Abstract
Background and Aims
Plant populations experiencing divergent pollination environments may be under selection to modify floral traits in ways that increase both attractiveness to and efficiency of novel pollinators. These changes may come at the cost of reducing overall effectiveness of other pollinators. The goal of this study was to examine differences in attractiveness and efficiency between Clarkia concinna and C. breweri, sister species of annual plants with parapatric distributions.
Methods
An assessment was made as to whether observed differences in visitors between natural populations are driven by differences in floral traits or differences in the local pollination environment. Differences in floral attractiveness were quantified by setting out arrays of both species in the geographical range of each species and exposing both species to nocturnal hawkmoths (Hyles lineata) in flight cages. Differences in visitor efficiency were estimated by measuring stigma–visitor contact frequency and pollen loads for diurnal visitors, and pollen deposition on stigmas for hawkmoths.
Key Results
The composition of visitors to arrayed plants was similar between plant species at any particular site, but highly divergent among sites, and reflected differences in visitors to natural populations. Diurnal insects visited both species, but were more common at C. concinna populations. Hummingbirds and hawkmoths were only observed visiting within the range of C. breweri. Despite attracting similar species when artificially presented together, C. concinna and C. breweri showed large differences in pollinator efficiency. All visitors except hawkmoths pollinated C. concinna more efficiently.
Conclusions
Differences in the available pollinator community may play a larger role than differences in floral traits in determining visitors to natural populations of C. concinna and C. breweri. However, floral traits mediate differences in pollinator efficiency. Increased effectiveness of the novel hawkmoth pollinator on C. breweri comes at relatively little cost in attractiveness to other visitors, but at large cost in their efficiency as pollinators.
Keywords: Clarkia concinna, Clarkia breweri, floral traits, Hyles lineata, parapatric divergence, pollinator effectiveness, pollinator efficiency, pollinator shift, speciation
INTRODUCTION
Changes in floral phenotypes to attract novel pollinators have been common and important in the evolutionary history of the angiosperms (Grant, 1949; Baker, 1959; Stebbins, 1970; Kay et al., 2006). Divergence in pollinator use can reduce gene flow among populations, potentially facilitating speciation (Grant, 1994; Schemske and Bradshaw, 1999; Kay and Sargent, 2009). The external pollinator environment is probably paramount in driving adaptation to novel pollinators (Stebbins, 1970; Harder and Barrett, 2007; Thomson and Wilson, 2008; Kay and Sargent, 2009), although there may be an important additional role for certain types of mutations that cause large changes in colour or reward in facilitating or constraining pollinator transitions (e.g. Bradshaw et al., 1998; Bradshaw and Schemske, 2003; Rausher, 2008). Geographical variation in the attributes of pollinator assemblages or the co-occurring plant community may result in range-wide differences in visitation rates of pollinators (Grant and Grant, 1965; Eckert, 2002; Aigner, 2005; Moeller, 2005; Hersch and Roy, 2007). These differences can drive selection for ecotypes that are better matched with the local pollination environment (Johnson, 1997; Johnson and Steiner, 1997; Perez-Barrales et al., 2007; Kay and Sargent, 2009; Anderson et al., 2010; Newman et al., 2012).
Adaptation to novel pollinators may come at the cost of reduced effectiveness of existing pollinators, as a single floral phenotype is unlikely to be optimal for all pollination environments. These pollinator effectiveness trade-offs may play a large role in determining the ease of transition to a novel floral phenotype (Aigner, 2001, 2004; Sargent and Otto, 2006; Muchhala, 2007; Kay and Sargent, 2009; Dell'Olivo and Kuhlemeler, 2013). Altering the floral phenotype to increase the effectiveness of a novel pollinator may result in a large decrease in effectiveness of a former pollinator (Fig. 1). In this so-called ‘concave’ trade-off, an intermediate phenotype may suffer reduced fitness due to poor effectiveness of both pollinators, making the evolutionary transition difficult. Alternatively, increasing the effectiveness of a novel pollinator may come with little or no cost in the effectiveness of the former pollinator (Fig. 1). If the transition involves a ‘convex’ trade-off or no trade-off at all, then a new pollinator may be more readily acquired (Aigner, 2001; Sargent and Otto, 2006). However, a convex trade-off is less likely to reduce gene flow between ancestral and derived morphologies, potentially slowing or preventing pollinator-driven speciation.
Fig. 1.
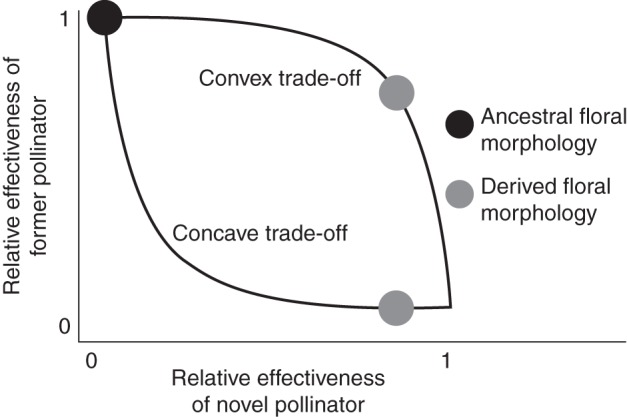
The shapes of two hypothetical trade-offs in pollinator effectiveness during acquisition of a novel pollinator. Evolution of a novel morphology that increases the effectiveness of a novel pollinator can come with relatively little loss in effectiveness of a former pollinator (convex trade-off) or a relatively large loss in effectiveness (concave trade-off). The acquisition of a novel pollinator with no loss in effectiveness of the former pollinator (no tradeoff) is also possible. The ancestral floral morphology is expected to show lower effectiveness of the novel pollinator regardless of the trade-off shape. Different components of effectiveness, such as attractiveness and efficiency, can have differently shaped trade-offs for the same pollination shift. Figure modified from Sargent and Otto (2006).
The contribution of a pollinator to a plant's reproductive fitness is a function of its visitation rate, the proportion of visits that result in successful deposition of pollen, the amount of pollen deposited given the amount removed and the quality of the pollen deposited. Although pollination biologists disagree on the terminology for these attributes, here we consider the overall effectiveness of a floral visitor as the combination of two components – attractiveness and efficiency (Stebbins, 1970; Herrera, 1987; Madjidian et al., 2008; Ne'eman et al., 2010). Attractiveness affects visitation rates and will depend on traits such as flower size (e.g. Kaczorowski et al., 2012), colour (e.g. Streisfeld and Kohn, 2007; Newman et al., 2012), scent (e.g. Hirota et al., 2012), and the amount and accessibility of the reward (e.g. Fulton and Hodges, 1999; Schemske and Bradshaw, 1999). Efficiency affects the per-visit likelihood or quantity of removal and subsequent deposition of pollen on a receptive conspecific stigma. Efficiency depends on the positioning of reproductive parts and morphological modifications that alter pollinator behaviour at the flower (Schemske and Horvitz, 1984; Inouye et al., 1994; Lau and Galloway, 2004; Anderson and Johnson, 2008). If traits affecting attractiveness and efficiency vary independently, the existence or shape of trade-offs can differ between these two aspects of pollinator effectiveness.
Before selection for increased efficiency of novel pollinators can occur, novel pollinators must first visit flowers at non-trivial rates. At the initial stages of a pollinator shift, we hypothesize that attractiveness trade-offs are usually convex or non-existent, facilitating the attraction of novel pollinators without excluding existing pollinators. There is some evidence for convex attractiveness trade-offs, as many seemingly specialized plant species attract a wide variety of visitors, and closely related species with divergent morphologies often still show substantial overlap in visitor assemblages (Dilley et al., 2000; Waser, 2001; Aigner, 2005; Smith et al., 2008; Botes et al., 2009; Kessler and Baldwin, 2011).
Once changes in attractiveness traits occur, selection can act to increase the efficiency of novel visitors. If these visitors differ substantially in size, morphology or behaviour, a floral phenotype that is efficiently pollinated by both former and novel pollinators may not be possible. Therefore, efficiency trade-offs may often be concave (Muchhala, 2007). If novel visitors have higher visitation rates than former visitors in the local environment, selection for novel efficiency traits may occur despite a concave trade-off. While differences in visitation rates between closely related species or populations are frequently studied, differences in pollinator efficiency are less commonly quantified (reviewed by Kay and Sargent, 2009).
Although it is difficult to determine the order in which floral traits evolved by studying the current evolutionary endpoints, we can assess the differences in attraction and efficiency of potential pollinators in carefully chosen sister taxa. In this study, we quantify differences in visitor effectiveness between Clarkia concinna and C. breweri (Onagraceae), two closely related sister species with parapatric ranges that occur across a steep environmental gradient of precipitation and habitat types in the coast ranges of California, USA. Clarkia concinna is probably more similar to the ancestral phenotype (Lewis and Lewis, 1955; Sytsma et al., 1990), and is visited by a variety of diurnal insects (MacSwain et al., 1973; Groom, 1998). Clarkia breweri is unique in the genus in having sweet-scented, night-opening flowers that are predicted to attract hawkmoths (MacSwain et al., 1973; Raguso, 1995; Raguso and Pichersky, 1995). We specifically chose to study parapatric species, as they are more likely to be representative of the early stages of geographical divergence in response to different pollination environments, and floral traits are unlikely to have been shaped by sympatric competition for pollinators (e.g. Armbruster et al., 1994), facilitation of pollination (e.g. Moeller, 2004) or selection against hybridization (i.e. reinforcement; e.g. Kay and Schemske, 2008).
