Abstract
Background
The hypothesis that pollinators have been important drivers of angiosperm diversity dates back to Darwin, and remains an important research topic today. Mounting evidence indicates that pollinators have the potential to drive diversification at several different stages of the evolutionary process. Microevolutionary studies have provided evidence for pollinator-mediated floral adaptation, while macroevolutionary evidence supports a general pattern of pollinator-driven diversification of angiosperms. However, the overarching issue of whether, and how, shifts in pollination system drive plant speciation represents a critical gap in knowledge. Bridging this gap is crucial to fully understand whether pollinator-driven microevolution accounts for the observed macroevolutionary patterns. Testable predictions about pollinator-driven speciation can be derived from the theory of ecological speciation, according to which adaptation (microevolution) and speciation (macroevolution) are directly linked. This theory is a particularly suitable framework for evaluating evidence for the processes underlying shifts in pollination systems and their potential consequences for the evolution of reproductive isolation and speciation.
Scope
This Viewpoint paper focuses on evidence for the four components of ecological speciation in the context of plant-pollinator interactions, namely (1) the role of pollinators as selective agents, (2) floral trait divergence, including the evolution of ‘pollination ecotypes‘, (3) the geographical context of selection on floral traits, and (4) the role of pollinators in the evolution of reproductive isolation. This Viewpoint also serves as the introduction to a Special Issue on Pollinator-Driven Speciation in Plants. The 13 papers in this Special Issue range from microevolutionary studies of ecotypes to macroevolutionary studies of historical ecological shifts, and span a wide range of geographical areas and plant families. These studies further illustrate innovative experimental approaches, and they employ modern tools in genetics and floral trait quantification. Future advances to the field require better quantification of selection through male fitness and pollinator isolation, for instance by exploiting next-generation sequencing technologies. By combining these new tools with strategically chosen study systems, and smart experimental design, we predict that examples of pollinator-driven speciation will be among the most widespread and compelling of all cases of ecological speciation.
Keywords: Geography, floral odour, flower colour, flower shape, specialization, reproductive isolation, next-generation sequencing, pollination ecotypes, pollination, nectar tube, adaptation, natural selection, geographical mosaic of selection, Grant–Stebbins model
INTRODUCTION
A key role for pollinators in the evolution of angiosperm diversity has long been hypothesized (Darwin, 1877; Crepet, 1984; Dodd et al., 1999; Vamosi and Vamosi, 2010). Indeed, mounting evidence indicates that pollinators have the potential to drive diversification at several different stages of the evolutionary process. For example, experimental studies of pollination function have frequently demonstrated the adaptive nature of floral traits, revealing the potential for pollinator-mediated microevolution (summarized in Harder and Johnson, 2009). Similarly, macroevolutionary evidence from phylogenetic studies shows that lineage splitting in many species-level angiosperm phylogenies is associated with shifts in pollination system and correlated shifts in floral traits (Whittall and Hodges, 2007; Valente et al., 2012; Van der Niet and Johnson, 2012; Forest et al., 2014).
Despite the emerging evidence that pollinators can strongly influence evolutionary processes, many questions still remain to be fully answered. In particular, the overarching issue of whether, and how, shifts in pollination system drive plant speciation represents a major gap in knowledge (Johnson, 2006; Kay and Sargent, 2009). Bridging this gap is crucial to fully understand whether pollinator-driven microevolution is seamlessly connected to the observed macroevolutionary patterns. A compelling framework for linking micro- and macroevolution is provided by the recently revitalized theory of ecological speciation (Simpson, 1953; Schluter, 2001; Nosil, 2012). This theory postulates that speciation proceeds when ecologically based divergent selection between populations in different environments leads to the evolution of barriers to gene flow (cf. Nosil, 2012), thus linking adaptation (microevolution) with speciation (macroevolution).
The theory of ecological speciation provides a series of testable predictions that can be framed for each step of the speciation process. Three predictions about the process of ecological speciation, as it would apply specifically to pollinator-driven speciation, are provided by a model, which was first conceptualized by Grant and Grant (1965) and Stebbins (1970), and further developed as the ‘Grant–Stebbins model’ by Johnson (2006, 2010). First, different pollinators are expected to act as agents of divergent selection on floral traits. This prediction follows from two observations: (1) pollinators directly mediate male and female fecundity (i.e. pollinators contribute to fitness) of a large proportion of plant species (Ollerton et al., 2011); (2) different functional pollinator groups (cf. Fenster et al., 2004) vary in their capabilities for perceiving floral attractant cues (e.g. Schiestl and Johnson, 2013) and in their morphology. Thus, when some specialization of pollination occurs (Johnson and Steiner, 2000; Kay and Sargent, 2009), divergent selection is expected to enhance the traits that mediate pollinator attraction, and improve their fit with flower reproductive organs under different pollinator climates (cf. Grant and Grant, 1965). Second, a geographical mosaic of divergent selection, where adaptation to the locally most-efficient pollinator may occur, is provided by variable pollinator distributions. This may lead to the formation of pollination ecotypes (e.g. Armbruster, 1985; Robertson and Wyatt, 1990; Johnson, 1997, 2010). However, divergence in floral traits that occurs as a result of adaptation to a new pollinator may, as a by-product, also lead to reduced attraction and/or pollination efficiency by the original pollinator (but see Aigner, 2001, who argued that these expected trade-offs do not always occur). If this occurs, a third prediction is that, given the critical dependence of plants on pollinators for reproduction, pollinator shifts contribute to reproductive isolation and thus speciation. Alternatively, absence of both original and any alternative pollinator may lead to the evolution of self-pollination (e.g. Moeller, 2006). If shifts to self-pollination are associated with the modification of floral traits important for pollinator-attraction or pollination efficiency (Goodwillie et al., 2010), such shifts are predicted to similarly lead to the evolution of reproductive isolation.
The predictions raised by ecological speciation theory are best tested by implementing integrated and multidisciplinary studies. For example, in butterflies (Chamberlain et al., 2009; Arias et al., 2012) and fish (Vines and Schluter, 2006; Roesti et al., 2012) strong support for ecological speciation has been gathered by integrating experiments that link trait divergence with adaptation, while at the same time applying molecular methods to confirm that gene flow is restricted among the diverging populations. Such integrative multidisciplinary studies of plant–pollinator interactions are relatively rare, which is surprising considering that this topic offers unique opportunities to better understand the mechanistic link between adaptation and reproductive isolation. The link between adaptation (trait divergence) and the formation of reproductive isolation is critical to confirm that ecological speciation has occurred (Schluter, 2000), yet it remains elusive in many systems. Owing to the dual role of floral traits as targets of pollinator–driven selection and mediating specialized interactions with different pollinators that may cause assortative mating, floral adaptation and the evolution of reproductive isolation may be opportunely linked. Thus, studies of plant–pollination system evolution promise to greatly improve our understanding of both the particular role of pollinators in angiosperm speciation and the ecological speciation process in general. Strong inferences about pollinator-driven ecological speciation can be drawn from multidisciplinary studies of diverging populations, ecotypes (or races) and incipient cryptic species, which may represent cases of ‘speciation in action’ (Grant and Grant, 1965; Fig. 1). Unlike comparative studies of older species, in which the environment may have been altered since the time of speciation and extinctions of intermediates may have erased the sequence of adaptive changes (Losos and Glor, 2003), studies of ecotypes and young sister species can offer insights into the actual environments that imposed selection and the precise sequences of adaptive changes.
Fig. 1.
Pollination races in Saltugilia splendens (previously known as Gilia splendens). (A) Saltugilia splendens ssp. splendens from Pine Mountain in Ventura County (California, USA), and (B) S. splendens ssp. grantii from Mt. Mooney, near the crest of the San Gabriel Mountains in Los Angeles County (California, USA). Saltugilia splendens was studied by Verne and Karen Grant, who recognized four pollination races: a widespread short-tubed form pollinated mainly by bombyliid flies (probably the form shown in A), a local long-tubed form with a narrow throat pollinated mainly by long-tongued flies (probably the form shown in B), a local long-tubed form with a wide throat mainly pollinated by hummingbirds, and a local small-flowered autogamous form. The Grants laid the foundation for a conceptual model of pollinator-driven speciation, according to which pollinator-driven divergence at the population level leads to the formation of races (ecotypes). When this process occurs repeatedly, adaptive radiation of pollination systems may occur. Extensive pollinator observations and experiments to estimate breeding systems in the Phlox family were used to demonstrate these processes (Grant and Grant, 1965). (A) Photograph from Lynn Watson, Santa Barbara, CA, USA. (B) Photograph from Bob Sikora, Alameda, CA, USA.
