mixed-model ANOVA to test for differences in floral traits between sympatric and allopatric parviflora populations; the model included region as a fixed effect and population within region as a random effect. Due to a strong correlation between petal length and width, we used principal components analysis to describe the major axes of petal variation and used those scores for the analysis. The first principal component (PC1) described overall petal size and explained approx. 88 and 79 % of the variation in the first and second greenhouse experiment, respectively. The second principal component (PC2) described differences in petal shape (i.e. petals that were thinner than expected based on size; Supplementary Data Table S3).
Second, we tested for phenotypic differentiation among phylogeographical clusters identified by Instruct v.3·2·09 (Gao et al., 2007). Populations were assigned to clusters based on the highest average assignment of all individuals sequenced in Pettengill and Moeller (2012b) for k = 2 and k = 4 clusters (Supplementary Data Table S1); k = 4 was the highest level of clustering in the Instruct analysis for which there was statistical support and geographical pattern. We eliminated two populations from our phenotypic analyses because they are located far north (1p) or south (27p) of the region where we conducted the remainder of our experimental work; those two populations also constitute a separate cluster in the k = 4 Instruct analysis and therefore that cluster was dropped from all subsequent analyses (hereafter k = 3). Sympatric and allopatric populations used for floral analysis in the second greenhouse common garden study aligned exactly with phylogeographical clusters (k = 2, sympatric = cluster 1, allopatric = cluster 2; k = 3, sympatric = cluster 1, allopatric = cluster 3, no populations belonged to cluster 2). Therefore, we will present only the regional analysis for this dataset. All analyses were conducted using the lmer function in the lme4 package for mixed model analysis in R (R Core Devlopment Team, 2012).
Finally, we tested for a relationship between floral traits and autofertility using binomial regression. In the model, fertilized and unfertilized fruits were treated as the binomial dependent variable and population and all floral characteristics (PC1 and PC2 for petal measurements, protandry and herkogamy) were used as independent predictor variables.
Geographical variation in pollen limitation and reproductive assurance
We performed floral manipulations to assess overall PL, reproductive assurance (RA), and pollinator-mediated pollen limitation. Overall PL is a measure of the extent to which seed set is limited by pollen receipt (from any source) and pollinator-mediated PL is a measure of the extent to which seed set is limited by receipt of pollen through pollinator visitation (outcrossing or geitonogamy). Higher values of overall PL indicate that plants have the resources to set more seeds if receipt of pollen were greater, whereas lower values of PL indicate that additional pollen receipt would not increase seed production. RA assesses the extent to which autonomous selfing elevates seed set compared with pollen receipt through pollinator visitation alone. Higher values of RA indicate that plants set a greater proportion of their seeds through autonomous selfing. Manipulations were conducted on naturally occurring plants in 15 populations throughout the range of parviflora during the 2012 flowering season (5–25 May). Due to dry conditions in 2012, not all populations from Pettengill and Moeller (2012b) had plants to sample; in particular, eastern populations at low elevation were uncommon in 2012. Nevertheless, our geographical sampling still spanned the east-to-west range of parviflora. Eight of the populations were in sites sympatric with Clarkia xantiana subsp. xantiana and seven of the populations were in allopatric areas of the range (Supplementary Data Table S4).
At each population three researchers manipulated plants in a systematic order as we moved through the population and encountered flowering individuals. In most populations, we sampled nearly every plant. Plants received one of three treatments to one flower per plant: (1) supplementation, (2) emasculation, (3) marked and unmanipulated. We have shown that conducting single flower manipulations (rather than whole-plant manipulations) does not bias estimates of PL or RA due to resource reallocation (Briscoe Runquist and Moeller, 2013). In total, we treated 1302 flowers (mean: 26 flowers per treatment per site) and recovered 1200 fruits; the remaining fruits were lost due to herbivory (i.e. a fruit was collected from every manipulated flower except for those plants and fruits damaged by herbivores). In the supplemented treatment, we applied pollen from two separate donors in succession by swiping collected anthers across the stigmatic surface. Anthers were harvested from plants found a minimum of 3 m away to limit biparental inbreeding. Because parviflora anthers can dehisce before the flower opens, flowers in the emasculation treatment were emasculated prior to opening. Previous work with parviflora has shown that flowers open normally and are not damaged after bud emasculation (Moeller, 2006). We allowed manipulated fruits to ripen for 2–3 weeks before collection. When collecting fruits, we determined the total number of fruits made by the plant and the position of the manipulated fruit on the plant. For the analysis, we used the number of seeds per flower as our metric of fecundity.
As with the analyses of phenotypic differentiation, we first tested for differences between allopatric and sympatric regions of the species' range and second tested for differences among phylogeographical (Instruct) clusters. Populations for which we conducted experiments but for which population genetic data were unavailable were assigned to Instruct clusters based on the closest population found in Pettengill and Moeller (2012b) (see Supplementary Data Table S4). We conducted two types of analyses based on floral manipulation experiments. First, we used ANOVA to test for differences among treatments (supplemented, unmanipulated, emasculated), between regions, and the interaction between floral treatments and geographical regions; models also included population within region as a random effect. Greater reproductive success in supplemented than unmanipulated treatments indicates overall pollen limitation of reproduction. Greater reproductive success in supplemented than emasculated treatments indicates that pollinator-mediated delivery of pollen per se limits reproduction (outcross or geitonogamous pollination). More importantly, the comparison of emasculated to supplemented flowers provides an estimate of the potential for outcrossing in a given environment, and allows for comparisons among environments. Last, greater reproductive success in unmanipulated than in emasculated treatments indicates that selfing provides reproductive assurance. A significant interaction between region and treatment would indicate that patterns of mating differ between sympatric and allopatric parviflora.
To compare reproductive ecology between populations, we also calculated an average standardized population summary score for the three metrics of PL and RA. We used population-level treatment averages to calculate the proportional increase in seed production of supplemented fruits to unmanipulated fruits for overall PL, supplemented fruits to emasculated fruits for pollinator-mediated PL and unmanipulated fruits to emasculated fruits for RA. Standardization allows us to assess the overall magnitude of the effect and further verify that regional differences in PL or RA are not merely the product of unaccounted for population differences in maximum reproductive potential. We used one-way ANOVAs to test for differences between geographical regions and among phylogeographical clusters for each of the three summary statistics.