Our study uses C. concinna and C. breweri to investigate the transition to a novel pollinator. We first quantify the between-species differences in floral morphology that may be involved in this transition. Next, we compare the visitor assemblages and the amount of diurnal and nocturnal pollen receipt in natural populations of both species. We expect nocturnal hawkmoths to visit and pollinate C. breweri, and diurnal insects to visit and pollinate C. concinna. We then compare visitation rates of different functional groups between plant species when placed together in each species' geographical range. We predict that, when provided a choice, hawkmoths would prefer C. breweri, and all other visitors will not show a strong preference. Finally, we compare estimates of pollinator efficiency for each functional group of pollinators between plant species. We predict that diurnal insects will more efficiently pollinate C. concinna and hawkmoths will more efficiently pollinate C. breweri.
MATERIALS AND METHODS
Study system
Clarkia concinna (Fisch. & C.A.Mey.) Greene and C. breweri Greene are sister species of self-compatible, annual herbs endemic to the Northern and Central California Coast Ranges, USA, with C. breweri replacing C. concinna at the south-eastern (drier) edge of the latter species' range (Lewis and Lewis, 1955; Gottlieb and Weeden, 1979; Fig. 2). The more broadly distributed C. concinna occurs in chaparral, mixed evergreen forest and mesic oak/pine woodland, whereas C. breweri is restricted to xeric, exposed hillsides in chaparral with a Pinus sabiniana overstorey. Sites with C. breweri populations average half the precipitation of C. concinna sites [42·8 cm (s.d. 9·1 cm) and 83·6 cm (s.d. 26·9 cm), respectively] and populations bloom approximately 1 month earlier (April–May versus May–July; T. Miller, unpubl. res.). Both species have four showy, lobed and clawed petals, a long, narrow hypanthium containing a nectar reward, four stamens and an inferior ovary (Fig. 3). Flowers are protandrous with the four-lobed stigma opening and becoming receptive 2–7 d after anthesis. Previous observations of natural populations indicate that C. concinna is visited by long-tongued flies, butterflies and bees (MacSwain et al., 1973; Groom, 1998). Clarkia breweri is much paler pink in colour than the bright magenta of C. concinna. Additionally, the former is more strongly scented, due to the production of sweetly scented linalool and its pyranoid oxide (in addition to novel aromatic volatiles) at rates 250 times higher than C. concinna per unit floral mass (Pichersky et al., 1994; Raguso and Pichersky, 1995).
Fig. 2.
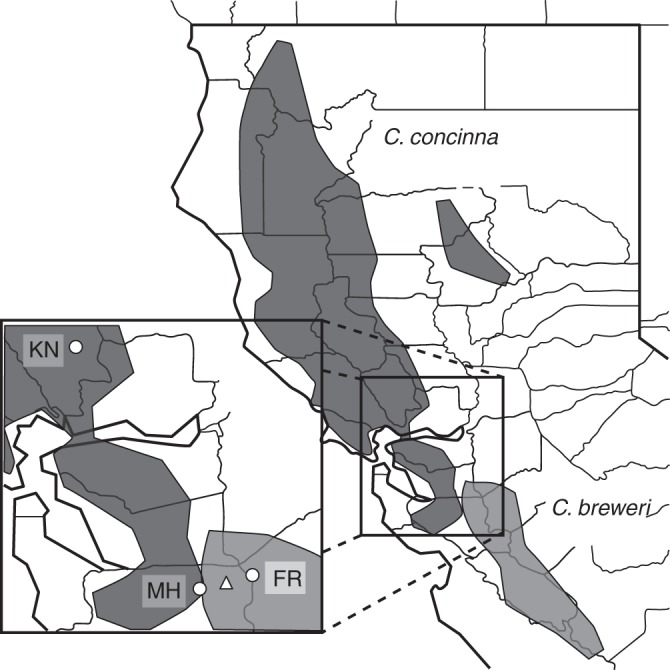
Range map of Clarkia concinna and Clarkia breweri in California, USA. Inset: circles denote the array locations. MH includes two array locations, MHN and MHS located on the north and south sides of Mount Hamilton. The triangle indicates the site of seed collection for C. breweri. Clarkia concinna seed was collected at KN. We conducted natural population observations for C. breweri at FR and the seed collection site. We conducted natural population observations for C. concinna at KN and five additional sites within 15 km.
Fig. 3.
Different functional groups show different efficiencies on C. concinna (left column of photos) and C. breweri (right column). (A, B) Small bees either collect pollen or nectar at the opening of the hypanthium tube. With both foraging behaviours, they frequently contact the stigma of C. concinna but rarely contact the highly exserted stigma of C. breweri. (C, D) Long-tongued flies insert their probosces into the hypanthium tube, while standing on the anthers and stigma of C. concinna and standing on the filaments and style in C. breweri, and therefore rarely contacting the stigma of C. breweri. Large bees (not shown) show similar behaviour and efficiency to long-tongued flies. (E) Hawkmoths are unable to insert their probosces far into the extremely narrow hypanthium tube of C. concinna, preventing them from fully contacting the stigma. Note the bent proboscis and the distance between the hawkmoth's body and the white stigma. (F) In contrast, hawkmoths fully insert their probosces into C. breweri's wider hypanthium tube, and their bodies and legs make contact with the stigma. Note the legs placed directly on the stigma. (G, H) Hummingbirds typically contact the slightly exserted stigma of C. concinna with their face and bill, but rarely do so for C. breweri, with its highly exserted and low-positioned stigma.
Both species provide nectar as a floral reward. The nectar differs between species in composition (mean sucrose/hexose ratios are 2 : 3 for C. concinna (n = 10) and 3 : 2 for C. breweri (n = 10), as determined via high-performance liquid chromatography; see Raguso et al., 2003 for methods), but total percentage sugar content is similar [brix, Bellingham & Stanley refractometer; C. breweri (n = 18) 33·9 ± 9·9 % per flower; C. concinna (n = 22) 35·0 ± 7·5 % per flower, t = 0·40, d.f. = 38, P = 0·69]. C. breweri produces more nectar per flower [nectar from greenhouse-grown plants mean ± SEM of standing crop: C. breweri (n = 18) 6·2 ± 2·8 µL per flower; C. concinna (n = 22) 2·3 ± 0·8 µL per flower, t = 6·32, d.f. = 38, P < 0·001]. The paler colour and fragrance of C. breweri (MacSwain et al., 1973), more copious nectar and the tendency of C. breweri's anthers to dehisce in the evening (K. Kay and D. Picklum, unpub. res., R. Raguso, unpubl. res.) suggest a nocturnal moth pollination syndrome. The current species distributions and novelty of the floral traits of C. breweri support a pattern of recent evolution of the xeric-adapted C. breweri from a mesic-adapted C. concinna-like ancestor (Lewis and Lewis, 1955; Sytsma et al., 1990).
Seed sources
We collected seed for all experiments from two natural populations in 2008 and 2010 near centres of abundance for both species (Fig. 2). Seed of C. breweri originated from a site 15 km east of the summit of Mount Hamilton along San Antonio Road, Santa Clara County, California (WGS 1984 coordinate system: 37·354 °N, 121·560 °W, elevation 630 m; MH). Seed of C. concinna originated from a site along Knoxville Road, south of Lake Berryessa, Napa County, California (38·497 °N, 121·242 °W, 160 m; KN). Both sites have habitats typical of their respective species. The seeds were stored in dry silica at 4 °C until planting. In the winters of 2010 and 2011, we planted seeds in 3·8-cm-diameter containers in a mixture of four parts Pro-Mix HP Mycorrhizae potting soil to one part perlite. Germination occurred in Conviron E-15 growth chambers (15 °C, 14-h light; 10-h 10 °C dark) and we moved plants to a greenhouse when secondary leaves had emerged. Plants were kept in the greenhouse at 13–21 °C until transport to the field sites.
Floral morphology
We characterized floral morphology by taking measurements with digital calipers from one flower with a freshly open stigma on 17 greenhouse-grown plants of each species. We measured corolla diameter from the tip of the left petal to the tip of the right petal, and petal width at the widest point of the top petal. We measured style and filament length from the hypanthium opening and hypanthium width just below the opening and just above the ovary. We used a discriminant analysis to compare floral measurements between species. All statistics were performed in JMP version 10·0 (SAS, USA).
Floral visitors to natural populations
To quantify differences in pollinator assemblages, we observed insect and hummingbird visitors to natural populations of both species. All observations occurred during peak flowering. Diurnal observations were conducted from mid-morning until late afternoon, corresponding with the peak in diurnal pollinator activity. Nighttime observations were conducted with the aid of a red-filtered flashlight from dusk until complete darkness, from approx. 2000 to 2200 h, with the exact interval dependent on the time of year. This time of day corresponds with the peak in hawkmoth activity. We observed C. breweri in April and May 1990–1994 at the seed collection site, and at an additional site along Del Puerto Canyon Road, near Frank Raines Regional Park, Stanislaus County, California (37·424 °N, 121·369 ° W, 310 m; FR; Fig. 2). Total observation time was 55 h (36 daytime and 19 nighttime hours over 37 d). We observed C. concinna in May 1990 and June 2010 at the seed collection site and five additional nearby sites (within 15 km). Visitor communities were qualitatively similar between years, so the 1990 and 2010 data were combined. Total observation time was 32 h (5 daytime hours over 2 d in 1990, 21 daytime and 6 nighttime hours over 6 d in 2010).