This Special Issue brings together a collection of new studies that focus on a range of topics related to pollinator-driven speciation. By spanning a wide taxonomic and geographic breadth, the aim of this issue is to build on the recent research efforts in the field (e.g. Johnson, 2006; Armbruster and Muchhala, 2009; Kay and Sargent, 2009) to help uncover some generalities about pollinator-driven speciation. Here, we first highlight recurrent themes from the papers in this Special Issue. In particular, we summarize progress on the four topics that are fundamental for a better understanding of pollinator-driven ecological speciation: (1) the role of pollinators as selective agents, (2) trait divergence under pollinator-driven selection, (3) the geographical mosaic of selection in which shifts in pollination system occur, and (4) the link between pollinator-driven selection and the evolution of reproductive isolation. Second, we outline future research directions and explore how technological advances may be integrated into current research programs to enhance our understanding of the processes underlying shifts in pollination system and speciation.
POLLINATORS AS SELECTIVE AGENTS
Distinguishing whether, and through what agents, natural selection has driven variation in floral traits remains a great challenge. Yet, resolving this issue is crucial for understanding the relative importance of pollinators versus other potential agents of selection. For example, in their study of floral polymorphism in a South African daisy, de Jager and Ellis (2014) show that pollinators and florivores exhibited similar floral preferences, probably leading to antagonistic selection on floral form. This study adds to the mounting evidence that floral variation should not automatically be attributed to pollinator-driven adaptation (Strauss and Whittall, 2006; Kessler et al., 2008), even in those cases where it appears the most likely a priori explanation, such as for variation in floral tube length (e.g. Huang and Fenster, 2007).
Evidence for pollinator-driven floral adaptation has been drawn from both descriptive and experimental approaches. Descriptive evidence is provided by ‘trait–environment’ correlations, such as strong matches between pollinator and floral dimensions (e.g. Anderson and Johnson, 2009; Cosacov et al., 2014; Newman et al., 2014; Van der Niet et al., 2014). However, descriptive evidence alone is not conclusive. This is because such correlations may also occur if floral traits first diverge in response to non-pollinator-mediated selection pressures, followed by ecological sorting or adaptation of pollinators to flowers, which will also lead to a close morphological fit between pollinators and flowers (e.g. Herrera et al., 2006). The adaptation of pollinators to flowers, rather than flowers to pollinators, might be expected when pollinator fitness is strongly influenced by an ability to access the reward in flowers of particular species (e.g. Pauw et al., 2009). These caveats can be addressed by attempting to reject alternative drivers. In a study of a Patagonian herb Calceolaria polyrhiza, Cosacov et al. (2014) show that the floral trait ‘corolla throat length’, which is critical for a fit between the pollinator body and plant reproductive organs, co-varies with pollinator distributions. Furthermore, this pattern is decoupled from variation in other floral parts, which correlate with environmental variables (see also Anderson and Johnson, 2008), providing a strong case for pollinator-mediated selection on corolla throat length.
Direct evidence for pollinators as selective agents is best provided by using an experimental approach. One of the strongest approaches for demonstrating adaptation to the local pollinator environment is by implementing reciprocal translocation experiments (cf. Clausen et al., 1940; Robertson and Wyatt, 1990; Ågren and Schemske, 2012; Boberg et al., 2014; Briscoe Runquist and Moeller, 2014; Sun et al., 2014). If ecotypes are adapted to their respective pollinator environments, the expectation is that ecotypes will be fittest in their own environment (evidenced by a significant interaction effect of ecotype × environment). These experiments thereby assess the effects of the entire pollinator community, which may be a particularly important consideration in more generalist pollination systems.
Although reciprocal translocation experiments often show the expected response of an ecotype × environment interaction (e.g. Waterman et al., 2011; Newman et al., 2012), results can also deviate from this expectation. For instance, consistent with expectations of adaptation to the local pollinator fauna, Sun et al. (2014) show that populations of an orchid growing at high altitudes in the Swiss Alps perform significantly better in terms of female fecundity in their own environment than at lower altitude. However, despite exhibiting reduced fecundity in the lowland, the mountain form still outperforms the local lowland form. Thus, if the present pollinator community is stable and representative, the lowland form may be maladapted. Alternatively, other selective forces, which may not be measured over the duration of the reciprocal translocation experiment, may have driven floral evolution of the lowland form.
Floral variation may occur in multiple features, including colour, shape and odour. While reciprocal translocation experiments can reveal whether the overall floral phenotype is adapted to the local pollinator environment, they may fail to reveal which floral trait(s) in particular has been under pollinator-mediated selection. Implementing an experimental design that controls for variation in other traits can test the fitness effect of variation in a particular floral trait. In a study of pollination ecotypes of the South African orchid species Eulophia parviflora, Peter and Johnson (2014) presented flowers of each ecotype to beetle pollinators in a choice experiment. This experimental design specifically focused on pollinator preference for olfactory cues, while controlling for a potential effect of visual cues. The results show that beetles prefer the odour of the ecotype they pollinate to the odour of the bee-pollinated ecotype. Ultimately, a combination of reciprocal translocation experiments with experiments designed to test the fitness of a specific trait in a particular pollinator context are likely to provide the strongest inference of whether and how pollinators have acted as selective agents. Such combined approaches have not been frequently applied, but hold great promise.
FLORAL TRAIT DIVERGENCE
The identification and study of the evolution of pollination ecotypes as potentially informative early stages in speciation requires a thorough understanding of floral trait divergence. A full assessment of which floral traits diverge as a result of pollinator-mediated selection is in part limited by the available technology for accurately measuring floral traits. Several recent technological developments have contributed strongly to the ability to measure traits that until recently could only be compared in a descriptive way. For example, the implementation of gas-chromatography coupled with mass spectrometry (GC-MS) has provided a powerful tool for quantifying variation in floral odour at the level of individual compounds (Raguso, 2008). Such analyses have revealed that floral odour variation can play a key role in pollinator shifts (Johnson et al., 2005; Peakall et al., 2010; Shuttleworth and Johnson, 2010; Peakall and Whitehead, 2014; Peter and Johnson, 2014; Sun et al., 2014; Van der Niet et al., 2014). Similarly, reflectance spectrophotometry provides an objective means for quantifying variation in flower colour, beyond the capabilities and biases of human vision. This has been used to show that flower colour is often divergent between pollination ecotypes (e.g. Newman et al., 2012, 2014; Sun et al., 2014). Finally, new 3-D methods for quantifying variation in shape of complex flowers have proven powerful to quantitatively confirm variation in floral shape (e.g. Van der Niet et al., 2010).
Despite these technological advances, it nevertheless remains challenging to understand why, and in what sequence, floral traits diverge. In the context of pollinator-driven speciation, a traditional dichotomy exists between traits that mediate pollinator attraction (attractant traits), and those that mediate pollination efficiency through the fit between pollinators and flowers (mechanical traits; cf. Grant, 1994). If ecotypes evolve through divergent use of the same pollinator, attractant traits are unlikely to vary. However, mechanical traits may diverge, for instance due to competition for pollen placement sites (Armbruster et al., 1994; Waterman et al., 2011). If competition for pollen placement sites on the pollinator drives floral divergence, it may lead to an adaptation whereby the pollen placement site is suboptimal in the context of pollinator morphology alone, but optimal in the presence of other plant species in the community. This needs to be accounted for in experiments that test for the adaptive significance of trait divergence.
The evolutionary changes associated with shifts within functional pollinator groups are often relatively few, and can be in attractant and/or mechanical traits. For instance, if pollinators vary locally in the length of their mouthparts, selection on traits that determine the distance between the floral reward and plant reproductive organs is expected. Indeed, Boberg et al. (2014) show that in the orchid Platanthera bifolia, variation in nectar spur length across its range in Scandinavia is best explained by variation in tongue length of the local moth assemblage (see also Newman et al., 2014; Peter and Johnson, 2014; Van der Niet et al., 2014). If local pollinators vary in the preference for attractant traits, shifts in colour (Newman et al., 2012) or odour (Peakall and Whitehead, 2014) may occur.
Shifts between functional pollinator groups are often characterized by correlated changes, leading to shifts in floral syndromes (Stebbins, 1970). In two studies from the South African flora, both Newman et al. (2014) and Van der Niet et al. (2014) show that floral divergence associated with shifts within functional pollinator groups mainly involve changes in floral tube length, while shifts between functional pollinator groups involve divergence in additional traits such as flower colour and odour. However, shifts between functional pollinator groups do not always lead to the expected syndrome changes. Miller et al. (2014) show in Clarkia that two sister species that are pollinated by diurnal insects versus hawkmoths both attract the same set of visitors at a given locality, while pollinator efficiency differs strongly. This leads them to hypothesize that attractant traits may be experiencing a different selection regime than efficiency traits. One possible explanation for the decoupling of attraction and efficiency traits is that some floral traits may evolve to filter nectar robbers and florivores, rather than to attract pollinators. This could explain the evolution of flower opening for only brief periods during day or night (Johnson et al., 2002; Goldblatt et al., 2004), as well as the evolution of some visual (Johnson et al., 2006) and olfactory (Kessler et al., 2008) cues. The evolution of traits to filter visitors has even been suggested for mechanical traits (Castellanos et al., 2004). Experimental research that specifically tests the effect of trait variation on flower visitors holds great promise for a deeper understanding of the function of floral traits, and for testing whether attraction or filtering of visitors is the greatest driver of divergence.