Reciprocal translocation experiment
We tested for regional adaptation in mating system by reciprocally translocating allopatric and sympatric genotypes in experimental arrays, conducting floral manipulations, and assessing pollen limitation and reproductive assurance. In May 2011, we constructed experimental populations of plants growing in conetainers (Stuewe and Sons, Inc.) in four field sites during the blooming period of the population: two allopatric sites (20p, 23p) and two sympatric sites (9p, 22p) (see Fig. 1). Plants were started in growth chambers and greenhouses at the University of Minnesota using the same conditions as described above for the floral phenotype common garden. The plants were then transported by truck to Kern Co., CA, where they were kept in an outdoor common garden setting when they were not actively used at the experimental field sites. In each experimental site, we constructed two populations that were placed at least 100 m apart: one population of allopatric genotypes from two populations (20p, 23p) and a second population of sympatric genotypes from three populations (9p, 22p, 77p). Allopatric and sympatric genotypes were separated into different arrays to prevent potential competitive effects of one set of genotypes and because the interaction between genotypes from the two regions was irrelevant to our questions. Populations were constructed using six conetainer racks placed in a circular array and contained 36 plants (six plants per rack) distributed evenly among source populations and rotated systematically through the array. We removed all flowers from plants that had opened before placement in the field and sub-irrigated plants using a cup under each conetainer. On each plant, we applied three different treatments (supplemented, unmanipulated, emasculated); each treatment was applied to one flower per plant. We systematically rotated the order of those treatments among plants. Plants remained in the field until all treatment flowers had senesced, which was approximately 2 weeks at each site. Plants were then moved to a common garden where fruits were allowed to mature and then collected. We allowed fruits to dry and then counted all fully formed seeds. The number of seeds per flower was used as the metric of fecundity for analysis. A companion experiment showed that resource reallocation does not occur among flowers in parviflora because of differences in pollen quantity or quality (Briscoe Runquist and Moeller, 2013).
For each plant, we calculated overall PL, pollinator-mediated PL and RA. Formulas used to calculate these indices are as follows: (1) overall PL: (supplemented seed-set – unmanipulated seed-set)/supplemented seed-set; (2) pollinator-mediated PL: (supplemented seed-set – emasculated seed-set)/supplemented seed-set; and (3) RA: (unmanipulated seed-set – emasculated seed-set)/unmanipulated seed-set. Estimates of overall PL and to a lesser extent pollinator-mediated PL and RA were skewed to the left and led to deviations from normality. To improve normality, we windsorized the data by setting all values in the bottom 5 % of the distribution at the 5 % value (Rivest, 1994). We used a split-plot mixed-model ANOVA, where the main-plot effect was experimental region (allopatric vs. sympatric) and the sub-plot effects were source genotype region (allopatric vs. sympatric) and their interaction with experimental region. All of those factors were considered as fixed effects. Experimental site nested within experimental region and source population nested within source genotype region were treated as random effects using the lmer function of the lme4 package in R (R Core Development Team, 2012). In all cases, we report the conservative estimate of degrees of freedom but determinations of significance were the same using anti-conservative degrees of freedom. A significant effect of experimental region on PL or pollinator-mediated PL could indicate that pollination environments differ (e.g. the availability of pollinators or mates for outcrossing). A significant experimental region by source genotype region effect could indicate local adaptation of mating system to regions if local genotypes have reduced PL or increased RA in their home environments.
RESULTS
Geographical variation in floral morphology
We detected significant divergence in parviflora flowers between sympatric and allopatric regions of the species' range. Allopatric flowers had 174 % larger petals (PC1) and 82 % greater herkogamy (Table 1 and Figs 1–3). In the second greenhouse common garden experiment, allopatric flowers were significantly larger in three out of the four measured floral characteristics (Supplementary Data Table S5 and Fig. S2): flowers had approx. 275 % larger petals (measured by PC1), 288 % greater measures of PC2 (i.e. allopatric petals were less strappy and wider for their length) and 55 % greater herkogamy (Supplementary Data Fig. S2). It is notable that sympatric parviflora flowers are more different from sympatric xantiana flowers (mean petal length 8·4 vs. 17·0 mm, respectively) than allopatric parviflora flowers (mean petal length 10·8 mm).
Table 1.
Fixed effects tables for ANOVAs testing for differences in floral traits between geographical regions (sympatric vs. allopatric) or phylogeographical clusters (k = 2 and k = 3); population nested within region or cluster was treated as a random factor
| Petal size (PC1) |
Petal shape (PC2) |
Protandry |
Herkogamy |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | SS | F | P | d.f. | SS | F | P | d.f. | SS | F | P | d.f. | SS | F | P | |
| Regions | 1, 52 | 5·53 | 9·24 | 0·0037 | 1, 52 | 0·08 | 0·45 | 0·5043 | 1, 52 | 0·03 | 0·96 | 0·3323 | 1, 52 | 0·38 | 7·76 | 0·0074 |
| k = 2 clusters | 1, 52 | 2·50 | 4·17 | 0·0463 | 1, 52 | 0·01 | 0·04 | 0·8357 | 1, 52 | 0·01 | 0·31 | 0·5771 | 1, 52 | 0·09 | 1·78 | 0·1881 |
| k = 3 clusters | 2, 51 | 6·73 | 5·61 | 0·0063 | 2, 51 | 0·02 | 0·06 | 0·9397 | 2, 51 | 0·09 | 1·37 | 0·2636 | 2, 51 | 0·18 | 1·79 | 0·1779 |
Bold values indicate significance of P < 0·05.