We classified visitors into six functional groups based on size and behaviour (Fenster et al., 2004): long-tongued flies (Bombyliidae and Acroceridae), small bees (Andrenidae, Megachilidae, Halictidae and Apidae in the genera Anthophora and Ceratina), large bees (Apidae in the genera Xylocopa, Bombus and Apis), lepidopterans (butterflies and moths excluding Sphingidae), hawkmoths (Sphingidae) and hummingbirds (Calypte anna and Archilochus alexandri). Short-tongued flies (Syrphidae and Calliphoridae) and beetles also visited both the natural populations and the floral arrays at low rates, but were not observed contacting reproductive structures of either species and thus are excluded from analyses. We observed a visitor for the duration of its foraging bout and counted the total number of visits. As the goal was to accumulate a large number of pollinator observations, the total number of flowers was not standardized, and varied between several to a few hundred depending on population density and observer position. We calculated the number of visits per hour of observation and the relative proportion of the observations for each functional class.
Pollinator exclosures
We compared pollen deposition from nocturnal and diurnal visitors in natural populations of both species by covering flowers during the day or night. Clarkia breweri exclosures (n = 66 per treatment) were performed in May 1991 at FR and in May 1993 at the seed collection site. Clarkia concinna exclosures (n = 27 per treatment) were performed in June 2013 at the Donald and Sylvia McLaughlin Natural reserve (38·869 °N, 122·378 °W, 510 m). To measure only pollinator-mediated pollen deposition, we emasculated flowers with unopened stigmas and covered them with bridal veil. Day exposed flowers were uncovered from dawn until dusk and night exposed flowers from dusk until dawn. The treatment continued until corolla dehiscence, 36–72 h after treatment initiation. Then, stigmas were collected and pollen grains counted. Clarkia pollen is easily distinguished from other pollen by its large size, pink colour and triangular shape, and we examined pollen loads when our species of interest was the only blooming Clarkia in the vicinity. Five-day exposed C. concinna stigmas were eaten by beetles and were not included in the analyses. For each species separately, we compared the proportion of stigmas with pollen deposition between treatments (day or night) with chi-squared tests. Stigmas with fewer than five grains of pollen were scored as no pollen deposition. We compared the natural logarithm of pollen deposited using an ANOVA with treatment (day or night), species and their interaction as factors. A significant interaction indicates a between-species difference in the timing of pollination.
Floral arrays
To assess whether observed differences in visitors to natural populations of the two species are driven by floral trait differences or by differences in local pollinator assemblages, we placed arrays of greenhouse-grown plants of the two species near two natural populations of each species. Plants were transported to the sites in their original containers, watered frequently in the field, and kept in tents, vehicles or under bridal veil when not being used in an array. Observations at experimental arrays occurred from April to June in 2010 and 2011, while the nearby Clarkia population was blooming. Two of the sites, FR and KN, were locations of natural population observations. Additionally, we conducted array observations at two sites on Mount Hamilton, Santa Clara County, California. The species ranges are adjacent at this location, with C. breweri occurring on the south-eastern slope and C. concinna on the north-western slope. One array location was near a C. breweri population (37·340 °N, 121·633 °W; elevation 1120 m; MHS), and the other was near a C. concinna population (37·343 °N, 121·649 °W; elevation 1090 m; MHN; Fig. 2).
Arrays consisted of 10–30 plants of each species. In 2010, we used an approximately equal number of plants of both species, placed on six trays composed entirely of one species each (three trays per species). Within a tray, plants were evenly spaced at approx. 15-cm intervals. Because C. concinna produces more flowers per plant than C. breweri, this arrangement led to an unbalanced number of flowers. Therefore, in 2011, we used approximately the same number of flowers of both species by reducing the proportion of C. concinna plants. We observed arrays for 96 daytime hours, which were divided into 2-h observation blocks (FR 2010: 4 d, 26 h, FR 2011: 2 d, 10 h; KN 2010: 3 d, 10 h, KN 2011, 2 d, 6 h; MHN 2010: 3 d, 18 h, MHN 2011: 1 d, 4 h; MHS 2010: 3 d, 18 h, MHS 2011: 1 d, 4 h). We also conducted 40 total hours of nighttime array observations at all four sites using infrared cameras equipped with motion detectors (FR 2010: 6 nights, 9 h; FR 2011: 3 nights, 6 h; KN 2010, 3 nights, 5 h; KN 2011: 3 nights, 6 h; MHN 2010: 2 nights, 3 h; MHN 2011: 2 nights, 4 h; MHS 2010: 2 nights, 3 h; MHS 2011: 2 nights, 4 h). However, we observed no hawkmoth visits and too few nocturnal moth visits to effectively analyse. During some observation nights at FR and KN, we also attempted to determine local hawkmoth activity using a black light to illuminate a white sheet. We failed to detect any hawkmoths despite previous success using this method at FR in 1991 (Raguso, 1995). Diurnal visitors were classified into functional groups as in the natural population observations. We measured visitation rates for each functional group during each observation period as visits per flower per hour. We then compared visitation rates of functional groups using a MANOVA with plant species, site, year and all interactions as factors. We compared the relative proportion of array visits for each site and species to diurnal natural population visits with a hierarchical cluster analysis using Ward's minimum variance method. If visitors to each species cluster with the natural population observations of the same species regardless of site, then the differences in natural population observations are due to divergent floral traits. Alternatively, if visitors to both species at a site cluster together with nearby natural population observations, then differences are due to differences in the local pollinator assemblages.
Diurnal floral visitor efficiency
To quantify the efficiency of each functional group of diurnal visitors, we measured stigma–visitor contact rates and visitor pollen loads. Although not as accurate as quantifying pollen deposition on emasculated stigmas (or the resulting seed production), using these two methods allowed us to obtain large sample sizes for all functional classes. Moreover, a visitor carrying pollen and contacting the stigma is much more likely to effect pollination than a visitor doing only one or neither of these acts. We measured contact rates during observations of both natural populations and floral arrays as the proportion of visits in which the visitor contacted the receptive surface of the stigma. In almost all cases of stigma contact, the anthers were also contacted, while the converse was not necessarily true. Contact was only assessed when the observer had an unobstructed view of the visitor. We quantified pollen loads on insects collected with a net as they departed from natural populations, placing them in a clean kill jar and then transferring them to individual micro-centrifuge tubes or larger plastic vials. We were not able to quantify hummingbird pollen loads. We dabbed the bodies of collected specimens with basic fuchsin glycerine jelly (Beattie, 1971; Kearns and Inouye, 1993), transferring the jelly to glass microscope slides and melting it with a butane lighter. We counted pollen grains under 80 × magnification. Additionally, we checked the bodies of specimens for any remaining pollen, which was also counted under a dissecting microscope. We compared differences in contact rates between species for each functional group with chi-squared tests. We compared between-species differences in log-transformed pollen loads for each functional group with t-tests.
Hawkmoth effectiveness
We used captive hawkmoths (Hyles lineata) to quantify floral preferences and relative efficiency of nocturnal pollinators. This species was the most common hawkmoth observed at C. breweri populations. Hawkmoth eggs and larvae were obtained opportunistically from Colorado Springs, Eldorado County, CO, during the summer of 2009 and were used to establish a laboratory colony maintained in a growth chamber (16 h light, 8 h dark, 24 ± 1 °C, 60 ± 5 % relative humidity) at Cornell University. This species is abundant across the Americas and disperses widely (Raguso et al., 1996), so there was no a priori reason to assume that a colony established from Colorado would not be representative of coastal California moths. Larvae were raised on a corn-meal-based diet as described by Goyret et al. (2009). We sorted approx. 60 pupae by sex into 0·4 × 0·4 × 1-m flight cages in separate growth chambers until they eclosed (14 h light, 10 h dark, 23 ± 1 °C, regular misting to increase relative humidity).
To test for floral preference, we allowed hawkmoths to forage on mixed arrays of C. breweri and C. concinna. Trials were performed in a 1·8 × 1·8 × 1·8-m flight cage in a windowless indoor room with approximately equal numbers of flowers of each species (between-species difference in flower number ≤2, 56–70 total flowers per trial) haphazardly arranged in the cage. Trials consisted of a feeding bout of an individual hawkmoth from the first visit of a flower until it left the array for longer than approx. 1 min. We classified an individual visit to a flower as either an approach (close investigation of flower but no insertion into the hypanthium) or a probe (tongue inserted into the hypanthium of the flower). Trials of five or fewer probes were not included, and hawkmoths were not reused. We compared the number of approaches, probes and total visits between plant species with separate paired t-tests using each trial (n = 10) as a replicate. There was no difference in preference between the sexes. Because we did not test hawkmoth behaviour on single species arrays, we were unable to measure innate differences in latency (time to first visit) between the plant species. Furthermore, because of the relatively small size of our flight cage, our work only addresses hawkmoth preference once they are in close proximity to the flowers, and have committed to foraging for nectar. There may be additional differences between C. breweri and C. concinna in the degree to which they affect feeding motivation, long-distance attraction and latency to visit by H. lineata and other hawkmoths, due to differences in floral scent, corolla shape and reflectivity (see Goyret et al., 2007; Hoballah et al., 2007; Schlumpberger and Raguso, 2008).