THE GEOGRAPHICAL MOSAIC OF POLLINATOR-MEDIATED SELECTION
One of the biggest challenges for understanding the evolution of pollination ecotypes is to determine the ultimate drivers of shifts in pollination system. Grant and Grant (1965) proposed that geographical variation in the pollinator climate is likely to be a primary driver. This ‘climate’ may be determined by absolute changes in pollinator distributions across plant species' ranges. When this is the case, strong coincidence between the boundaries of ecotypes and their respective pollinator ranges is expected (e.g. Johnson and Steiner, 1997). In this Special Issue, such patterns of association are reported for Calceolaria ecotypes in the South American Andes (Cosacov et al., 2014), and bird-pollinated Erica ecotypes in South Africa (Van der Niet et al., 2014). Both these examples involve relatively specialized pollination systems. It has long been unclear whether subtle variation in the spatial distribution of pollinator assemblages for plant species with generalized pollination systems could also lead to the formation of pollination ecotypes. In a study of Erysimum mediohyspanicum (Brassicaceae) from the Iberian Peninsula, Gómez et al. (2014) use pollination network tools to identify the existence of structure among pollinator assemblages across the plant species range. The authors show that several different pollination modules, or niches, exist. Each is correlated with variation in floral traits, lending support for the evolution of generalized pollination ecotypes in response to variation in the pollinator climate.
The strongest evidence that pollinator distributions control the geographical mosaic of selection is provided by a concerted adaptive response by multiple plant species that experience the same variable pollinator climate. Indeed, this has been shown for several plant ecotypes exploiting a range of long-tongued fly pollinators in the Cape Floristic Region (Johnson and Steiner, 1997; Pauw et al., 2009; Johnson, 2010). Similarly, Newman et al. (2014) show concerted adaptive responses at two geographic scales in the South African flora: first among populations of 17 plant species to the same nemestrinid long-tongued fly species, Prosoeca longipennis, that locally varies in tongue length; and second between populations of nine plant species that are fly-pollinated within the fly range, but that are adapted to a different pollination system if they also occur outside the fly range.
An alternative to pollinator shifts as a response to changes in the pollinator climate is the evolution of ecotypes that evolve increased self-pollination. Briscoe Runquist and Moeller (2014) test the hypothesis that populations of Clarkia xantiana ssp. parviflora on the western margin of the range have evolved a highly selfing phenotype in response to chronically low pollinator availability. Instead, they find no differences in reproductive ecology between eastern and western portions of the range, suggesting that other factors such as reinforcement selection due to interactions with its sister taxon, subspecies xantiana, has driven mating system evolution in sympatry.
Variation in the local plant community may also drive shifts in pollination systems through a variety of processes irrespective of pollinator distributions. The local availability of pollinators may vary due to facilitation and competition between plant species that share pollinators, creating a variable selection mosaic. Competition between plant species for pollination may lead to two different outcomes. (1) Reproductive character displacement may occur when there is competition for pollen placement sites on a pollinators' body, but competition does not lead to reduced visitation (Armbruster et al., 1994). (2) Shifts in pollination system may occur when competition leads to reduced visitation, or if the evolution of reproductive character displacement is constrained (Armbruster, 1985). Both these types of shifts are particularly expected if competition occurs in contact zones between closely related species (Armbruster and Muchhala, 2009).
If incipient species capable of hybridizing co-occur, reinforcement may arise as an example of a special case in which community factors further drive the evolution of pollination systems (Van der Niet et al., 2006; Hopkins and Rausher, 2012). In Lapeirousia (Iridaceae) from the Cape Floristic Region of South Africa, shifts in pollination system were thought to have reinforced speciation between populations that had first diverged on different soil types (Goldblatt and Manning, 1996; Van der Niet et al., 2006). These inferences were based on the reconstructed evolution of soil types and pollinators, using a phylogeny derived from a cladistic analysis of morphological characters. Forest et al. (2014) have revisited this hypothesis, this time using DNA sequence data for phylogeny reconstruction. The resulting phylogenetic pattern supports a model of speciation that was driven most frequently by shifts in pollination system (Fig. 2), independent of shifts in soil type, contrary to the original model of speciation from Goldblatt and Manning (1996).
Fig. 2.

Long-tongued fly pollination in painted petal irises (Lapeirousia) from South Africa. (A) Moegistorhynchus longirostris visiting Lapeirousia anceps, and (B) Prosoeca sp. nov. visiting L. oreogena. Lapeirousia species which belong to specialized pollination guilds, for instance those pollinated by different species of long-tongued fly, are characterized by specific suites of floral traits, such as a particular colour and nectar-tube length. Frequent shifts in pollination system have therefore led to a spectacular radiation in floral traits (Forest et al., 2014). (A) Photograph by Bruce Anderson, Stellenbosch, South Africa. (B) Photograph by Dennis Hansen, Zürich, Switzerland.
Other ecological shifts may indirectly trigger shifts in pollination system. In such cases neither the pollinator climate, nor the plant community directly affects the evolution of pollination ecotypes. Peter and Johnson (2014) show that a shift from bee- to beetle-pollination in a deceptive orchid most likely ultimately occurred as a result of an altitude-mediated shift in flowering time, rather than being due directly to an absence of bees. The later flowering at higher altitudes is hypothesized to have decreased the availability of naive pollinators, which, in turn, may have altered the pollinator selection regime. Likewise, Van der Niet et al. (2014) show that the shift from bird- to moth-pollination in the shrub Erica plukenetii from the Cape Floristic Region (South Africa) cannot be explained solely by the absence of the bird pollinators. It seems more likely that changes in the plant habit, perhaps an indication of adaptation to the local environment, resulted in suboptimal efficiency of bird pollinators, leading to a shift to more efficient moth pollinators.
While there is now strong evidence that variation in the pollinator climate is one driver of pollination ecotype evolution, it is by no means the only one (already acknowledged by Stebbins, 1970; Fig. 3). Determining the selective landscape that drives shifts in pollination systems remains one of the most important areas for future research in the field. At the same time, this kind of research requires a complex set of community data, and progress will probably be slow until we can find more effective ways to assess and monitor plant and pollinator communities.
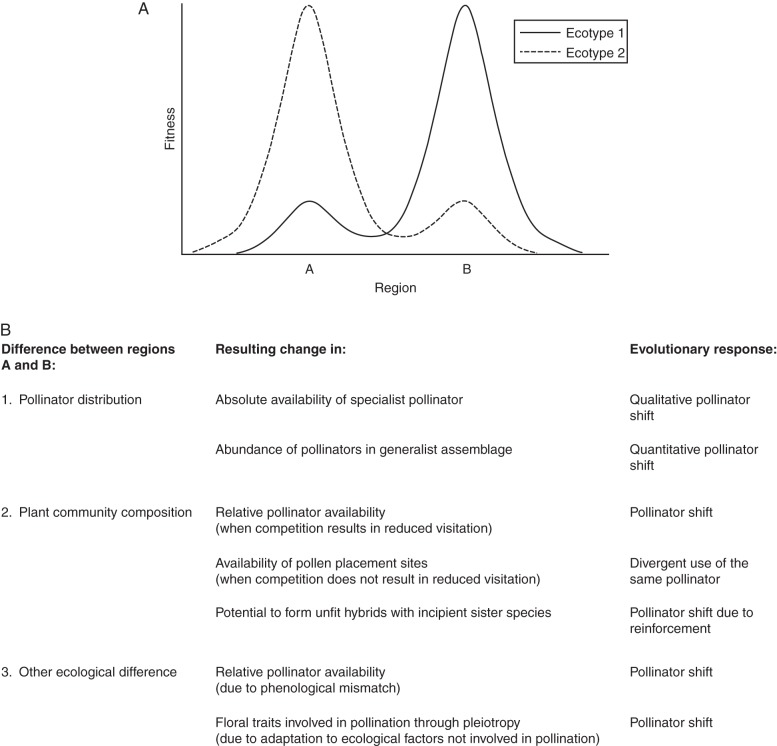
Fig. 3.
The geographical mosaic of selection that leads to divergence in floral traits that characterizes pollination ecotypes. (A) The fitness of ecotypes 1 and 2 depends on the area in which they occur. Ecotype 1 is adapted to the conditions in region B, while ecotype 2 is adapted to the conditions in region A. (B) Several factors may determine divergent selection regimes between regions A and B. While all factors ultimately affect the local availability and/or efficiency of pollinators, the distribution of pollinators is not the only determinant. The evolutionary response depends on the change that is caused by differences between regions A and B. In this scheme the evolution to self-pollination as a response to pollen limitation is not considered, although it is a potential outcome under each scenario.