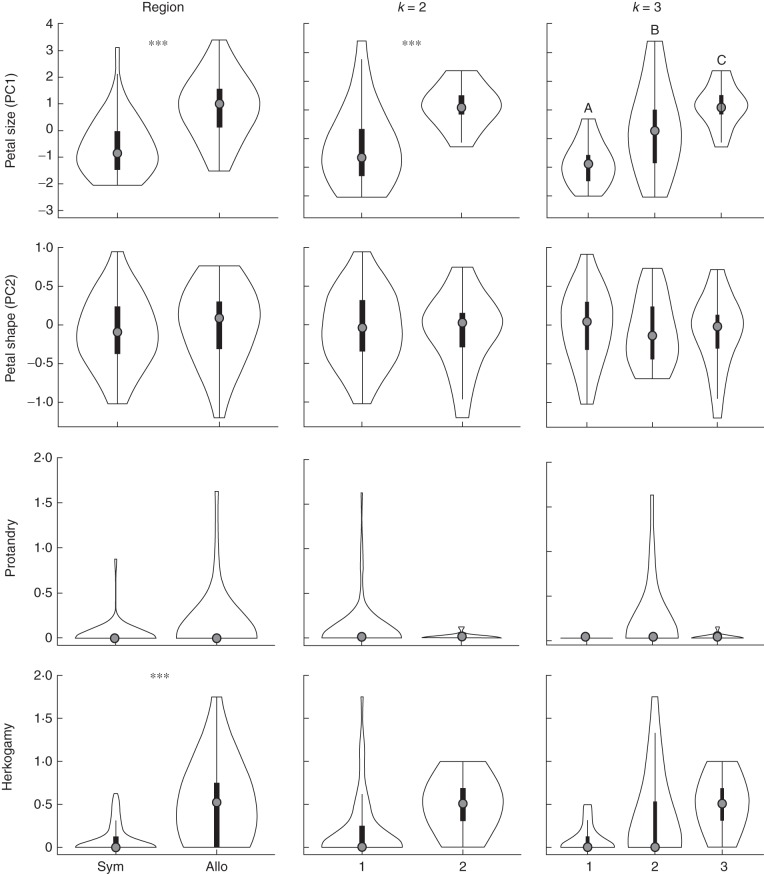
Fig. 2.

Flowers of parviflora from sympatric populations (left) and allopatric populations (right). The number under each flower photo indicates the populations (Supplementary Data Figure S1).
Fig. 3.
Floral divergence between regions and phylogeographical clusters (k = 2 and k = 3) for petal size (PC1), petal shape (PC2), protandry and herkogamy. Plots are violin plots where the external lines describe the probability density of the data using a smoothing kernel and the internal boxplot shows the upper and lower 25 and 75 % quantiles. The grey dot in the middle represents the mean. In panels with only one comparison (columns 1 and 2) asterisks denote significant differences (*0·05 ≥ P > 0·01; **0·01 ≥ P > 0·0001; ***P <0·0001). In column 3 letters represent significantly different groupings determined by post hoc Tukey tests.
Floral divergence was evident at both levels of phylogeographical clustering (k = the number of phylogeographical clusters identified by Instruct). Figure 1 shows the cluster to which each population belongs at k = 2 and k = 3. For k = 2, flowers in cluster 2, which includes a subset of allopatric populations, had 128 % larger petals than cluster 1, which includes all sympatric populations and a smaller number of allopatric populations (Table 1 and Fig. 2). There was no significant difference in petal shape, protandry or herkogamy (Table 1). For k = 3, all three clusters differed significantly in petal size. There was a decline in flower size from east to west, parallelling the sequence of range expansion revealed by phylogeographical clusters (Table 1 and Fig. 3). Most notably, two of the populations with the largest flowers had the highest probability of being ancestral from the Beast (Lemey et al., 2009) phylogeography analysis. Similar to k = 2, there were no significant differences among clusters in petal shape, protandry or herkogamy (Table 1 and Fig. 3).
Floral phenotype is statistically related to the proportion of flowers that set fruit through autonomous selfing (autofertility). Plants in the first greenhouse experiment with larger values of PC2 (i.e. shorter and wider petals) and reduced herkogamy set a greater proportion of fruit (χ2 = 7·537, df = 1, P = 0·0060 and χ2 = 4·731, df = 1, P = 0·0296, respectively). Although PC1 was not significant in this analysis, in a separate model that includes petal length and petal width in the place of PC1 and PC2, both floral characteristics are significantly correlated with autofertility such that plants with shorter and wider petals set a greater proportion of fruits through autonomous selfing.
Geographical variation in PL and RA
Allopatric and sympatric parviflora differed significantly in patterns of PL and RA, as indicated by the significant treatment by region interaction (Table 2a). In the allopatric region, treatments did not differ significantly suggesting there is no PL or RA (Supplementary Data Fig. S3). In the sympatric region, all three treatments differed significantly, indicating both PL and RA (Supplementary Data Fig. S3). In sympatry, supplemented flowers set on average 19 % more seed that unmanipulated flowers, indicating that sympatric parviflora suffer from overall PL. However, autonomous self-pollination does provide a mechanism of RA, as unmanipulated fruits set 59 % more seeds than emasculated fruits. There was limited potential for outcrossing in sympatric parviflora as evidenced by supplemented fruits producing 90 % more seed than emasculated fruits (i.e. high pollinator-mediated PL). Differences in PL or RA between regions were not influenced by regional differences in total seed production (Table 2a).
Table 2.
Fixed effects tables for ANOVAs testing for the effects of floral treatments (supplemented, unmanipulated, emasculated), geographical region (sympatric vs. allopatric) or phylogeographical cluster (k = 2 and k = 3), and their interaction on seed set; population nested within region or cluster was treated as a random factor.
| d.f. | SS | F | P | |
|---|---|---|---|---|
| Geographical designation | ||||
| Region | 1, 1076 | 414 | 0·55 | 0·4603 |
| Treatment | 2, 1076 | 96,748 | 63·77 | <0·0001 |
| Region × Treatment | 2, 1076 | 12,019 | 7·92 | 0·0004 |
| k = 2 clusters | ||||
| Cluster | 1, 1076 | 309 | 0·40 | 0·5255 |
| Treatment | 2, 1076 | 96,926 | 63·36 | <0·0001 |
| Cluster × Treatment | 2, 1076 | 4,591 | 3·00 | 0·0502 |
| k = 3 clusters | ||||
| Cluster | 2, 1073 | 6,077 | 4·01 | 0·0185 |
| Treatment | 2, 1073 | 97,157 | 64·02 | <0·0001 |
| Cluster × Treatment | 4, 1073 | 12,708 | 4·19 | 0·0023 |
Bold values indicate significance of P < 0·05.