To estimate hawkmoth efficiency, we conducted single-species pollen deposition trials in 0·4 × 0·4 × 1-m flight cages. Nine separate trials for each species were conducted with 1–4 emasculated flowers with open stigmas and 2–9 unmanipulated flowers with fresh pollen per trial. The ratio of emasculated to perfect flowers did not differ between species [mean of 0·43 (± 0·15 s.e.) for C. breweri and 0·56 (± 0·22 s.e.) for C concinna, t = 1·42, P = 0·17]. We placed an individual hawkmoth in the cage and allowed it to forage until it had probed all emasculated flowers after first visiting at least one perfect flower. The number of probes per emasculated flower did not differ between species [mean of 2·80 (± 1·71 s.e.) for C. breweri and 2·92 (± 1·34 s.e.) for C concinna, t = 0·16, P = 0·87]. We collected and squashed stigmas on microscope slides, and counted pollen under 80 × magnification. Efficiency for each trial was calculated as the natural logarithm of the mean number of grains of pollen deposited per probe. Efficiency was compared between plant species with a two-tailed t-test.
RESULTS
Floral morphology
Greenhouse-grown Clarkia breweri and C. concinna differ markedly in floral traits (Table 1). Discriminant analysis correctly classified the species identity of all flowers based on floral morphology (n = 34, log likelihood <0·001). Clarkia breweri flowers have smaller corolla diameters, but wider individual petals than C. concinna. Reproductive parts, especially the anthers, are exserted further and the hypanthium is wider in C. breweri.
Table 1.
Comparison of floral morphology between Clarkia breweri and Clarkia concinna (mm, mean with s.e. in parentheses) with measurements taken on newly female flowers of greenhouse-grown plants from the study populations (one flower per plant, 17 plants per species)
| Trait | C. breweri | C. concinna |
|---|---|---|
| Corolla diameter | 48·4 (1·2) | 56·9 (1·3) |
| Petal width | 19·4 (0·7) | 12·9 (0·4) |
| Style length | 23·1 (0·7) | 14·6 (0·4) |
| Stamen length | 13·9 (0·7) | 11·2 (0·3) |
| Top hypanthium width | 2·05 (0·07) | 1·51 (0·07) |
| Bottom hypanthium width | 1·24 (0·04) | 0·83 (0·02) |
| Floral colour | Pale pink | Bright magenta |
| Floral scent | Strong, sweet | Weak |
Floral visitors to natural populations
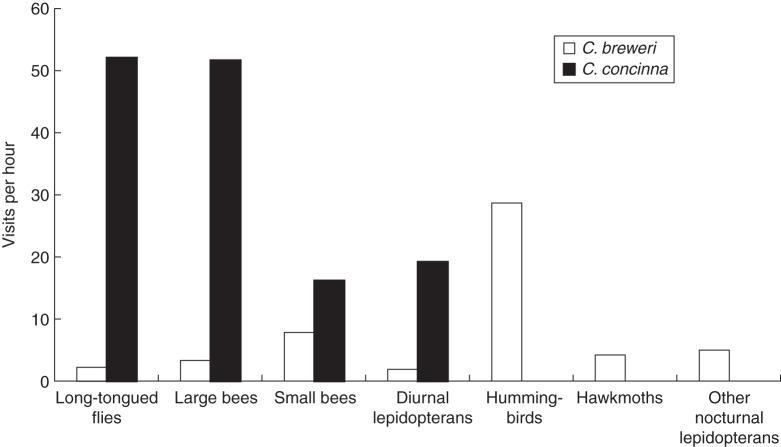
We observed 2913 visits over 32 h to C. concinna and 1695 visits over 55 h to C. breweri (Fig. 4). The majority of visits to C. concinna at all six observed populations were from long-tongued flies and large bees, with additional visits by diurnal lepidopterans and small bees. No nocturnal visitors or hummingbirds were observed. The majority of diurnal visits to C. breweri at both populations were from hummingbirds, a group that did not visit C. concinna natural populations. Clarkia breweri also received a small number of visits from all other diurnal functional groups, as well as hawkmoths and other nocturnal lepidopterans.
Fig. 4.
Visits per hour by different functional groups to natural populations of Clarkia breweri and C. concinna. Nocturnal lepidopteran visits were divided into hawkmoths (Sphingidae), and other nocturnal lepidopterans (primarily Noctuidae). No hummingbirds, hawkmoths or other nocturnal lepidopterans were observed at C. concinna populations.
Pollinator exclosures
C. concinna flowers were primarily pollinated during the day (95 % of day-exposed stigmas had pollen deposition, 19 % of night-exposed stigmas; χ2 = 28·8, P < 0·001), and C. breweri flowers during the night (26 % day-exposed, 59 % night-exposed; χ2 = 15·0, P < 0·001). More pollen was deposited during the day for C. concinna, and during the night for C. breweri (d.f. = 3, 177; treatment f = 1·85, P = 0·07; species f = 0·98, P = 0·32, treatment by species f = 7·09, P < 0·001; Fig. 5).
Fig. 5.
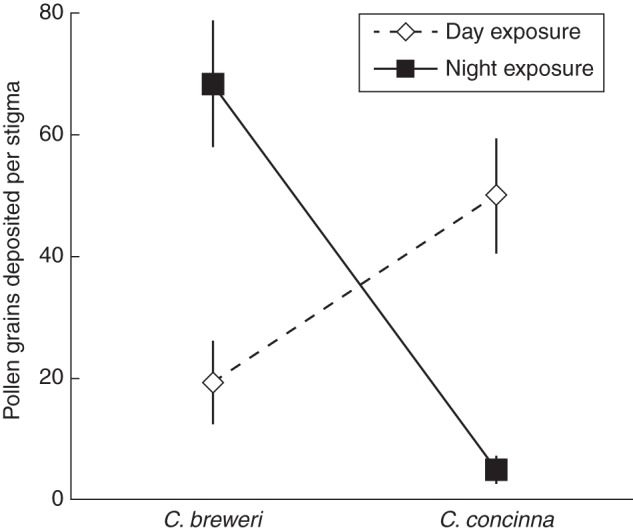
Pollen deposition per stigma on emasculated flowers of Clarkia breweri and C. concinna during day and night pollinator exclosure treatments. Most pollen was deposited on C. concinna during the day and on C. breweri during the night. Error bars, ±s.e.
Floral arrays
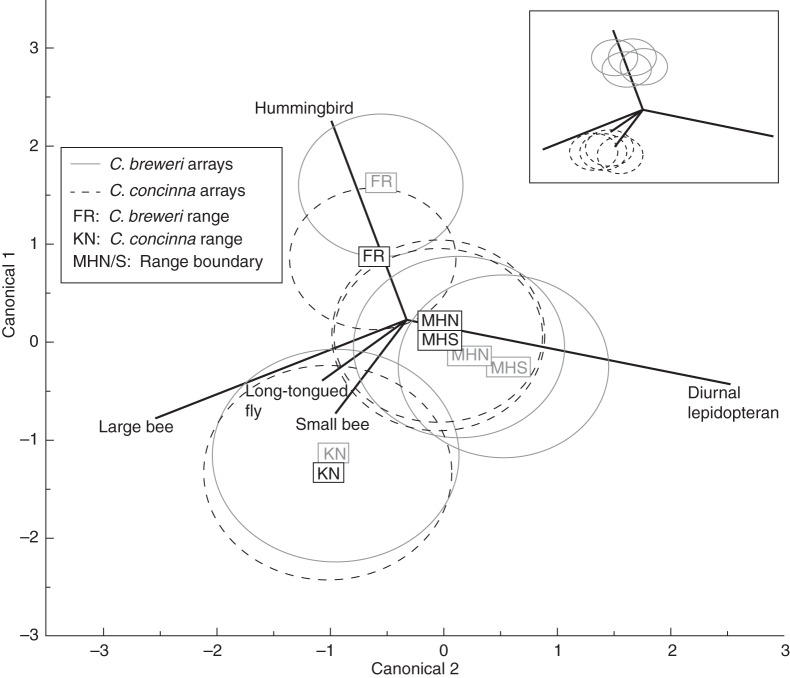
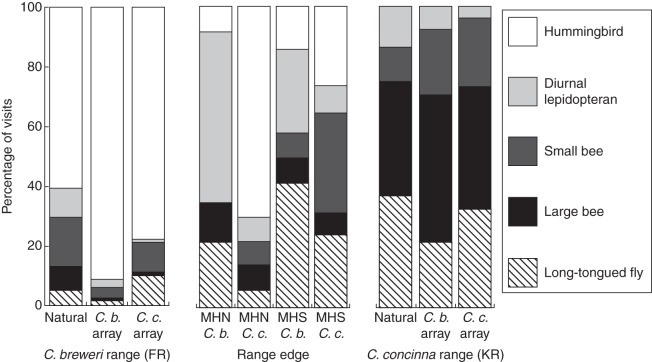
The identity of the plant species had no effect on the composition of visitors to the array flowers, nor was there an effect of species by year or by location interactions. Location, year and the location by year interaction all significantly affected visitor composition to the arrays (Table 2, Fig. 6). Therefore, visitation rates were determined by where and when the array was observed rather than the species identity of the flower. The three-way interaction of species, location and year was also significant. The effect of year may be attributed to between-year differences in the pollination environment or the changes in our experimental design. No functional class showed any between-species difference in visitation rates (long-tongued flies: t = 0·47, d.f. = 92·0, P = 0·64; large bees: t = 0·18, d.f. = 73·9, P = 0·85; small bees: t = 1·04, d.f. = 77·6, P = 0·29; diurnal lepidopterans: t = 1·55, d.f. = 66·9, P = 0·12; hummingbirds: t = 0·88, d.f. = 84·5, P = 0·37). The composition of visitors to flowers of both species at FR clustered with C. breweri natural populations, and the composition of visitors to both species at KN clustered with C. concinna natural populations (Fig. 7).