THE EVOLUTION OF REPRODUCTIVE ISOLATION
How ecological divergence leads to the evolution of reproductive isolation perhaps remains the least well-understood step in ecological speciation (cf. Nosil, 2012). In a pollinator-driven speciation context the key question is whether or not the evolution of pollination ecotypes can in turn promote reproductive isolation? Pollinator-mediated reproductive isolation may evolve if trait divergence resulting from adaptation to a new pollination system causes cessation of pollination by the original pollinator. Before we can ascertain whether this process can occur, it is paramount to recognize that not all shifts in pollination system are the same. One potentially helpful way to think about pollination shifts is to consider the nature of the pollination system shift in relation to four axes of variation (Fig. 4). After defining the type of pollination system shift involved, clues about whether or not reproductive isolation may evolve can be provided by asking whether or not pollination ecotypes would mate assortatively in sympatry. Below, we consider this question in the context of the four axes of variation. While these axes are not strictly mutually exclusive, we believe that applying this new framework facilitates framing of clear and testable predictions for the expected evolutionary outcomes.
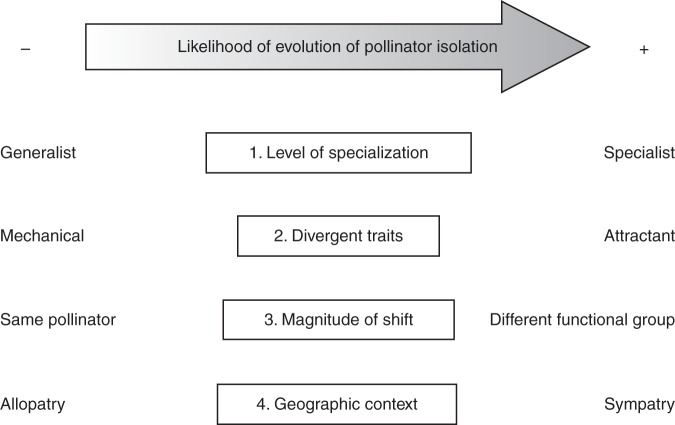
Fig. 4.
Four axes along which shifts in pollination system can vary. Axis 1 represents the degree of pollinator specialization, varying from generalist through functionally specialized (e.g. only bees), to extreme specialist. Axis 2 represents the type and extent of trait divergence, varying from divergence in mechanical traits to variation in attractant traits. Axis 3 represents the magnitude of pollination system shifts, varying from divergent use of the same pollinator through minor shifts between similar pollinator species to major shifts among functional pollinator groups (e.g. bees to birds). Axis 4 represents the geographic context of pollination system shift, varying from allopatric, through parapatric to sympatric ecotype ranges. The evolution of pollinator isolation is more likely for shifts in pollination system characterized by the intrinsic and extrinsic conditions listed on the right of each axis compared to those listed on the left.
First of all, the level of pollinator specialization determines whether there is likely to be overlap between the original and new pollination systems (Waser, 1998, 2001; Aigner, 2001). The more generalized the pollination systems, the more likely that any pollination system shifts are subtle, and therefore probably inadequate for promoting assortative mating in sympatry. Indeed, it is of interest that the majority of systems in which pollinator-driven speciation appears most likely involve lineages with highly specialized pollination systems, such as orchids and other plant groups with specialized reward systems or floral morphologies (Van der Niet and Johnson, 2012).
Second, the type and extent of trait divergence may strongly affect the evolution of reproductive isolation. Shifts in traits that mediate pollinator attraction may lead to rapid ethological isolation. Compelling examples of ethological isolation are provided by sexually deceptive orchids, where minor chemical changes are predicted to mediate pollinator switching and strong reproductive isolation in the absence of any other isolating mechanisms (Peakall et al., 2010; Xu et al., 2011). Indeed, in their study of the Australian orchid genus Chiloglottis, Peakall and Whitehead (2014) show that floral odour compounds essential for pollinator attraction are the only trait changes that can reliably distinguish between three sympatric and morphologically very similar taxa. These fully cross-compatible chemically defined cryptic taxa are also shown by comprehensive nuclear and chloroplast DNA analysis to be reproductively isolated.
Conversely, if floral traits diverge to match the different morphologies of contrasting pollinators, or to facilitate pollen placement on different parts of the same pollinator species, mechanical isolation may occur. The genus Pedicularis was considered by Grant (1994) as an exemplar of mechanical isolation according to the second type. However, Armbruster et al. (2014) report that pollen placement on bumble bees is not precise enough to achieve mechanical isolation among three sympatric species of Pedicularis in south-west China. A difference between ethological and mechanical isolation is that ethologically isolated ecotypes attract different pollinators, while mechanically isolated ecotypes may attract the same pollinator. If the same pollinator is attracted, strong mechanical precision is required for assortative mating. Such isolation is expected only in plant groups with extremely precise pollen placement such as orchids (see Waterman et al., 2011), but not in the majority of plants.
Third, the magnitude of shifts in pollination system is also expected to strongly influence the likelihood of reproductive isolation evolution. For instance, shifts within functional pollinator groups probably lead only to relatively minor trait divergence. An example is the frequent observation of variation in the distance between the location of the reward and plant reproductive structures, as a function of the length of the pollinator mouthpart or body size (e.g. Boberg et al., 2014; Cosacov et al., 2014; Newman et al., 2014; Peter and Johnson, 2014; Van der Niet et al., 2014). It is doubtful whether such mechanical shifts can lead to assortative mating in sympatry. Unless pollen placement is highly precise, mechanical isolation is probably inadequate for the evolution of reproductive isolation (Armbruster et al., 2014). An exception is provided again by sexually deceptive orchids, where a change in a single floral odour compound may attract a closely related insect, but still allow reproductive isolation to evolve (Peakall and Whitehead, 2014). In general, however, changes between functional pollinator groups are most likely to drive more profound divergence in attractant and mechanical traits, with a higher likelihood that they will lead to assortative mating in sympatry.
Finally, the geography of shifts in pollination system is important to consider. If shifts occur across vast geographical distances (e.g. Cosacov et al., 2014), or on different islands (e.g. Martén-Rodriguez et al., 2011), pollinator-mediated reproductive isolation may or may not evolve, according to the three previous axes of variation. In general, if shifts occur in close geographical proximity, gene flow may counteract local adaptation to pollinators unless selection is very strong, or if shifts confer reproductive isolation. This leads to the counter-intuitive idea that the closer the geographical proximity in which shifts occur, the higher the likelihood that pollinator-mediated reproductive isolation would evolve, simply because only those shifts that lead to reproductive isolation would have a chance to survive in the face of gene flow (e.g. Peakall and Whitehead, 2014).
We predict that when considered within this framework of the four axes of variation, most known allopatric pollination ecotypes will not be sufficiently reproductively isolated through pollinator-mediated isolation in the case of secondary contact. If reproductive isolation was lacking, such ecotypes would not count as final products of ecological speciation. However, an important point to consider is that plants often diverge through adaptation to pollinators that themselves have allopatric distributions. For instance, the populations of plant species that vary in nectar tube length as a function of proboscis length of a particular species of long-tongued fly in South Africa are distributed allopatrically, along with allopatrically distributed fly populations. Each pollination niche is thus limited to one particular site and secondary sympatry is unlikely to occur. In those cases it is not relevant to ask whether or not there would be assortative mating in sympatry. If the pollination niches along which pollination ecotypes have diverged in allopatry also overlap in places, then secondary contact could potentially lead to introgression of one ecotype into the other. The maintenance of both pollination ecotypes then depends on the stability of the selection environment that drove the evolution of ecotypes in the first place. Whether or not pollinator-mediated reproductive isolation would be possible between currently allopatric pollination ecotypes ultimately needs to be tested by reciprocal translocation experiments and paternity analyses. For most cases to date these are lacking, highlighting the need for more research in this area.
FUTURE DIRECTIONS
This Special Issue showcases a series of papers ranging from microevolutionary studies of ecotypes to macroevolutionary studies of historical pollinator shifts, which offer important insights that improve our understanding of pollinator-driven speciation. However, many unanswered questions remain. As exemplified by modern research on ecological speciation more generally (e.g. Rice et al., 2011), exploiting new technologies to help us more effectively address the outstanding questions is of paramount importance. At the same time, applying new technologies is not a substitute for the strategic choice of study systems or the application of simple, yet elegant experimental design in the field. The discovery and investigation of new systems may offer valuable clues about evolutionary processes. Hence, the venerable fields of natural history and taxonomy should not be neglected as starting points for the identification of exciting new study systems (e.g. Grant and Grant, 1965) (Fig. 1). Below, we describe four important topics for future research and highlight where the application of new technologies might prove particularly helpful.