We also detected differences in PL and RA among the finer-scale phylogeographical clusters (k = 3) but not at the coarser scale (k = 2). For k = 2, as with the regional model, there was an overall treatment effect (Table 2b); however, there was a non-significant treatment by cluster interaction (Table 2b and Supplementary Data Fig. S3). For k = 3, clusters differed in both the magnitude of seed production and in the pattern of PL and RA. A significant cluster by treatment interaction indicated that clusters differed in patterns of PL and RA (Table 2c). We found evidence for PL and RA in cluster 1, the western-most, sympatric lineage, but not in clusters 2 and 3, which include primarily eastern, allopatric populations (Supplementary Data Fig. S3). Within cluster 1, supplemented flowers set 17 % more seeds than unmanipulated flowers and supplemented flowers set 89 % more seeds than emasculated flowers, indicating overall and pollinator-mediated PL. Unmanipulated flowers set 63 % more seeds than emasculated flowers, indicative of RA.
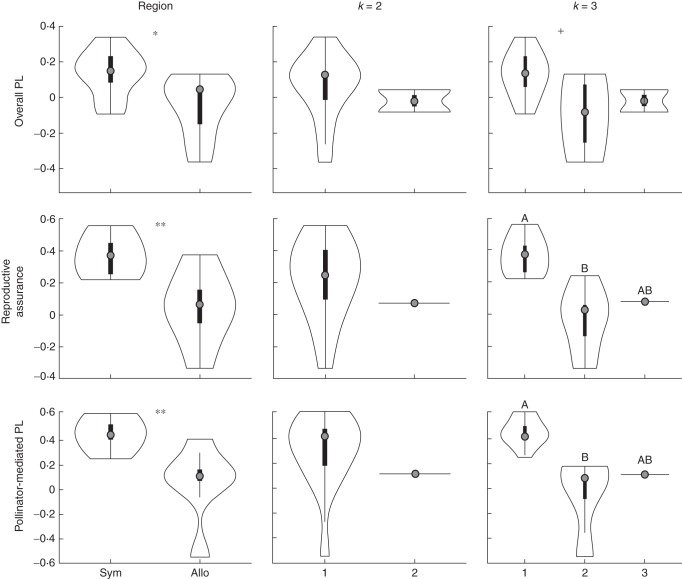
We find the same patterns when PL and RA are calculated for each population based on treatment means and ANOVAs are conducted on population-level values of PL and RA. When populations are grouped by region, sympatric populations have significantly greater PL and RA (Table 3a) and median values for allopatric populations are nearly zero (Fig. 4). Although there are similar trends for k = 2, differences were not significantly different (Table 3b and Fig. 4). At the finer level of clustering (k = 3), cluster 1 populations had significantly greater RA and pollinator-mediated PL and marginally significantly greater overall PL (Table 3c and Fig.4).
Table 3.
ANOVAs testing for the effects of geographical region (sympatric vs. allopatric) or phylogeographical clusters (k = 2 and k = 3) on population summary measures of reproductive ecology [overall pollen limitation (PL), reproductive assurance (RA) and pollinator-mediated PL]
| Overall PL |
RA |
Pollinator-mediated PL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | SS | F | P | d.f. | SS | F | P | d.f. | SS | F | P | |
| Regions | 1 | 0·14 | 5·46 | 0·0361 | 1 | 0·40 | 11·59 | 0·0047 | 1 | 0·57 | 11·18 | 0·0058 |
| k = 2 clusters | 1 | 0·01 | 0·31 | 0·5887 | 1 | 0·02 | 0·35 | 0·5630 | 1 | 0·03 | 0·32 | 0·5842 |
| k = 3 clusters | 2 | 0·16 | 3·02 | 0·0866 | 2 | 0·53 | 10·43 | 0·0024 | 2 | 0·75 | 9·44 | 0·0041 |
Bold values indicate significance of P < 0·05.
Fig. 4.
Population-level measures of overall pollen limitation (PL), reproductive assurance (RA) and pollinator-mediated PL between geographical regions and phylogeographical clusters (k = 2 and k = 3). Plots are violin plots where the external lines describe the probability density of the data using a smoothing kernel and the internal boxplot shows the upper and lower 25 and 75 % quantiles. The grey dot in the middle represents the mean. Significant differences are denoted by asterisks in panels with only two bars (*0·05 ≥ P > 0·01; **0·01 ≥ P > 0·0001; ***P <0·0001). Letters represent significantly different means detected by post hoc Tukey tests. Note that overall PL for k = 3 has a marginally significant difference between clusters 1 and 2 (P = 0·08), marked with a plus sign (+).
Reciprocal translocation experiment
The reciprocal translocation experiment showed that the differences in PL and RA that we detected between regions in the study of natural populations (above) is due only to the effect of genotype and not due to differences in the pollination environment. Across both sympatric and allopatric experimental sites, sympatric genotypes exhibited consistently greater RA and pollinator-mediated PL than allopatric genotypes (Table 4b, c and Fig. 5). In both regions, sympatric genotypes had >48 % RA and >49 % pollinator-mediated PL than allopatric genotypes. There was a marginally significant interaction between site region and genotype region for pollinator-mediated PL, where allopatric genotypes had proportionately less pollinator-mediated PL in allopatric than sympatric sites (Table 4c, Fig. 5).
Table 4.
Fixed effects tables for split-plot ANOVAs for the reciprocal translocation experiment
| Overall PL |
RA |
Pollinator-mediated PL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | SS | F | P | d.f. | SS | F | P | d.f. | SS | F | P | |
| Destination region | 1, 2 | 0·02 | 3·29 | 0·2156 | 1, 2 | 0·06 | 3·97 | 0·1843 | 1, 2 | 0·09 | 3·7 | 0·1940 |
| Source region | 1, 246 | 0·07 | 0·58 | 0·4472 | 1, 242 | 0·92 | 7·32 | 0·0073 | 1, 243 | 0·47 | 4·20 | 0·0414 |
| Destination × Source region | 1, 246 | 0·25 | 2·15 | 0·1442 | 1, 242 | 0·04 | 0·30 | 0·5822 | 1, 243 | 0·35 | 3·14 | 0·0775 |
We tested the effects of destination geographical region (sympatric vs. allopatric), which refers to the location of sites where experimental populations were constructed, source region (sympatric vs. allopatric), which refers to plant genotypes in constructed populations, and their interaction on three summary statistics of reproductive ecology: overall pollen limitation (PL), reproductive assurance (RA) and pollinator-mediated PL. Source population nested within source region and experimental site nested within destination region were treated as random factors. Bold values indicate significance of P < 0·05.