Table 2.
MANOVA of the response of floral visitation rates (visits per flower per hour) of five functional groups of visitors to species (C. concinna or C. breweri), location (FR, KN, MHN, MHS), year (2010 or 2011) and all interactions
| Effect | Wilks' λ | F-value | No. of variables | d.f. | P |
|---|---|---|---|---|---|
| Species | 0·11 | 2·10 | 2 | 477 | 0·089 |
| Location | 0·25 | 11·66 | 4 | 12, 204 | < 0·0001 |
| Year | 0·20 | 3·86 | 2 | 477 | 0·0065 |
| Species × location | 0·84 | 1·17 | 8 | 12, 204 | 0·30 |
| Species × year | 0·10 | 1·94 | 4 | 477 | 0·112 |
| Location × year | 0·39 | 7·26 | 8 | 12, 204 | < 0·0001 |
| Species × location × year | 0·63 | 3·16 | 16 | 12, 204 | 0·0004 |
Significant differences (P < 0·05) are highlighted in bold.
Fig. 6.
Canonical correlation plot of diurnal functional group visitation rates (visits per flower per hour) to the array plants with centroids shown for each species–location combination. MANOVA finds a significant effect of geographical location (and year) on visitation to the array plants, but not of species. Year, site and species centroids are omitted for clarity. Inset: expected results if variation in visitation was driven primarily by the differences in floral traits between the species.
Fig. 7.
Percentage of diurnal visitors to C. breweri (C. b.) and C. concinna (C. c.) flowers at natural populations of each species (Natural) and mixed arrays at four sites (FR, KN, MHN, MHS). FR and KN arrays were located near observed natural populations of C. breweri and C. concinna, respectively. Hierarchical cluster analysis of the proportion of visits grouped both species in arrays at FR with the C. breweri natural populations, and both species in arrays at KN with the C. concinna natural populations. MHN and MHS arrays were near peripheral populations of C. concinna and C. breweri, respectively, and experienced relatively lower overall visitation rates.
Diurnal floral visitor efficiency
Diurnal visitors of all five functional groups were potentially efficient pollinators of C. concinna, but not of C. breweri (Fig. 8). All functional groups contacted the stigmas of C. concinna flowers more frequently than those of C. breweri (Fig. 8A). Contact rates ranged from 35 to 90 % in C. concinna and 4 to 12 % in C. breweri (long-tongued flies χ2 = 33·26; large bees χ2 = 134·61; small bees χ2 = 13·12; lepidopterans χ2 = 19·98; hummingbirds χ2 = 152·17; P < 0·001 for all tests). Long-tongued flies and large bees both had larger pollen loads of C. concinna than C. breweri (Fig. 8B; long-tongued flies: t = 9·80, d.f. = 20·82, P < 0·001; large bees: t = 11·01, d.f. = 11·60, P < 0·001). Small bees carried a substantial amount of pollen of both species, although amounts were marginally higher for C. concinna (t = 1·77, d.f. = 12·98, P = 0·10). Diurnal lepidopterans carried little pollen of either species (t = 0·94, d.f. = 18·80, P = 0·35).
Fig. 8.
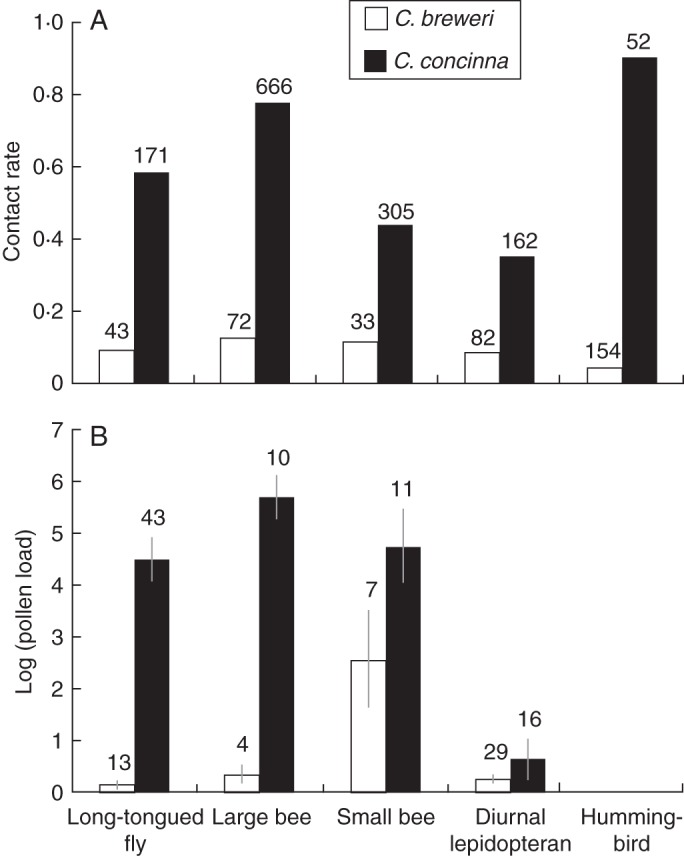
Two estimates of diurnal pollinator efficiency. (A) Proportion of visits for each visitor functional group to C. breweri and C. concinna that involved stigma contact. Observations are from both natural populations and floral arrays. Numbers above columns are sample sizes. All functional groups had significantly higher contact rates to C. concinna. (B) Pollen loads for each functional group to C. breweri and C. concinna. Insects collected are from natural populations. Hummingbird pollen load was not measured. Long-tongued flies and large bees were carrying significantly more C. concinna pollen, and small bees were carrying marginally more C. concinna pollen. Numbers above columns are sample sizes.
Hawkmoth effectiveness
Hawkmoths were more effective pollinators of C. breweri than of C. concinna (Fig. 9). When presented with both species, hawkmoths visited them at approximately equal rates (t = 0·86, d.f. = 9, P = 0·41). However, more visits to C. breweri resulted in probes (t = 2·22, d.f. = 9, P = 0·052), whereas more visits to C. concinna ended in approaches without a successful probe (t = 3·14, d.f. = 9, P = 0·012). Single species efficiency trials showed the deposition of ten times more pollen per visit on the stigmas of C. breweri than C. concinna (t = 3·86, d.f. = 15·51, P = 0·001).
Fig. 9.
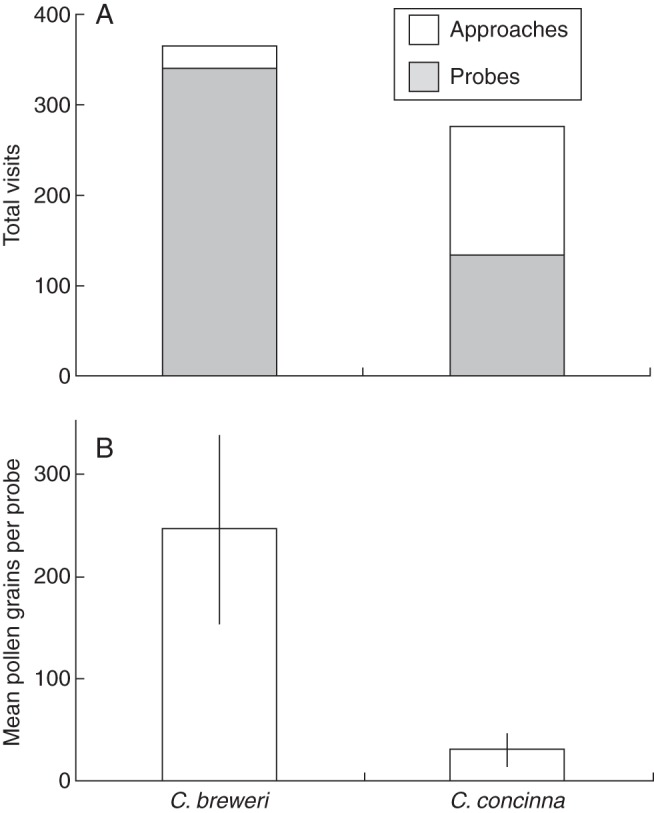
Two measures of hawkmoth pollinator effectiveness. (A) Total visits to Clarkia breweri and C. concinna in choice trials. Hawkmoths visited both species equally (P = 0·41), but successfully probed C. breweri more (P = 0·052), and approached C. concinna without probing more (P = 0·012). (B) Number of pollen grains deposited on the stigmas of both species per probe in single species pollen deposition trials. Hawkmoths deposited more pollen per visit on the stigmas of Clarkia breweri than Clarkia concinna (P < 0·001). Error bars, ±1s.e.