Pollinators as selective agents through male fitness
One of the largest gaps remaining in experimental investigations of pollinator adaptation is a lack of studies that measure selection through male fitness. In the majority of studies to date, fitness proxies that describe female fitness, such as fruit or seed set are used (Briscoe Runquist and Moeller, 2014; Sun et al., 2014). However, there is growing evidence that under certain conditions female fitness may be unaffected by floral trait variation (e.g. under conditions of resource limitation of fecundity), yet male fitness can vary considerably (e.g. Aximoff and Freitas, 2010; de Jager and Ellis, 2014). In such cases, experiments based on female fitness alone would fail to detect pollinator-mediated adaptation for male fitness. An equally serious problem for interpreting pollinator-mediated floral adaptation occurs if male and female fitness responses to pollinators are non-aligned (e.g. Kulbaba and Worley, 2012) or even antagonistic (e.g. Ellis and Johnson, 2010).
Clearly, more experiments to measure male fitness responses to floral trait variation are needed, to assess if there are important differences in magnitude and direction to female fitness. Such studies may be further extended by applying paternity analysis (e.g. Rymer et al., 2010; Kulbaba and Worley, 2012), which will provide a more precise estimate of male fitness variation among individuals. The high cost of marker development in non-model species has probably been one contributor to the slow uptake of paternity analysis in plant systems of particular interest for studies of pollinator-driven adaptation (Karron et al., 2012). Fortunately, next-generation sequencing (NGS) now enables cost-effective genome-wide discovery of variable genetic markers such as microsatellites (Dalca and Brudno, 2010; Davey et al., 2011; Gardner et al., 2011). Thus, with large panels of highly polymorphic microsatellites now potentially accessible in any species for a fraction of previous costs, paternity analysis can be more cost effective than ever before.
Identifying variation in pollinator preference
One of the key elements of the Grant–Stebbins model is that pollinators vary in their ability to perceive floral cues. Evidence for this has been derived from the careful analysis of floral traits in species that belong to the same pollination guild (Johnson, 2010). This, as well as laboratory and field studies of responses by individual pollinator species to floral advertising cues (Chittka and Raine, 2006), has revealed extensive convergent evolution of traits associated with pollinator attraction. However, although much progress has been made in understanding insect behaviour, the visual and olfactory capabilities of the majority of pollinators are still unknown. While new technology now allows for detailed assessment of floral colour, models of insect vision are still only available for a handful of model species (Dyer et al., 2011). A better understanding of the vision of non-model pollinators will add considerable explanatory power to the observation of colour variation in many plant species (e.g. Jersakova et al., 2012), and is critical to move this field forward.
Similarly, GC-MS analyses of floral odour have contributed a great deal towards describing the wide variety of odour compounds produced by flowers (Knudsen et al., 2006). However, the number of pollinators for which it is known whether they can perceive odour compounds, and whether these function as attractants or not, is still at its infancy. Methods that couple GC-MS with electro-antennographic detection (EAD), combined with field-based bioassays have provided very powerful insights into the function of floral odour divergence (Schiestl et al., 1999, 2003; Peakall et al., 2010), and hold great promise for application in other systems.
An important prerequisite for the development of tools and experiments to improve understanding of pollinator perception as a force underlying floral divergence, is the availability of suitable floral variation. Creatively exploiting biotechnology may offer ways to obtain floral variants that differ in a particular trait of interest, while controlling for variation in other traits. Specifically, the use of genetically transformed plants created through a series of targeted hybridization events has been useful for teasing apart the complex interactions between plants and pollinators in several model systems. In a series of papers, the role of colour variation in mediating differential pollinator attraction, ultimately contributing to speciation (Ramsey et al., 2003), was successfully established using near-isogenic lines in two closely related Mimulus species (Schemske and Bradshaw, 1999; Bradshaw and Schemske, 2003). In other model systems, transformed plants have been used to manipulate floral traits such as nectar volume in Petunia (Brandenburg et al., 2012) and floral odour in Nicotiana (Kessler et al., 2008), to study plant–pollinator interactions. Extension of this approach to other systems is expected to lead to some of the most powerful insights into the effect of trait variation on pollinator behaviour and efficiency. Unfortunately these approaches will be challenging to implement in non-model organisms. Nonetheless, one feature that could be employed more widely is the clonal propagation of lines with target traits of interest (Whitehead et al., 2012). Clonal lines could be particularly effectively employed in experimental arrays (e.g. Karron and Mitchell, 2012), or within the reciprocal translocation experiments we have already advocated.
If floral variation does not occur naturally, or if it cannot be obtained through genetic transformation, pollinator-driven trait divergence can still be studied using two alternative approaches. First of all, the effect of variation in a particular trait on pollinator behaviour or efficiency can be evaluated through direct floral manipulations by researchers. This approach has been successfully applied to study variation in flower angle (Fulton and Hodges, 1999), inflorescence shape (Johnson et al., 2003), flower shape (Castellanos et al., 2004), flower colour (Waser and Price, 1985) and floral odour (Shuttleworth and Johnson, 2010), and is particularly powerful if combined with assessments of effects on both male and female plant fitness (e.g. Hansen et al., 2012). Secondly, variable artificial flowers can be used to study the effect of variation in specific floral traits on pollinator responses. Use of artificial flowers is particularly useful for studies of rare plant species that are of conservation concern (e.g. Newman et al., 2012).
Determining the direction of evolutionary shifts at the ecotype level
Although clues about the environment in which selection for shifts in pollination system occur can be inferred from the distribution of pollinators and potential competing plant species, it is also crucial to know the direction of evolutionary shifts. This information is currently lacking from many pollination ecotypes studies, but can add considerable explanatory power for understanding the underlying driver of the evolutionary shift (e.g. Van der Niet et al., 2014).
Insight into the direction of evolutionary shifts can be straightforward when fully resolved phylogenies are available. However, in the studies of most interest here (i.e. divergent population, ecotypes, incipient and cryptic species), the taxa will rarely have diverged to the point that full phylogenetic resolution is possible. Therefore, it will be essential to draw on both the laboratory tools and the statistical analysis options from across the fields of population genetics, phylogeography and phylogenetics in order to help determine the polarity of evolutionary change. With these tools it may also be possible to assess the direction of migration and colonization (e.g. Pettengill and Moeller, 2012; Briscoe Runquist and Moeller, 2014; Van der Niet et al., 2014). In applying tools from across these fields to investigate genetic variation at the multiple hierarchical levels of populations, ecotypes and species, it will be important to take full advantage of the opportunities afforded by NGS. For example, NGS offers both simultaneous discovery and assay of hundreds to thousands of single-nucleotide polymorphisms (SNPs) for tens to hundreds of samples. Restriction-site-associated DNA sequencing (RAD-Seq; Davey and Blaxter, 2010; Davey et al., 2011) and related techniques such as genotype-by-sequencing (Elshire et al., 2011) offer the potential for detecting thousands of polymorphisms simultaneously across multiple individuals in a single NGS run. With access to such large numbers of genetic markers and samples, robust and highly informative hierarchical genetic analyses will be possible for any study system of interest. Also important in such genetic analyses are the complementary insights offered by nuclear versus cpDNA markers (Ebert and Peakall, 2009; Peakall and Whitehead, 2014).
Quantifying reproductive isolation
Perhaps the most substantive challenge remaining for studies of pollinator-driven speciation will be to assess whether pollinator-driven divergence can lead to pollinator isolation. This could be tested by first establishing whether ecotype boundaries coincide with barriers to gene flow, which may be identified by findings of substantive genetic differentiation between ecotypes. Population genetic analysis that takes full advantage of NGS methods should prove particularly powerful for this task. However, an appropriate choice of loci and genetic analyses are essential to ensure that the genetic signals evaluated reflect current rather than historic gene flow.
Of course, alternative barriers other than reproductive isolation, including geographical isolation, may also limit gene flow. In such cases it is difficult to establish whether pollinator-driven adaptation is the cause of speciation, or whether pollinator-driven adaptation only evolves if pre-existing barriers to gene flow are present (e.g. as a consequence of speciation). To explicitly evaluate whether pollinator isolation occurs, tests for assortative mating in sympatry are required. Sympatry can be simulated by performing reciprocal translocation experiments. Ethical issues due to potential for genetic pollution and logistical challenges may preclude straightforward implementation of translocation experiments. These problems can be solved by performing experiments that simulate sympatry under controlled conditions in enclosed areas such as greenhouses. Paternity analysis of progeny from ecotypes exposed in sympatry under different pollinator regimes could reveal whether assortative pollinator-mediated mating occurs. To control for a potential confounding effect of non-pollinator-driven postmating isolation, paternity analyses would need to be supplemented with direct observations of pollinator behaviour, quantification of pollinator-mediated heterospecific pollen deposition, and an assessment of cross-compatibility using hand-pollinations (cf. Nagy, 1997).