Fig. 5.
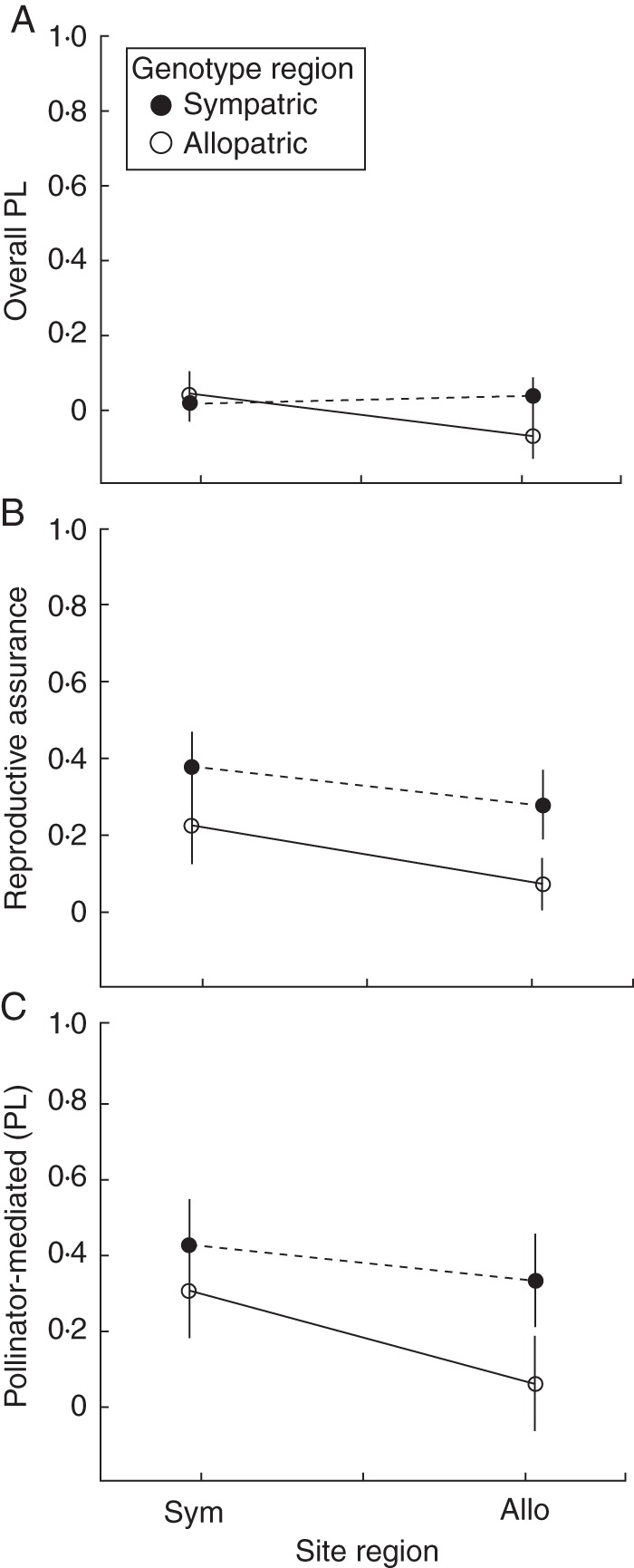
Pollen limitation and reproductive assurance from reciprocal translocation experiments. Categories on the x-axis indicate the region in which the experimental populations were located. Sympatric and allopatric genotypes are indicated by filled and open circles, respectively.
DISCUSSION
Reproductive character displacement (RCD) between (partially) compatible plant taxa is considered to be one potentially important outcome of reinforcement selection (Rundle and Schluter, 1998; Coyne and Orr, 2004; Springer and Crespi, 2004). Although RCD is not uncommon in plants (van der Niet et al., 2006), for most systems it is poorly understood whether the evolution of RCD has (1) been caused by interactions among congenerics and selection against traits that facilitate hybrid formation (reinforcement) or (2) is a by-product of selection caused by other aspects of the environment that are correlated with the transition from allopatry to sympatry. Here, we test the latter hypothesis for RCD in Clarkia xantiana subsp. xantiana and subsp. parviflora – that the highly reduced floral form (and higher propensity for selfing) and RCD of sympatric parviflora is a by-product of adaptation to limited pollinator availability in sympatry compared with allopatry. Field experiments in natural populations and reciprocal translocation experiments between allopatric and sympatric regions suggest that pollination environments do not differ substantially between sympatric and allopatric regions. Therefore, the strongly reduced flower size of sympatric parviflora is probably the result of other selective pressures.
Our previous phylogenetic and molecular population genetic studies have shown that xantiana and parviflora recently diverged in allopatry and subsequently came back into contact in secondary sympatry as a result of Holocene range expansion (Pettengill and Moeller 2012a, b). Specifically, parviflora populations at the far eastern portion of the range had the highest probability of ancestry (Lemey et al., 2009), and those populations occur where palaeo-distribution models predict suitable habitat was located at the last glacial maximum (Pettengill and Moeller, 2012b). Population genetic structure occurs across an east-to-west gradient from allopatry to sympatry, with little differentiation from north to south. This population structure is consistent with a pattern of westward range expansion since the last glacial maximum. Collectively, this historical information has provided polarity to the patterns of floral evolution we observed in this study. Notably, the most ancestral populations have the largest flowers and most closely resemble the outcrossing sister subspecies, xantiana. From the ancestral, eastern populations to the derived, western populations there is a continuous decline in flower size and herkogamy (anther–stigma distance) from east to west, both of which are associated with greater autofertility in sympatric populations. Our two common garden studies show that this floral variation has a strong genetic basis and that the geographical pattern of floral differentiation is repeatable across independent studies.