DISCUSSION
The large differences in diurnal visitation to natural populations of Clarkia concinna and C. breweri are largely the result of differences in available pollinator community, not differences in floral attractiveness (Figs 4 and 6). All diurnal visitors are attracted to both species equally when given a choice. Nocturnal hawkmoths, however, show a preference for probing C. breweri (Fig. 9A). Assuming that the floral phenotype of C. breweri represents a derived condition, the addition of novel traits known to attract hawkmoths (strong floral scent, paler floral colour) do not deter former pollinators, indicating the absence of an attractiveness trade-off. Pollinator efficiency follows a different pattern, apparently due to the increased floral dimensions of C. breweri. All diurnal visitors show the potential to consistently pollinate C. concinna, but not C. breweri (Fig. 8). In contrast, hawkmoths were much more efficient at depositing pollen on C. breweri than C. concinna (Fig. 9B). The addition of hawkmoths as an effective pollinator for C. breweri comes at a large cost in reducing the efficiency of former pollinators.
Natural populations
Our observations of visitors to natural populations (Fig. 4) match previous work showing C. concinna is visited by a diverse range of insects (MacSwain et al., 1973; Groom, 1998) and predictions that C. breweri is visited by hawkmoths (MacSwain et al., 1973). Diurnal insects do visit C. breweri, although at low rates. Surprisingly, hummingbirds are the most common visitors to C. breweri populations, despite this flower not fitting a typical hummingbird syndrome. C. breweri provides a nectar reward, and local hummingbirds have learned to exploit this resource. Hummingbirds, although present throughout the range of both species, did not visit natural C. concinna populations in this experiment, and have not been observed visiting at other C. concinna populations (Groom, 1998; T. Miller, unpubl. res.). Community context is a potential explanation for the lack of hummingbird visitation at natural C. concinna populations. Hummingbirds may ignore Clarkia if more rewarding plant species are abundant.
Despite the relatively low frequency of nocturnal visits to C. breweri, the pollinator exclosures reveal all diurnal visitors, including hummingbirds, are depositing much less outcrossed pollen on C. breweri stigmas than nocturnal visitors (Fig. 5). This finding fits with other studies that show the most common visitor is not necessarily the most effective pollinator (e.g. Mayfield et al., 2001; Fumero-Caban and Melendez-Ackerman, 2007; Frick et al., 2013). We also may have underestimated hawkmoth visitations due to the relative difficulty of observing pollinators in low-light conditions. Unsurprisingly, C. concinna received the vast majority of outcrossed pollen during the day. The small amount of nocturnal pollen receipt may be from early- or late-flying bees, or nocturnal insects such as crane flies.
Pollinator preference
Our lack of a significant plant species effect on functional group visitation at the arrays suggests that there is essentially no trade-off involved in attracting novel hawkmoth visitors (see Aigner, 2001; Sargent and Otto, 2006). The lack of preference by all diurnal visitors contrasts with previous work on bee to hummingbird transitions (Schemske and Bradshaw, 1999; Castellanos et al., 2004), and hummingbird to hawkmoth transitions (Streisfeld and Kohn, 2007). These transitions include the evolution of traits toward the novel pollinator as well as away from former pollinators (Schemske and Bradshaw, 1999; Thomson, 2003; Thomson and Wilson, 2008). The evolution of deterrent traits may depend critically on geographical and community context, and may be more likely when the visitor spectrum includes larcenists or florivores whose activities directly reduce reproductive fitness (Theis et al., 2007; Galen et al., 2011). As diurnal visitors do occasionally deposit C. breweri pollen, they may serve as co-pollinators, especially in years when hawkmoth abundance is low. Supplemental pollination by alternative visitors in supposedly specialized plants is becoming increasingly well documented (Kessler and Baldwin, 2011). However, less efficient visitors may act as parasites, precluding nectar and pollen use by more efficient visitors (Thomson, 2003; Castellanos et al., 2004; Johnson et al., 2006; Thomson and Wilson, 2008). Because the two Clarkia species never co-occur, competition for pollinators or selection against interspecific pollen transfer is not expected, eliminating these as mechanisms that can promote deterrent traits (Armbruster et al., 1994; Kay and Schemske, 2008).
Hawkmoths show a clear preference for probing C. breweri flowers (Fig. 9A). This preference could be due to the broader paler corollas of C. breweri that probably aid in visibility and orientation (Hoballah et al., 2007; Venail et al., 2010). However, hawkmoths visited (approaches plus probes) both species at similar rates. Because we presented hawkmoths with both Clarkia species in a small flight cage, our study only measured short-distance attraction once hawkmoths had committed to feeding. Although there is some evidence that hawkmoths can use scent to choose between flowers at close distances (Klahre et al., 2011), the scent of C. breweri may primarily be a long-range attractant for hawkmoths, with visual cues guiding floral probing at shorter distances (Goyret et al., 2007). Scent may also induce hawkmoths to visit flowers in general (Raguso and Willis, 2002, 2005), and once latency is overcome, they may visit indiscriminately. This ‘releasing’ function of scent can explain hawkmoth approaches to C. concinna and similarly unscented flowers in other field array experiments (Fulton and Hodges, 1999; Hirota et al., 2012). If scent is important in overcoming latency, the presence of scented plants in the surrounding community may be crucial in the initial stages of acquiring hawkmoth pollinators.
Pollinator efficiency
In contrast to visitation rates, we found large between-species differences in pollinator efficiency for all functional groups (Figs 7 and 8B). The more exserted anthers and stigma, wider hypanthium, and greater herkogamy (spatial separation of anthers and stigma) of C. breweri probably evolved through selection for improved hawkmoth efficiency. These morphological changes came at the cost of a substantial reduction in efficiency for all other visitors, including hummingbirds. While few studies have directly quantified trade-offs in pollinator efficiency, we believe they will usually be concave between different pollinator functional groups. It is unlikely that one floral morphology will effect equal pollen transfer across pollinators with divergent sizes, shapes and behaviours. For instance, Muchhala (2007) showed that intermediate phenotypes between bat- and bird-pollinated species of Burmeistera have the lowest amounts of pollen transferred due to a poor fit with either type of visitor. In Polemonium brandegei, hummingbirds select for exserted sex organs, while hawkmoths select for recessed sex organs, due to differences in pollen removal and placement. Year-to-year variation in pollinator abundance maintains within-population variation in these traits (Kulbaba and Worley, 2008).
The foraging behaviour of visitors and floral morphology are informative in explaining potential differences in efficiency. Most hawkmoth probes of C. breweri resulted in strong contact with the anthers, leaving pollen hanging from the legs and lower abdomen of the insect (Clarkia pollen grains are typically connected with viscin threads). The long style of C. breweri puts the receptive surface of the stigma in better alignment with the dangling pollen than the shorter C. concinna style (Fig. 3E, F). Additionally, the wider hypanthium tube of C. breweri may better accommodate the insertion of the hawkmoth proboscis. We observed hawkmoths failing to probe C. concinna flowers due to difficulties in aligning their probosces with the narrow hypanthium opening. Large bees, lepidopterans and long-tongued flies all foraged for nectar either by inserting their tongue into the hypanthium or by lapping nectar exuded at the hypanthium opening. The large distance between the anthers and the nectar reward in C. breweri, compared with C. concinna, greatly reduced anther contact, and thus the pollen load, carried by these groups (Fig. 3C, D). Small bees were the only functional group that commonly foraged on both nectar and pollen of both species. When foraging for nectar, small bees rarely contacted the stigma of either species. Individual small bees that were actively collecting pollen carried large pollen loads of both species and frequently contacted C. concinna stigmas; however, the greater herkogamy in C. breweri decreased the stigma contact rate (Fig. 3A, B). Although hummingbirds are a novel visitor of C. breweri natural populations, they contact C. concinna stigmas much more frequently due to their upright foraging position (Fig. 3G, H). An additional experiment that quantified hummingbird efficiency on C. breweri through florescent dye transfer found similarly low efficiencies (Raguso, 1995). However, hummingbirds can provide at least some supplemental pollination to hawkmoth-specialized plants, such as C. breweri, when hawkmoths are rare (Aigner and Scott, 2002).
Additional considerations
We documented substantial between-year variation in pollination environments. Previous studies have also found high amounts of temporal variation, highlighting the need for studying pollination across multiple years (Kay and Schemske, 2003; Price et al., 2005; Herrera, 2008; Petanidou et al., 2008). Hawkmoths, in particular, are known to show high year-to-year variation in abundance (Miller, 1981; Campbell et al., 1997; Sime and Baldwin, 2003; Kulbaba and Worley, 2008), and the lack of hawkmoth visitors to our floral arrays may reflect this variability. In addition to supplemental pollination by diurnal visitors, self-fertilization may provide an important mechanism for the persistence of C. breweri in years with low hawkmoth abundances. All Clarkia species can self, and this ability may be essential in allowing an annual plant to evolve reliance on an episodically abundant pollinator. We expect selfing rates to show greater among-year variance in C. breweri than C. concinna (Kay and Picklum, 2013), and to show a negative correlation with hawkmoth abundance in C. breweri.
Low-abundance hawkmoth years may be compensated for by the high quality of pollen delivered by hawkmoths in years of high abundance. Pollen quality was not measured in this study, but can have large effects on seed set and progeny fitness, making it an important component of overall pollinator effectiveness (Ramsey and Vaughton, 2000; Chacoff et al., 2008). Pollen carried by hawkmoths may be higher quality, as they can move large amounts of pollen over longer distances than other pollinators (Raguso and Willis, 2003). As hawkmoths do not groom or consume pollen, they may also transfer a higher proportion of removed pollen than bees. Thus, quantifying pollen quality and transfer efficiency would probably increase our estimates of the overall effectiveness of hawkmoths as pollinators of C. breweri.