It is becoming increasingly clear that in many cases of ecological speciation, selection occurs simultaneously along multiple ecological gradients (Nosil, 2012; Fig. 5). It is thus likely that divergence, including that mediated by pollinators, occurs along multiple ecological niche gradients throughout a plants' distribution. In this case, selection will be multifarious and pollinator isolation would then simultaneously contribute to speciation along with other reproductive barriers, such as flowering time differences and immigrant inviability due to local adaptation (Leimu and Fischer, 2008). A multifaceted evolutionary process, such as this, may also better explain the coexistence of closely related species (note, however, that Pauw, 2013, does provide a model for stable co-existance between species that differ only in pollination system). Therefore, it will always be critical to consider pollinator isolation in combination with other potential isolating mechanisms. A macroevolutionary expectation that follows from a process of multifarious selection is that closely related species will differ at multiple ecological barriers to reproduction. Indeed, comparisons of isolating barriers between closely related species confirm this is the case (e.g. Ramsey et al., 2003; Lowry et al., 2008; Scopece et al., 2013). Implementing extensive reciprocal translocation experiments to simultaneously assess fitness and reproductive isolation at various life history stages will need to be performed to more fully understand the relative contribution of pollinator isolation to total reproductive isolation. Such experiments have, to our knowledge, not yet been performed with pollination ecotypes.
Fig. 5.
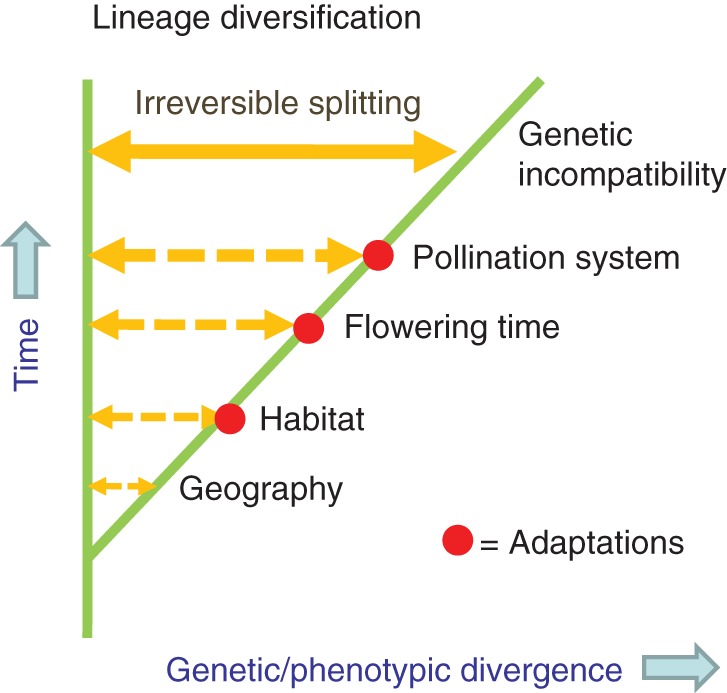
Hypothetical example of allopatric speciation via the intermediate stage of ecotypes. Ecological adaptations (including shifts in pollination system) resulting in genetic and/or phenotypic divergence can occur at any point in time, and in any sequence, after initial geographic isolation. Usually these adaptations will not lead to complete reproductive isolation, but since they occur between allopatric populations gene flow is limited. Full reproductive isolation, sufficient to maintain identity of forms during secondary contact, builds up gradually with time.
CONCLUSIONS
Since the seminal works of Grant and Grant (1965) and Stebbins (1970) on the evolution of pollination systems, much progress has been made. The emergence of experimental approaches, and the availability of new technology that allows for better quantification of floral trait divergence, combined with the genetic analysis of shifts in pollination system, have both contributed towards progress in the field. While several decades of research have largely confirmed the validity of the Grant–Stebbins model's principles, it is now clear that floral trait divergence can arise through non-pollinator-driven processes as well. Experiments have also revealed that floral traits can evolve through complex mechanisms that reflect both pollinator preference and filters. Further, it is evident that the geographical mosaic of selection on floral traits may be determined by pollination efficiency, variation in pollinator distribution and variation in plant community composition, as well as non-pollinator-driven ecological shifts.
One particularly critical question remains to be fully addressed: can shifts in pollination systems lead necessarily to ecological speciation? The outcome of speciation was considered a logical consequence by Grant (1994), but has been seriously questioned by Waser (1998, 2001). Nevertheless, regardless of whether reproductive isolation evolves as a direct consequence of shifts in pollination system, or whether these shifts contribute only partially to the speciation process, it is becoming increasingly clear that pollinators have contributed strongly to the diversification of flowering plants. In contrast to the unique mechanisms of ecological speciation that characterize many other systems, pollinator-driven ecological speciation may have occurred frequently, via similar processes, and across a large number of independent clades (Van der Niet and Johnson, 2012). Thus, pollinator-driven speciation may yet prove to be amongst the most widespread and most compelling cases of ecological speciation.
ACKNOWLEDGEMENTS
We would like to thank Annals of Botany Regional Editor Jeff Karron and Managing Editor David Frost for their tremendous efforts into facilitating this Special Issue. Ruth Cozien, Karl Duffy, Jeff Karron and Pat Heslop-Harrison provided useful comments on an earlier draft of this Viewpoint. Lynn Watson and Bob Sikora kindly provided photographs of Saltugilia splendens. Bruce Anderson and Dennis Hansen provided photographs of long-tongued fly pollinators in South Africa. This work was supported by funding to R.P. from the Australian Research Council project DP1094453, and to S.D.J. from the Department of Science and Technology of the National Research Foundation (NRF) of South Africa. Any opinion, finding and conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept liability in this regard.
LITERATURE CITED
- Ågren J, Schemske DW. Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytologist. 2012;194:1112–1122. doi: 10.1111/j.1469-8137.2012.04112.x. [DOI] [PubMed] [Google Scholar]
- Aigner PA. Optimality modeling and fitness trade-offs: when should plants become pollinator specialists? Oikos. 2001;95:177–184. [Google Scholar]
- Anderson B, Johnson SD. The geographical mosaic of coevolution in a plant–pollinator mutualism. Evolution. 2008;62:220–225. doi: 10.1111/j.1558-5646.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Anderson B, Johnson SD. Geographical covariation and local convergence of flower depth in a guild of fly-pollinated plants New Phytologist. 2009;182:533–540. doi: 10.1111/j.1469-8137.2009.02764.x. [DOI] [PubMed] [Google Scholar]
- Arias CF, Rosales C, Salazar C, et al. Sharp genetic discontinuity across a unimodal Heliconius hybrid zone. Molecular Ecology. 2012;21:5778–5794. doi: 10.1111/j.1365-294X.2012.05746.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS. Patterns of character divergence and the evolution of reproductive ecotypes of Dalechampia scandens (Euphorbiaceae) Evolution. 1985;39:733–752. doi: 10.1111/j.1558-5646.1985.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Muchhala N. Associations between floral specialization and species diversity: cause, effect, or correlation? Evolutionary Ecology. 2009;23:159–179. [Google Scholar]
- Armbruster WS, Edwards ME, Debevec EM. Floral character displacement generates assemblage structure of Western Australian triggerplants (Stylidium) Ecology. 1994;75:315–329. [Google Scholar]
- Armbruster WS, Shi X-Q, Huang S-Q. Do specialized flowers promote reproductive isolation? Realized pollination accuracy of three sympatric Pedicularis species. Annals of Botany. 2014;113 doi: 10.1093/aob/mct187. 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aximoff IA, Freitas L. Is pollen removal or seed set favoured by flower longevity in a hummingbird-pollinated Salvia species? Annals of Botany. 2010;106:413–419. doi: 10.1093/aob/mcq141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg E, Alexandersson R, Johnsson M, Maad J, Ågren J, Nilsson LA. Pollinator shifts and the evolution of spur length in the moth-pollinated orchid Platanthera bifolia. Annals of Botany. 2014;113 doi: 10.1093/aob/mct217. 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Brandenburg A, Kuhlemeier C, Bshary R. Hawkmoth pollinators decrease seed set of a low-nectar Petunia axillaris line through reduced probing time. Current Biology. 2012;22:1635–1639. doi: 10.1016/j.cub.2012.06.058. [DOI] [PubMed] [Google Scholar]
- Briscoe Runquist RD, Moeller DA. Floral and mating system divergence in secondary sympatry: testing an alternative hypothesis to reinforcement in Clarkia. Annals of Botany. 2014;113 doi: 10.1093/aob/mct218. 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos MC, Wilson P, Thomson JD. ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology. 2004;17:876–885. doi: 10.1111/j.1420-9101.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Hill RI, Kapan DD, Gilbert LE, Kronforst MR. Polymorphic butterfly reveals the missing link in ecological speciation. Science. 2009;326:847–850. doi: 10.1126/science.1179141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L, Raine NE. Recognition of flowers by pollinators. Current Opinion in Plant Biology. 2006;9:428–435. doi: 10.1016/j.pbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Clausen J, Keck DD, Hiesey WM. Experimental studies on the nature of species. I. Effect of varied environments on Western North American plants. Washington, DC: Carnegie Institution, publication no. 520; 1940. [Google Scholar]