Previous population genetic studies showed that sympatric populations along a ∼50 × 5-km zone at the far western edge of parviflora's distribution form a distinct lineage, as determined by Instruct analyses at k = 3 (Pettengill and Moeller, 2012b). In this study, we showed that this distinct sympatric lineage also has significantly reduced flowers compared with populations that belong to the other phylogeographical clusters. One sympatric population at the northernmost edge of the species' range (6p) is not part of the distinct sympatric cluster and interestingly also does not have the highly reduced flower size characteristic of other sympatric parviflora populations. Our previous molecular population genetic studies suggest that significant introgression has occurred between xantiana and parviflora at this site (Pettengill and Moeller, 2012a, b). This introgression may have minimized differences between the two taxa in floral phenotypes and at putatively neutral sequenced loci. Introgression may be particularly pronounced at this most northern site because the flowering period of both subspecies can be compressed into a narrower window of time resulting in greater overlap (D. A. Moeller, personal observation).
Floral manipulations in natural populations showed that sympatric populations had greater levels of PL and RA than allopatric populations, suggesting that the potential for outcrossing is more limited for sympatric than allopatric populations. The regional differences in reproductive ecology parallel geographical patterns of variation in floral traits, particularly the east–west gradient in flower size (PC1) described above. Our findings are similar to the results of a series of studies in Collinsia parviflora where the magnitude of RA is correlated with among-population variation in flower size (Elle and Carney, 2003; Kennedy and Elle, 2008).
Identifying the causes of geographical covariation between floral traits and reproductive ecology is complicated by the fact that two alternative hypotheses are difficult to distinguish with studies of natural populations: (1) pollen limitation and/or reproductive assurance could be strong in small-flowered populations because of the pollination environment (pollinators or mates limit the potential for outcrossing) or (2) reduced floral traits hinder pollinator visitation even though pollinators are sufficiently available in the environment. In most studies of intraspecific geographical variation in floral form (e.g. Robertson and Wyatt, 1990; Johnson, 1997; Johnson and Steiner, 1997; Moeller, 2006; Perez-Barrales et al., 2007; Alonso et al., 2007), this problem hampers the ability to distinguish among the possible causes of trait changes. Quantifying geographical variation in pollinator communities or pollinator visitation rates and establishing that they covary with floral variation does not eliminate the possibility that floral differences themselves have subsequently altered interactions with potential pollinators. Reciprocal translocation experiments are important for testing the hypothesis that the pollination environment per se drove the evolution of floral form and for eliminating alternative hypotheses. Reciprocal transplant experiments are the norm for testing for local adaptation to abiotic environments (Leimu and Fischer, 2008; Hereford, 2009) but are less commonly used in studies of adaptation to biotic environments, particularly floral adaptation to pollination environments (but see Boberg et al., 2014; Sun et al., 2014). In our experiment, plants from the sympatric and allopatric region were reciprocally translocated using replicated artificial populations of potted plants and PL and RA were assessed. We found that genotype and not geographical region was the dominant factor predicting variation in PL and RA. Sympatric genotypes exhibited greater PL and RA than allopatric genotypes in both allopatric and sympatric sites. We did not find a significant effect of geographical region on any metric of reproductive ecology, indicating that pollination environments do not differ substantially between sympatric and allopatric portions of the species' range. Previous work has similarly suggested that pollinator availability does not differ between the allopatric and sympatric portions of the species' range; in fact, specialist bee pollinators, which are among the most effective pollinators, are limited only to the sympatric portion of parviflora's range (Fausto et al., 2001; Moeller, 2006).
Overall, our studies indicate that floral evolution in parviflora occurred during the process of range expansion from eastern Pleistocene refugia to the current western range limit (the zone of sympatry with xantiana). The results of field experiments in natural and reciprocally translocated populations are inconsistent with the hypothesis that floral evolution occurred as an adaptive evolutionary response to local pollinator environments. Instead, we found that sympatric genotypes were more pollen limited and that selfing provided greater reproductive assurance in all environments, presumably because the strongly reduced size of floral organs hindered pollinator visitation. It is difficult to rule out the possibility that mating system evolution occurred as a response to mate limitation, rather than pollinator limitation, during the colonization process involved in westward range expansion. Nevertheless, our results suggest that floral evolution in parviflora occurred as a response to other aspects of the biotic or abiotic environment. One possibility is that interactions between parviflora and its sister taxon, xantiana, via shared pollinators drove the evolution of reduced flower size in parviflora as a mechanism of reinforcement. The apparent reproductive character displacement between the large-flowered xantiana and smaller-flowered parviflora is consistent with the possibility that selection has favoured traits in parviflora that minimize the receipt of congeneric pollen from xantiana and thereby limit the formation of hybrids. We are actively testing this hypothesis using experiments that dissect the contribution of floral variation to rates of hybridization and that quantify the fitness effects of hybridization across the life cycle.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Justin Iverson, Jason Kopp and James Pettengill for assistance with field and greenhouse experiments. Funding was provided by grants from the National Science Foundation (DEB-1025004) and the University of Minnesota to D.A.M.