Future directions
Although our results reveal clear differences in between-species patterns of attraction and efficiency, we make the important caveat that we are using current end points to infer past processes. The generality of our results can only be understood through carefully controlled comparisons of both visitation rates and efficiency across multiple types of pollinator transitions. A comparison of attractiveness and efficiency trade-offs between allopatric/parapatric and sympatric sister species is especially warranted. Our prediction from such a comparison is that trade-offs in visitation rates between species that do not co-occur are more likely to be convex or absent than in sympatric species, while efficiency trade-offs are frequently concave irrespective of geographical distributions. Another important avenue of future work is to create intermediate phenotypes to directly measure visitation rates and efficiency of possible ancestral states. Testing explicit predictions about processes involved in adopting novel pollinators will allow us to better understand how pollinator transitions contribute to plant speciation and the evolution of floral phenotypes.
ACKNOWLEDGEMENTS
We thank B. Bowman, V. Ford, L. Gottlieb and B. LeNeve for assistance locating populations; M. Minor, J. Bahr, A. Philbin, R. Price, K. Cnop and especially D. Picklum for assistance with field and flight cage experiments; A. Shapiro and R. Thorp for help with insect identification; K. Eberwein and M. Ramos for permission to work at FR; J. Velzy and D. Polk for expert plant care; and J. Yost and two anonymous reviewers for comments on an earlier draft. This work was partially funded by a Committee on Research Grant to K.M.K. from UCSC. R.A.R. was supported by a Sigma Xi Grant-in-Aid of Research, the California Native Plant Society, the Theodore Roosevelt Fund of the American Museum of Natural History and by NSF grant MCB-9218989 to Eran Pichersky, University of Michigan. Data presented here include part of a dissertation submitted in partial fulfilment of the requirements for the degree of doctor of philosophy, University of Michigan, 1995. We dedicate this paper to the memory of L. D. Gottlieb for his inspiring work on the genus Clarkia and for supporting and encouraging our work on these fascinating species.
LITERATURE CITED
- Aigner PA. Optimality modeling and fitness trade-offs: when should plants become pollinator specialists? Oikos. 2001;95:177–184. [Google Scholar]
- Aigner PA. Floral specialization without trade-offs: optimal corolla flare in contrasting pollination environments. Ecology. 2004;85:2560–2569. [Google Scholar]
- Aigner PA. Variation in pollination performance gradients in a Dudleya species complex: can generalization promote floral divergence? Functional Ecology. 2005;19:681–689. [Google Scholar]
- Aigner PA, Scott PE. Use and pollination of a hawkmoth plant Southwestern Naturalist. Nicotiana attenuata, by migrant hummingbirds. 2002;47:1–11. [Google Scholar]
- Anderson B, Johnson SD. The geographical mosaic of coevolution in a plant pollinator mutualism. Evolution. 2008;62:220–225. doi: 10.1111/j.1558-5646.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Alexandersson R, Johnson SD. Evolution and coexistence of pollination ecotypes in an African Gladiolus (Iridaceae) Evolution. 2010;64:960–972. doi: 10.1111/j.1558-5646.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. Floral character displacement generates assemblage structure of Western-Australian triggerplants (Stylidium) Ecology. 1994;75:315–329. [Google Scholar]
- Baker HG. Reproductive methods as factors in speciation in flowering plants. Cold Spring Harbour Symposia on Quantitative Biology. 1959;24:177–191. doi: 10.1101/sqb.1959.024.01.019. [DOI] [PubMed] [Google Scholar]
- Beattie AJ. A technique for the study of insect-borne pollen. Pan-Pacific Entomologist. 1971;47:82. [Google Scholar]
- Botes C, Johnson SD, Cowling RM. The birds and the bees: using selective exclusion to identify effective pollinators of African tree aloes. International Journal of Plant Science. 2009;170:151–156. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at flower colour locus produces a pollinator sfit in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Otto KG, Frewen BE, McKay JK, Schemske DW. Quantitative trait loci affecting differences in floral morphology between two species of monkeyflower (Mimulus) Genetics. 1998;149:367–382. doi: 10.1093/genetics/149.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR, Waser NM, Melendez-Ackerman EJ. Analyzing pollinator mediated selection in a plant hybrid zone: hummingbird visitation patterns on three spatial scales. American Naturalist. 1997;149:295–315. [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology. 2004;17:876–885. doi: 10.1111/j.1420-9101.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- Chacoff NP, Garcia D, Obeso JR. Effects of pollen quality and quantity on pollen limitation in Crataegus monogyna (Rosaceae) in NW Spain. Flora. 2008;203:499–507. [Google Scholar]
- Dell'Olivo A, Kuhlemeler C. Asymmetric effects of loss and gain of a floral trait on pollinator preference. Evolution. 2013;67:3023–3031. doi: 10.1111/evo.12178. [DOI] [PubMed] [Google Scholar]
- Dilley JD, Wilson P, Mesler MR. The radiation of Calochortus: generalist flowers moving through a mosaic of potential pollinators. Oikos. 2000;89:209–222. [Google Scholar]
- Eckert CG. Effect of geographical variation in pollinator fauna on the mating system of Decodon verticillatus (Lythraceae) International Journal of Plant Sciences. 2002;163:123–132. [Google Scholar]
- Fenster CB, Armbuster WS, Wilson P, Dudash MR, Thompson JD. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics. 2004;35:375–403. [Google Scholar]
- Frick WF, Price RD, Heady PA, III, Kay KM. Insectivorous bat pollinates columnar cactus more effectively per visit than specialized nectar bat. American Naturalist. 2013;181:137–144. doi: 10.1086/668595. [DOI] [PubMed] [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society B—Biological Sciences. 1999;266:2247–2254. [Google Scholar]
- Fumero-Caban JJ, Melendez-Ackerman EJ. Relative pollination effectiveness of floral visitors of Pitcairnia angustifolia (Bromeliaceae) American Journal of Botany. 2007;94:419–424. doi: 10.3732/ajb.94.3.419. [DOI] [PubMed] [Google Scholar]
- Galen C, Kaczorowski R, Todd SL, Geib J, Raguso RA. Dosage-dependent impacts of a floral volatile on pollinators, larcenists and the potential for floral evolution in the alpine skypilot. The American Naturalist. Polemonium viscosum. 2011;177:258–272. doi: 10.1086/657993. [DOI] [PubMed] [Google Scholar]
- Gottlieb LD, Weeden NF. Gene duplication and phylogeny in Clarkia. Evolution. 1979;33:1024–1039. doi: 10.1111/j.1558-5646.1979.tb04759.x. [DOI] [PubMed] [Google Scholar]
- Goyret J, Markwell PM, Raguso RA. The effect of olfactory and visual stimuli decoupling on the foraging behavior of Manduca sexta. Journal of Experimental Biology. 2007;210:1398–1405. doi: 10.1242/jeb.02752. [DOI] [PubMed] [Google Scholar]
- Goyret J, Kelber A, Pfaff M. Flexible responses to visual and olfactory stimuli by foraging Manduca sexta: larval nutrition affects adult behaviour. Proceedings of the Royal Society B—Biological Sciences. 2009;276:2739–2745. doi: 10.1098/rspb.2009.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. Pollination systems as isolating mechanisms in angiosperms. Evolution. 1949;3:82–97. doi: 10.1111/j.1558-5646.1949.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Grant V. Mechanical and ethological isolation between Pedicularis groelandica and P. attallens (Scrophulariaceae) Biologisches Zentralblatt. 1994;113:43–51. [Google Scholar]
- Grant V, Grant KA. Flower Pollination in the Phlox Family. New York: Columbia University Press; 1965. [Google Scholar]
- Groom MJ. Allee effects limit population viability of an annual plant. American Naturalist. 1998;151:487–496. doi: 10.1086/286135. [DOI] [PubMed] [Google Scholar]
- Harder LD, Barrett SCH. The ecology and evolution of flowers. New York: Oxford University Press; 2007. [Google Scholar]
- Herrera CM. Components of pollinator ‘quality’: comparative analysis of a diverse insect assemblage. Oikos. 1987;50:79–90. [Google Scholar]
- Herrera CM. Variation in mutualisms: the spatio-temporal mosaic of a pollinator assemblage. Biological Journal of the Linnean Society. 2008;35:95–125. [Google Scholar]
- Hersch EI, Roy BA. Context dependent pollinator behavior: an explanation for patterns of hybridization among three species of indian paintbrush. Evolution. 2007;61:111–124. doi: 10.1111/j.1558-5646.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- Hirota SK, Nitta K, Kim Y, et al. Relative role of flower color and scent on pollinator attraction: experimental test using F1 and F2 hybrids of daylily and nightlily. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0039010. e39010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoballah ME, Gübitz T, Stuurman J, et al. Single gene mediated shift in pollinator attraction in Petunia. The Plant Cell. 2007;19:779–790. doi: 10.1105/tpc.106.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye DW, Gill DE, Dudash MR, Fenster CB. A model and lexicon for pollen fate. American Journal of Botany. 1994;81:1517–1530. [Google Scholar]
- Johnson SD. Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society. 1997;123:225–235. [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kaczorowski RL, Seliger AR, Gaskett AC, Wigsten SK, Raguso RA. Corolla shape vs size in flower choice by a nocturnal hawkmoth pollinator. Functional Ecology. 2012;26:577–587. [Google Scholar]