- Cosacov A, Cocucci AA, Sérsic AN. Geographical differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza: do pollinators matter? Annals of Botany. 2014;113 doi: 10.1093/aob/mct239. 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepet WL. Advanced (constant) insect pollination mechanisms: pattern of evolution and implications vis-à-vis angiosperm diversity. Annals of the Missouri Botanical Garden. 1984;71:607–630. [Google Scholar]
- Dalca AV, Brudno M. Genome variation discovery with high-throughput sequencing data. Briefings in Bioinformatics. 2010;11:3–14. doi: 10.1093/bib/bbp058. [DOI] [PubMed] [Google Scholar]
- Darwin CR. The various contrivances by which orchids are fertilised by insects. London: John Murray; 1877. [Google Scholar]
- Davey JW, Blaxter ML. RADSeq: next-generation population genetics. Briefings in Functional Genomics. 2010;9:416–423. doi: 10.1093/bfgp/elq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nature Reviews Genetics. 2011;12:499–510. doi: 10.1038/nrg3012. [DOI] [PubMed] [Google Scholar]
- Dodd ME, Silvertown J, Chase MW. Phylogenetic analysis of trait evolution and species diversity variation among angiosperm families. Evolution. 1999;53:732–744. doi: 10.1111/j.1558-5646.1999.tb05367.x. [DOI] [PubMed] [Google Scholar]
- Dyer AG, Paulk AC, Reser DH. Colour processing in complex environments: insights from the visual system of bees. Proceedings of the Royal Society B, Biological Sciences. 2011;278:952–959. doi: 10.1098/rspb.2010.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Peakall R. Chloroplast simple sequence repeats (cpSSRs): technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Molecular Ecology Resources. 2009;9:673–690. doi: 10.1111/j.1755-0998.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- Ellis AG, Johnson SD. Gender differences in the effects of floral spur length manipulation on fitness in a hermaphroditic orchid. International Journal of Plant Sciences. 2010;171:1010–1019. [Google Scholar]
- Elshire RJ, Glaubitz JC, Sun Q, et al. A robust, simple genotyping-by-Sequencing (GBS) approach for high diversity species. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019379. pe19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology Evolution and Systematics. 2004;35:375–403. [Google Scholar]
- Forest F, Goldblatt P, Manning JC, Baker D, Colville JF, Devey DS, Jose S, Kaye M, Buerki S. Pollinator shifts as triggers of speciation in painted petal irises (Lapeirousia: Iridaceae) Annals of Botany. 2014;113 doi: 10.1093/aob/mct248. 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society B, Biological Sciences. 1999;266:2247–2252. [Google Scholar]
- Gardner MG, Fitch AJ, Bertozzi T, Lowe AJ. Rise of the machines – recommendations for ecologists when using next generation sequencing for microsatellite development. Molecular Ecology Resources. 2011;11:1093–1101. doi: 10.1111/j.1755-0998.2011.03037.x. [DOI] [PubMed] [Google Scholar]
- Goldblatt P, Manning JC. Phylogeny and speciation in Lapeirousia subgenus Lapeirousia (Iridaceae: Ixioideae) Annals of the Missouri Botanical Garden. 1996;83:346–361. [Google Scholar]
- Goldblatt P, Nanni I, Bernhardt P, Manning JC. Floral biology of Hesperantha (Iridaceae : Crocoideae): how minor shifts in floral presentation change the pollination system. Annals of the Missouri Botanical Garden. 2004;91:186–206. [Google Scholar]
- Gómez JM, Muños-Pajares AJ, Abdelaziz M, Lorite J, Perfectti F. Evolution of pollination niches and floral divergence in the generalist plant Erysimum mediohispanicum. Annals of Botany. 2014;113 doi: 10.1093/aob/mct186. 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwillie C, Sargent RD, Eckert CG, et al. Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system evolution. New Phytologist. 2010;185:311–321. doi: 10.1111/j.1469-8137.2009.03043.x. [DOI] [PubMed] [Google Scholar]
- Grant V. Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences of the USA. 1994;91:3–10. doi: 10.1073/pnas.91.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V, Grant KA. Flower pollination in the Phlox family. New York: Columbia University Press; 1965. [Google Scholar]
- Hansen DM, Van der Niet T, Johnson SD. Floral signposts: testing the significance of visual ‘nectar guides’ for pollinator behaviour and plant fitness. Proceedings of the Royal Society B, Biological Sciences. 2012;279:634–639. doi: 10.1098/rspb.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder LD, Johnson SD. Darwin's beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist. 2009;183:530–545. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Castellanos MC, Medrano M. Geographical context of floral evolution: towards an improved research programme in floral diversification. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 278–294. [Google Scholar]
- Hopkins R, Rausher MD. Pollinator-mediated selection on flower color allele drives reinforcement. Science. 2012;335:1090–1092. doi: 10.1126/science.1215198. [DOI] [PubMed] [Google Scholar]
- Huang SQ, Fenster CB. Absence of long-proboscid pollinators for long-corolla-tubed Himalayan Pedicularis species: implications for the evolution of corolla length. International Journal of Plant Sciences. 2007;168:325–331. [Google Scholar]
- de Jager ML, Ellis AG. Floral polymorphism and the fitness implications of attracting pollinating and florivorous insects. Annals of Botany. 2014;113 doi: 10.1093/aob/mct189. 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jersakova J, Jurgens A, Smilauer P, Johnson SD. The evolution of floral mimicry: identifying traits that visually attract pollinators. Functional Ecology. 2012;26:1381–1389. [Google Scholar]
- Johnson SD. Pollination ecotypes of Satyrium hallackii (Orchidaceae) in South Africa. Botanical Journal of the Linnean Society. 1997;123:225–235. [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 295–310. [Google Scholar]
- Johnson SD. The pollination niche and its role in the diversification and maintenance of the southern African flora. Philosophical Transactions of the Royal Society B, Biological Sciences. 2010;365:499–516. doi: 10.1098/rstb.2009.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae) Evolution. 1997;51:45–53. doi: 10.1111/j.1558-5646.1997.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Steiner KE. Generalization versus specialization in plant pollination systems. Trends in Ecology & Evolution. 2000;15:190–193. doi: 10.1016/s0169-5347(99)01811-x. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Edwards TJ, Carbutt C, Potgieter C. Specialization for hawkmoth and long-proboscid fly pollination in Zaluzianskya section Nycterinia (Scrophulariaceae) Botanical Journal of the Linnaean Society. 2002;138:17–27. [Google Scholar]
- Johnson SD, Alexandersson R, Linder HP. Experimental and phylogenetic evidence for floral mimicry in a guild of fly-pollinated plants. Biological Journal of the Linnean Society. 2003;80:289–304. [Google Scholar]
- Johnson SD, Steiner KE, Kaiser R. Deceptive pollination in two subspecies of Disa spathulata (Orchidaceae) differing in morphology and floral fragrance. Plant Systematics and Evolution. 2005;255:87–98. [Google Scholar]
- Johnson SD, Hargreaves AL, Brown M. Dark bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Karron JD, Mitchell RJ. Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals of Botany. 2012;109:563–570. doi: 10.1093/aob/mcr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Ivey CT, Mitchell RJ, Whitehead MR, Peakall R, Case AL. New perspectives on the evolution of plant mating systems. Annals of Botany. 2012;109:493–503. doi: 10.1093/aob/mcr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay KM, Sargent RD. The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annual Review of Ecology Evolution and Systematics. 2009;40:637–656. [Google Scholar]
- Kessler D, Gase K, Baldwin IT. Field experiments with transformed plants reveal the sense of floral scents. Science. 2008;321:1200–1202. doi: 10.1126/science.1160072. [DOI] [PubMed] [Google Scholar]
- Knudsen JT, Eriksson R, Gershenzon J, Stahl B. Diversity and distribution of floral scent. Botanical Review. 2006;72:1–120. [Google Scholar]
- Kulbaba MW, Worley AC. Selection on floral design in Polemonium brandegeei (Polemoniaceae): female and male fitness under hawkmoth pollination. Evolution. 2012;66:1344–1359. doi: 10.1111/j.1558-5646.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- Leimu R, Fischer M. A meta-analysis of local adaptation in plants. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0004010. pe4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losos JB, Glor RE. Phylogenetic comparative methods and the geography of speciation. Trends in Ecology & Evolution. 2003;18:220–227. [Google Scholar]
- Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH. The strength and genetic basis of reproductive isolating barriers in flowering plants. Philosophical Transactions of the Royal Society B, Biological Sciences. 2008;363:3009–3021. doi: 10.1098/rstb.2008.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martén-Rodriguez S, Kress WJ, Temeles EJ, Meléndez-Ackerman E. Plant–pollinator interactions and floral convergence in two species of Heliconia from the Caribbean Islands. Oecologia. 2011;167:1075–1083. doi: 10.1007/s00442-011-2043-8. [DOI] [PubMed] [Google Scholar]