LITERATURE CITED
- Alonso C, Mutikainen P, Herrera CM. Ecological context for breeding system variation: sex, size and pollination in a (predominantly) gynodioecious shrub. Annals of Botany. 2007;100:1547–1556. doi: 10.1093/aob/mcm254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman TL, Knight TM, Steets JA, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Blair WF. Mating call and stage of speciation in the Microhyla olivacea – M. carolinensis complex. Evolution. 1955;9:469–480. [Google Scholar]
- Boberg E, Alexandersson R, Jonsson M, Maad J, Ågren J, Nilsson LA. Pollinator shifts and the evolution of spur length in the moth-pollinated orchid Platanthera bifolia. Annals of Botany. 2014;113 doi: 10.1093/aob/mct217. 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Briscoe Runquist RD, Moeller DA. Resource reallocation does not influence estimates of pollen limitation or reproductive assurance in Clarkia xantiana ssp. parviflora. American Journal of Botany. 2013 doi: 10.3732/ajb.1300050. in press. [DOI] [PubMed] [Google Scholar]
- Brys R, Geens B, Beeckman T, Jacquemyn H. Differences in dichogamy and herkogamy contribute to higher selfing in contrasting environments in the annual Blackstonia perfoliata (Gentianaceae) Annals of Botany. 2013;111:651–661. doi: 10.1093/aob/mct031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch JW, Joly S, Schoen DJ. Demographic signatures accompanying the evolution of selfing in Leavenworthia alabamica. Molecular Biology and Evolution. 2011;28:1717–1729. doi: 10.1093/molbev/msq352. [DOI] [PubMed] [Google Scholar]
- Butlin RK. Reinforcement of premating isolation. In: Otte D, Endler JA, editors. Speciation and its consequences. Sunderland, MA: Sinauer Associates; 1989. pp. 158–179. [Google Scholar]
- Cosacov A, Cocucci AA, Sérsic AN. Geographical differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza: do pollinators matter? Annals of Botany. 2014;113 doi: 10.1093/aob/mct239. 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- de Jager ML, Ellis AG. Floral polymorphism and the fitness implications of attracting pollinating and florivorous insects. Annals of Botany. 2014;113 doi: 10.1093/aob/mct189. 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics and the origin of species. New York: Columbia Univeristy Press; 1937. [Google Scholar]
- Dobzhansky T. Speciation as a stage in evolutionary divergence. The American Naturalist. 1940;74:302–321. [Google Scholar]
- Dornier A, Munoz F, Cheptou PO. Allee effect and self-fertilization in hermaphrodites: reproductive assurance in a structured metapopulation. Evolution. 2008;62:2558–2569. doi: 10.1111/j.1558-5646.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Dart S. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 183–203. [Google Scholar]
- Eckhart VM, Geber MA. Character variation and geographic distribution of Clarkia xantiana (Onagraceae): flowers and phenology distinguish two subspecies. Madrono. 1999;46:117–125. [Google Scholar]
- Eckhart V, Geber M, Morris W, Fabio E, Tiffin P, Moeller D. The geography of demography: long-term demographic studies and species distribution models reveal a species border limited by adaptation. American Naturalist. 2011;178:S26–S43. doi: 10.1086/661782. [DOI] [PubMed] [Google Scholar]
- Elle E, Carney R. Reproductive assurance varies with flower size in Collinsia parviflora (Scrophulariaceae) American Journal of Botany. 2003;90:888–896. doi: 10.3732/ajb.90.6.888. [DOI] [PubMed] [Google Scholar]
- Fausto J, Eckhart V, Geber M. Reproductive assurance and the evolutionary ecology of self-pollination in Clarkia xantiana (Onagraceae) American Journal of Botany. 2001;88:1794–1800. [PubMed] [Google Scholar]
- Fishman L, Wyatt R. Pollinator-mediated competition, reproductive character displacement, and the evolution of selfing in Arenaria uniflora (Caryophyllaceae) Evolution. 1999;53:1723–1733. doi: 10.1111/j.1558-5646.1999.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Foxe JP, Slotte T, Stahl EA, Neuffer B, Hurka H, Wright SI. Recent speciation associated with the evolution of selfing in Capsella. Proceedings of the National Academy of Sciences USA. 2009;106:5241–5245. doi: 10.1073/pnas.0807679106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society B-Biological Sciences. 1999;266:2247–2252. [Google Scholar]
- Gao H, Williamson S, Butamante CD. A Markov Chain Monte Carlo approach for joint inference of population structure and inbreeding rates from multilocus genotype data. Genetics. 2007;176:1635–1651. doi: 10.1534/genetics.107.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EE, Igic B. Tempo and mode in plant breeding system evolution. Evolution. 2012;66:3701–3709. doi: 10.1111/j.1558-5646.2012.01730.x. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Muñoz-Pajares AJ, Abdelaziz M, Lorite J, Perfectti F. Evolution of pollination niches and floral divergence in the generalist plant Erysimum mediohispanicum. Annals of Botany. 2014;113 doi: 10.1093/aob/mct186. 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom MJ. Allee effects limit population viability of an annual plant. American Naturalist. 1998;151:487–496. doi: 10.1086/286135. [DOI] [PubMed] [Google Scholar]
- Grossenbacher DL, Whittall JB. Increased floral divergence in sympatric monkeyflowers. Evolution. 2011;65:2712–2718. doi: 10.1111/j.1558-5646.2011.01306.x. [DOI] [PubMed] [Google Scholar]
- Hackney EE, McGraw JB. Experimental demonstration of an Allee effect in American ginseng. Conservation Biology. 2001;15:129–136. [Google Scholar]
- Harrison RG. The language of speciation. Evolution. 2012;66:3643–3657. doi: 10.1111/j.1558-5646.2012.01785.x. [DOI] [PubMed] [Google Scholar]
- Hereford J. A quantitative survey of local adaptation and fitness trade-offs. American Naturalist. 2009;173:579–588. doi: 10.1086/597611. [DOI] [PubMed] [Google Scholar]
- Herlihy CR, Eckert CG. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature. 2002;416:320–323. doi: 10.1038/416320a. [DOI] [PubMed] [Google Scholar]
- Hopkins R. Reinforcement in plants. New Phytologist. 2013;197:1095–1103. doi: 10.1111/nph.12119. [DOI] [PubMed] [Google Scholar]
- Hopkins R, Rausher MD. Pollinator-mediated selection on flower color allele drives reinforcement. Science. 2012;335:1090–1092. doi: 10.1126/science.1215198. [DOI] [PubMed] [Google Scholar]
- Howard DJ. Reinforcement: origin, dynamics, and the fate of an evolutionary hypothesis. In: Harrison RG, editor. Hybrid zones and the evolutionary process. New York: Oxford University Press; 1993. pp. 46–69. [Google Scholar]
- Ippolito A, Fernandes GW, Holtsford TP. Pollinator preferences for Nicotiana alata, N-forgetiana, and their F-1 hybrids. Evolution. 2004;58:2634–2644. doi: 10.1111/j.0014-3820.2004.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Jain SK. Evolution of inbreeding in plants. Annual Review of Ecology and Systematics. 1976;7:469–495. [Google Scholar]
- Johnson SD. Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society. 1997;123:225–235. [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 295–310. [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Kalisz SD, Vogler DW, Hanley KM. Context-dependent autonomous self-fertilization yields reproductive assurance and mixed mating. Nature. 2004;430:884–887. doi: 10.1038/nature02776. [DOI] [PubMed] [Google Scholar]