- Kay KM, Picklum DA. Drought alters the expression of mating system traits in two species of Clarkia. Evolutionary Ecology. 2013;27 online early, doi:10.1007/s10682-013-9630-6. [Google Scholar]
- Kay KM, Sargent RD. The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annual Review of Ecology, Evolution, and Systematics. 2009;40:637–656. [Google Scholar]
- Kay KM, Schemske DW. Pollinator assemblages and visitation rates for 11 species of neotropical Costus (Costaceae) Biotropica. 2003;35:198–207. [Google Scholar]
- Kay KM, Schemske DW. Natural selection reinforces speciation in a radiation of neotropical rainforest plants. Evolution. 2008;62:2628–2642. doi: 10.1111/j.1558-5646.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Kay KM, Voelckel C, Yang JY, Hufford KM, Kaska DD, Hodges SA. Floral characters and species diversification. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. New York: Oxford University Press; 2006. pp. 311–325. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Boulder, CO: University of Colorado Press; 1993. [Google Scholar]
- Kessler D, Baldwin IT. Back to the past for pollination biology. Current Opinion in Pollination Biology. 2011;14:429–434. doi: 10.1016/j.pbi.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Klahre U, Gurba A, Hermann K, et al. Pollinator choice in Petunia depends on two major genetic loci for floral scent production. Current Biology. 2011;21:730–739. doi: 10.1016/j.cub.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Kulbaba MW, Worley AC. Floral design in Polemonium brandegei (Polemoniaceae): genetic and phenotypic variation under hawkmoth and hummingbird pollination. International Journal of Plant Science. 2008;169:509–522. [Google Scholar]
- Lau JA, Galloway LF. Effects of low-efficiency pollinators on plant fitness and floral trait evolution in Campanula americana (Campanulaceae) Oecologia. 2004;141:577–583. doi: 10.1007/s00442-004-1677-1. [DOI] [PubMed] [Google Scholar]
- Lewis H, Lewis ME. The genus Clarkia. Berkeley: University of California Press; 1955. [Google Scholar]
- MacSwain J, Raven PH, Thorp R. Comparative behavior of bees and Onagraceae. IV. Clarkia bees of the western United States. University of California Publications in Entomology. 1973;70:1–80. [Google Scholar]
- Madjidian JA, Morales CL, Smith HG. Displacement of a native by an alien bumblebee: lower pollinator efficiency overcome by higher visitation frequency. Oecologia. 2008;156:835–845. doi: 10.1007/s00442-008-1039-5. [DOI] [PubMed] [Google Scholar]
- Mayfield MM, Waser NM, Price MV. Exploring the ‘Most effective pollinator principle’ with complex flowers: bumblebees and Ipomopsis aggregata. Annals of Botany. 2001;88:591–596. [Google Scholar]
- Miller RB. Hawkmoths and the geographic patterns of floral variation in Aquilegia caerulea. Evolution. 1981;35:763–774. doi: 10.1111/j.1558-5646.1981.tb04936.x. [DOI] [PubMed] [Google Scholar]
- Moeller DA. Facilitative interactions among plants via shared pollinators. Ecology. 2004;85:3289–3301. [Google Scholar]
- Moeller DA. Pollinator community structure and sources of spatial variation in plant–pollinator interaction in Clarkia xantiana ssp. xantiana. Oecologia. 2005;142:28–37. doi: 10.1007/s00442-004-1693-1. [DOI] [PubMed] [Google Scholar]
- Muchhala N. Adaptive trade-off in floral morphology mediates specialization for flowers pollinated by bats and hummingbirds. American Naturalist. 2007;169:494–504. doi: 10.1086/512047. [DOI] [PubMed] [Google Scholar]
- Ne'eman G, Jürgens A, Newstrom-Lloyd L, Potts S, Dafni A. A framework for comparing pollinator performance: effectiveness and efficiency. Biological Reviews. 2010;85:435–451. doi: 10.1111/j.1469-185X.2009.00108.x. [DOI] [PubMed] [Google Scholar]
- Newman E, Anderson B, Johnson SD. Flower colour adaptation in a mimetic orchid. Proceedings of the Royal Society B—Biological Sciences. 2012;279:2309–2313. doi: 10.1098/rspb.2011.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Barrales R, Arroyo J, Armbruster WS. Differences in pollinator faunas may generate geographic differences in floral morphology and integration in Narcissus papyraceus (Alarcissio papyraceris) Oikos. 2007;116:1904–1918. [Google Scholar]
- Petanidou T, Kallimanis AS, Tzanopoulos J, Sgardelis SP, Pantis JD. Long-term observation of a pollination network: fluctuation in species and interactions, relative invariance of network structure and implications for estimates of specialization. Ecology Letters. 2008;11:564–575. doi: 10.1111/j.1461-0248.2008.01170.x. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Raguso RA, Lewinsohn E, Croteau R. Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiology. 1994;106:1533–1540. doi: 10.1104/pp.106.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MV, Waser NM, Irwin RE, Campbell DR. Temporal and spatial variation in pollination of a montane herb: a seven-year study. Ecology. 2005;86:2106–2116. [Google Scholar]
- Raguso RA. Mechanisms of floral scent production and hawkmoth pollination in Clarkia breweri (Onagraceae) Ann Arbor, MI: University of Michigan; 1995. PhD dissertation. [Google Scholar]
- Raguso RA, Pichersky E. Floral volatiles from Clarkia breweri, and C. concinna (Onagraceae): recent evolution of floral scent and moth pollination. Plant Systematics and Evolution. 1995;194:55–67. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by naïve hawkmoths. Animal Behaviour. 2002;64:685–695. [Google Scholar]
- Raguso RA, Willis MA. Hawkmoth pollination in Arizona's Sonoran Desert: behavioral responses to floral traits. In: Boggs CL, Watt WB, Ehrlich PR, editors. Evolution and ecology taking flight: butterflies as model systems. Chicago: University of Chicago Press; 2003. pp. 43–65. Rocky Mountain Biological Lab Symposium Series. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths. Animal Behaviour. 2005;65:407–418. [Google Scholar]
- Raguso RA, Light DM, Pichersky E. Electroantennogram responses of Hyles lineata (Sphingidae: Lepidoptera) to floral volatile compounds from Clarkia breweri (Onagraceae) and other moth-pollinated flowers. Journal of Chemical Ecology. 1996;22:1735–1766. doi: 10.1007/BF02028502. [DOI] [PubMed] [Google Scholar]
- Raguso RA, Henzel C, Buchmann SL, Nabhan GP. Trumpet flowers of the Sonoran Desert: floral biology of Peniocereus cacti and sacred datura. International Journal of Plant Biology. 2003;164:877–892. [Google Scholar]
- Ramsey M, Vaughton G. Pollen quality limits seed set in Burchardia umbellata (Colchicaceae) American Journal of Botany. 2000;87:845–852. [PubMed] [Google Scholar]
- Rausher MD. Evolutionary transitions in floral color. International Journal of Plant Science. 2008;169:7–21. [Google Scholar]
- Sargent RD, Otto SP. The role of local species abundance in the evolution of pollinator attraction in flowering plants. American Naturalist. 2006;167:67–80. doi: 10.1086/498433. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemske DW, Horvitz CC. Variation among floral visitors in pollination ability—a precondition for mutualism specialization. Science. 1984;225:519–521. doi: 10.1126/science.225.4661.519. [DOI] [PubMed] [Google Scholar]
- Schlumpberger BO, Raguso RA. Geographic variation in floral scent of Echinopsis ancistrophora (Cactaceae); evidence for constraints on hawkmoth attraction. Oikos. 2008;117:801–814. [Google Scholar]
- Sime K, Baldwin I. Opportunistic out-crossing in Nicotiana attenuata (Solanaceae), a predominantly self-fertilizing native tobacco. BMC Ecology. 2003;3:6. doi: 10.1186/1472-6785-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Hall SJ, Izquierdo PR, Baum DA. Comparative pollination biology of sympatric and allopatric Andean Iochroma (Solanaceae) Annals of the Missouri Botanical Garden. 2008;95:600–617. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms I: Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Streisfeld MA, Kohn JR. Environment and pollinator-mediated selection on parapatric floral races of Mimulus aurantiacus. Journal of Evolutionary Biology. 2007;20:122–132. doi: 10.1111/j.1420-9101.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- Sytsma KJ, Smith JF, Gottlieb LD. Phylogenetics in Clarkia (Onagraceae): restriction site mapping of chloroplast DNA. Systematic Botany. 1990;15:280–295. [Google Scholar]
- Theis NB, Lerdau M, Raguso RA. The challenge of attracting pollinators while evading floral herbivores: patterns of fragrance emission in Cirsium arvense and Cirsium repandum (Asteraceae) International Journal of Plant Science. 2007;168:587–601. [Google Scholar]
- Thomson JD. When is it mutualism? (An American Society of Naturalists Presidential Address) American Naturalist. 2003;162:S1–9. [Google Scholar]
- Thomson JD, Wilson P. Explaining evolutionary shifts between bee and hummingbird pollination: convergence, divergence, and directionality. International Journal of Plant Science. 2008;169:23–38. [Google Scholar]
- Venail J, Dell'Olivo A, Kuhlemeier C. Speciation genes in the genus Petunia. Philosophical Transactions of the Royal Society B. 2010;365:461–468. doi: 10.1098/rstb.2009.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser NM. Pollinator behavior and plant speciation: looking beyond the ‘ethological isolation’ paradigm. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination: animal behavior and floral evolution. Cambridge: Cambridge University Press; 2001. pp. 318–336. [Google Scholar]