- Miller TJ, Raguso RA, Kay KM. Novel adaptation to hawkmoth pollinators in Clarkia reduces efficiency, not attraction of diurnal visitors. Annals of Botany. 2014;113 doi: 10.1093/aob/mct237. 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller DA. Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology. 2006;87:1510–1522. doi: 10.1890/0012-9658(2006)87[1510:gsopcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nagy ES. Frequency-dependent seed production and hybridization rates: implications for gene flow between locally adapted plant populations. Evolution. 1997;51:703–714. doi: 10.1111/j.1558-5646.1997.tb03654.x. [DOI] [PubMed] [Google Scholar]
- Newman E, Anderson B, Johnson SD. Flower colour adaptation in a mimetic orchid. Proceedings of the Royal Society B, Biological Sciences. 2012;279:2309–2313. doi: 10.1098/rspb.2011.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Manning JC, Anderson B. Matching floral and pollinator traits through guild convergence and pollinator ecotype formation. Annals of Botany. 2014;113 doi: 10.1093/aob/mct203. 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P. Ecological speciation. Oxford: Oxford University Press; 2012. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120:321–326. [Google Scholar]
- Pauw A. Can pollination niches facilitate plant coexistence? Trends in Ecology & Evolution. 2013;28:30–37. doi: 10.1016/j.tree.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Pauw A, Stofberg J, Waterman RJ. Flies and flowers in Darwin's race. Evolution. 2009;63:268–279. doi: 10.1111/j.1558-5646.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Ebert D, Poldy J, et al. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchids: implications for pollinator-driven speciation. New Phytologist. 2010;188:437–450. doi: 10.1111/j.1469-8137.2010.03308.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Whitehead MR. Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Annals of Botany. 2014;113 doi: 10.1093/aob/mct199. 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. A pollinator shift explains floral divergence in an orchid species complex in South Africa. Annals of Botany. 2014;113 doi: 10.1093/aob/mct216. 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettengill JB, Moeller DA. Phylogeography of speciation: allopatric divergence and secondary contact between outcrossing and selfing Clarkia. Molecular Ecology. 2012;21:4578–4592. doi: 10.1111/j.1365-294X.2012.05715.x. [DOI] [PubMed] [Google Scholar]
- Raguso RA. Wake up and smell the roses: the ecology and evolution of floral scent. Annual Review of Ecology Evolution and Systematics. 2008;39:549–569. [Google Scholar]
- Ramsey J, Bradshaw HD, Schemske DW. Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae) Evolution. 2003;57:1520–1534. doi: 10.1111/j.0014-3820.2003.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Rice AM, Rudh A, Ellegren H, Qvarnstrom A. A guide to the genomics of ecological speciation in natural animal populations. Ecology Letters. 2011;14:9–18. doi: 10.1111/j.1461-0248.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- Robertson JL, Wyatt R. Evidence for pollination ecotypes in the yellow-fringed orchid, Platanthera ciliaris. Evolution. 1990;44:121–133. doi: 10.1111/j.1558-5646.1990.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Roesti M, Hendry AP, Salzburger W, Berner D. Genome divergence during evolutionary diversification as revealed in replicate lake-stream stickleback population pairs. Molecular Ecology. 2012;21:2852–2862. doi: 10.1111/j.1365-294X.2012.05509.x. [DOI] [PubMed] [Google Scholar]
- Rymer PD, Johnson SD, Savolainen V. Pollinator behaviour and plant speciation: can assortative mating and disruptive selection maintain distinct floral morphs in sympatry? New Phytologist. 2010;188:426–436. doi: 10.1111/j.1469-8137.2010.03438.x. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Bradshaw HD. Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences of the USA. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Johnson SD. Pollinator-mediated evolution of floral signals. Trends in Ecology & Evolution. 2013;28:307–315. doi: 10.1016/j.tree.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, et al. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. The chemistry of sexual deception in an orchid–wasp pollination system. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- Schluter D. The ecology of adaptive radiation. Oxford: Oxford University Press; 2000. [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends in Ecology & Evolution. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. [DOI] [PubMed] [Google Scholar]
- Scopece G, Croce A, Lexer C, Cozzolino S. Components of reproductive isolation between Orchis mascula and Orchis pauciflora. Evolution. 2013;67:2083–2093. doi: 10.1111/evo.12091. [DOI] [PubMed] [Google Scholar]
- Shuttleworth A, Johnson SD. The missing stink: sulphur compounds can mediate a shift between fly and wasp pollination systems. Proceedings of the Royal Society B, Biological Sciences. 2010;277:2811–2819. doi: 10.1098/rspb.2010.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG. The major features of evolution. New York: Columbia University Press; 1953. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Strauss SY, Whittall JB. Non-pollinator agents of selection on floral traits. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 120–138. [Google Scholar]
- Sun M, Gross K, Schiestl FP. Floral adaptation to local pollinator guilds in a terrestrial orchid. Annals of Botany. 2014;113 doi: 10.1093/aob/mct219. 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente LM, Manning JC, Goldblatt P, Vargas P. Did pollination shifts drive diversification in Southern African Gladiolus? Evaluating the model of pollinator-driven speciation. American Naturalist. 2012;180:83–98. doi: 10.1086/666003. [DOI] [PubMed] [Google Scholar]
- Vamosi JC, Vamosi SM. Key innovations within a geographical context in flowering plants: towards resolving Darwin's abominable mystery. Ecology Letters. 2010;13:1270–1279. doi: 10.1111/j.1461-0248.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- Van der Niet T, Johnson SD. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends in Ecology & Evolution. 2012;27:353–361. doi: 10.1016/j.tree.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Van der Niet T, Johnson SD, Linder HP. Macroevolutionary data suggest a role for reinforcement in pollination system shifts. Evolution. 2006;60:1596–1601. doi: 10.1554/05-705.1. [DOI] [PubMed] [Google Scholar]
- Van der Niet T, Zollikofer CPE, de Leon MSP, Johnson SD, Linder HP. Three-dimensional geometric morphometrics for studying floral shape variation. Trends in Plant Science. 2010;15:423–426. doi: 10.1016/j.tplants.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Van der Niet T, Pirie MD, Shuttleworth A, Johnson SD, Midgley JJ. Do pollinator distributions underlie the evolution of pollination ecotypes in the Cape shrub Erica plukenetii? Annals of Botany. 2014;113 doi: 10.1093/aob/mct193. 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines TH, Schluter D. Strong assortative mating between allopatric sticklebacks as a by–product of adaptation to different environments. Proceedings of the Royal Society B, Biological Sciences. 2006;273:911–916. doi: 10.1098/rspb.2005.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waser NM. Pollination, angiosperm speciation, and the nature of species boundaries. Oikos. 1998;82:198–201. [Google Scholar]
- Waser NM. Pollinator behaviour and plant speciation: looking beyond the ‘ethological isolation’ paradigm. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination: animal behavior and floral evolution. Cambridge: Cambridge University Press. 2001. pp. 318–336. [Google Scholar]
- Waser NM, Price MV. The effect of nectar guides on pollinator preference – experimental studies with a montane herb. Oecologia. 1985;67:121–126. doi: 10.1007/BF00378462. [DOI] [PubMed] [Google Scholar]
- Waterman RJ, Bidartondo MI, Stofberg J, et al. The effects of above- and belowground mutualisms on orchid speciation and coexistence. American Naturalist. 2011;177:E54–E68. doi: 10.1086/657955. [DOI] [PubMed] [Google Scholar]
- Whitehead MR, Phillips RD, Peakall R. Pollination: the price of attraction. Current Biology. 2012;22:R680–R682. doi: 10.1016/j.cub.2012.06.072. [DOI] [PubMed] [Google Scholar]
- Whittall JB, Hodges SA. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature. 2007;447:706–U12. doi: 10.1038/nature05857. [DOI] [PubMed] [Google Scholar]
- Xu SQ, Schluter PM, Scopece G, et al. Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution. 2011;65:2606–2620. doi: 10.1111/j.1558-5646.2011.01323.x. [DOI] [PubMed] [Google Scholar]