- Kay KM. Reproductive isolation between two closely related hummingbird-pollinated neotropical gingers. Evolution. 2006;60:538–552. [PubMed] [Google Scholar]
- Kay KM, Schemske DW. Natural selection reinforces speciation in a radiation of neotropical rainforest plants. Evolution. 2008;62:2628–2642. doi: 10.1111/j.1558-5646.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Kennedy BF, Elle E. The reproductive assurance benefit of selfing: importance of flower size and population size. Oecologia. 2008;155:469–477. doi: 10.1007/s00442-007-0924-7. [DOI] [PubMed] [Google Scholar]
- Lamont BB, Klinkhamer PGL, Witkowski ETF. Population fragmentation may reduce fertility to zero in Banksia goodii: a demonstration of the Allee effect. Oecologia. 1993;94:446–450. doi: 10.1007/BF00317122. [DOI] [PubMed] [Google Scholar]
- Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0004010. e4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds it roots. PLoS Computational Biology. 2009;5 doi: 10.1371/journal.pcbi.1000520. e1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DA, Schaal BA. Corolla color as an inhibitor of interspecific hybridization in Phlox. American Naturalist. 1970;104:273–283. [Google Scholar]
- Lloyd DG. Self-fertilization and cross-fertilization in plants. 2 The selection of self-fertilization. International Journal of Plant Sciences. 1992;153:370–380. [Google Scholar]
- Martin NH, Willis JH. Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution. 2007;61:68–82. doi: 10.1111/j.1558-5646.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Facilitative interactions among plants via shared pollinators. Ecology. 2004;85:3289–3301. [Google Scholar]
- Moeller DA. Pollinator community structure and sources of spatial variation in plant–pollinator interactions in Clarkia xantiana ssp. xantiana. Oecologia. 2005;142:28–37. doi: 10.1007/s00442-004-1693-1. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moeller DA, Geber MA. Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution. 2005;59:786–799. doi: 10.1554/04-656. [DOI] [PubMed] [Google Scholar]
- Morgan MT, Wilson WG. Self-fertilization and the escape from pollen limitation in variable pollination environments. Evolution. 2005;59:1143–1148. [PubMed] [Google Scholar]
- Newman E, Manning J, Anderson B. Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany. 2014;113:373–384. doi: 10.1093/aob/mct203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell JR, Barrett SCH. Baker's law revisited: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Whitehead MR. Floral odour chemistry defines species boundaries and inderpins strong reproductive isolation in sexually deceptive orchids. Annals of Botany. 2014;113 doi: 10.1093/aob/mct199. 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. A pollinator shift explains floral divergence in an orchid species complex in South Africa. Annals of Botany. 2014;113 doi: 10.1093/aob/mct216. 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Barrales R, Arroyo J, Armbruster WS. Differences in pollinator faunas may generate geographic differences in floral morphology and integration in Narcissus papyraceus (Alarcissiopapyraceris) Oikos. 2007;116:1904–1918. [Google Scholar]
- Pettengill J, Moeller D. Tempo and mode of mating system evolution between incipient Clarkia species. Evolution. 2012a;66:1210–1225. doi: 10.1111/j.1558-5646.2011.01521.x. [DOI] [PubMed] [Google Scholar]
- Pettengill JB, Moeller DA. Phylogeography of speciation: allopatric divergence and secondary contact between outcrossing and selfing Clarkia. Molecular Ecology. 2012b;21:4578–4592. doi: 10.1111/j.1365-294X.2012.05715.x. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R version 2·15·1: “Roasted Marshmallows”. Vienna: The R Foundation for Statistical Computing; 2012. www.r-project.org . [Google Scholar]
- Rausher MD. Evolutionary transitions in floral color. International Journal of Plant Sciences. 2008;169:7–21. [Google Scholar]
- Rivest LP. Statistical properties of windsorized means for skewed distributions. Biometrika. 1994;81:373–383. [Google Scholar]
- Robertson JL, Wyatt R. Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris. Evolution. 1990;44:121–133. doi: 10.1111/j.1558-5646.1990.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Rundle HD, Schluter D. Reinforcement of stickleback mate preferences: sympatry breeds contempt. Evolution. 1998;52:200–208. doi: 10.1111/j.1558-5646.1998.tb05153.x. [DOI] [PubMed] [Google Scholar]
- Runions CJ, Geber MA. Evolution of the self-pollinating flower in Clarkia xantitana (Onangraceae). I. Size and development of floral organs. American Journal of Botany. 2000;87:1439–1451. [PubMed] [Google Scholar]
- Schemske DW, Bierzychudek P. Spatial differentiation for flower color in the desert annual Linanthus parryae: was Wright right? Evolution. 2007;61:2528–2543. doi: 10.1111/j.1558-5646.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Schluter PM. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annual Review of Entomology. 2009;54(425–446) doi: 10.1146/annurev.ento.54.110807.090603. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Morgan MT, Bataillon T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Philosophical Transactions of the Royal Society London-B. 1996;351:1281–1290. [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annual Review of Ecology Evolution and Systematics. 2003;34:339–364. [Google Scholar]
- Springer SA, Crespi BJ. Adaptive gamete-recognition divergence in a hybridizing Mytilus population. Evolution. 2004;61:772–783. doi: 10.1111/j.1558-5646.2007.00073.x. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms. 1. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Stebbins GL. Flowering plants: evolution above the species level. Cambridge, MA: Harvard University Press; 1974. [Google Scholar]
- Strauss SY, Whittall JB. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 120–138. [Google Scholar]
- Sun M, Gross K, Schiestl FP. Floral adaptation to local pollinator guilds in a terrestrial orchid. Annals of Botany. 2014;113 doi: 10.1093/aob/mct219. 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Niet T, Johnson SD, Linder HP. Macroevolutionary data suggest a role for reinforcement in pollination system shifts. Evolution. 2006;60:1596–1601. doi: 10.1554/05-705.1. [DOI] [PubMed] [Google Scholar]
- van der Niet T, Pirie MD, Shuttleworth A, Johnson SD, Midgley JJ. Do pollinator distributions underlie the evolution of pollination ecotypes in the Cape shrub Erica plukenetii. Annals of Botany. 2014;113:301–315. doi: 10.1093/aob/mct193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J, Mackenzie S. Why are all colour combinations not equally represented as flower-colour polymorphisms? New Phytologist. 2001;151:237–241. doi: 10.1046/j.1469-8137.2001.00159.x. [DOI] [PubMed] [Google Scholar]
- Wyatt R. Phylogenetic aspects of the evolution of self-pollination. In: Gottlieb LD, Jain SK, editors. Plant evolutionary biology. Dordrecht: Springer; 1988. pp. 109–131. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.