Abstract
Rationale
Nicotine influences many cognitive processes, especially those requiring high attentional loads, yet the impact of nicotine on all aspects of information processing has not been well delineated.
Objective
The aim of the study was to determine the relative behavioral and functional effects of nicotine on dissociable aspects of information processing (i.e., selective attention and motor intention).
Methods
Adult smokers (N=25) and healthy controls (N=23) performed the intention/attention task (IAT) twice, during functional magnetic resonance imaging. The IAT assesses the relative differences in performance evoked by prime stimuli that provide information regarding either the correct hand with which to respond (i.e., intentional primes) or the likely location of a target stimulus (i.e., attentional primes). Smokers were scanned 2 h after nicotine (21 mg) or placebo patch placement. The order of nicotine and placebo sessions was randomized and counter-balanced. Controls were also scanned twice, with no patch placement in either session.
Results
While drug condition had no significant effect on reaction time, smokers were overall more accurate than controls. Moreover, nicotine significantly increased the response to intentional primes in brain regions known to mediate response readiness, e.g., inferior parietal lobe, supramarginal gyrus, and striatum.
Conclusions
While limited to participant accuracy, these data suggest that the behavioral effects of nicotine in smokers are not only limited to information processing input (i.e., selective attention) but are also generalizable to output functions (i.e., motor intention). Moreover, nicotine’s effects on intention appear to be mediated by a facilitation of function in brain regions associated with information processing output.
Keywords: Nicotine, Smoking, Intention, Response readiness, Selective attention, Functional MRI, Information processing
Introduction
Nicotine is the primary psychoactive ingredient and addictive component in tobacco. It exerts its effects via activation of nicotinic acetylcholine receptors (nAchR), which are widely distributed throughout the brain (see Clementi et al. 2000 for review). Neurons of the mesocorticolimbic dopamine system that project from the ventral tegmental area to anterior limbic forebrain areas, such as the nucleus accumbens and cingulate cortex, express high affinity nAChRs on both cell bodies and terminals (Zoli et al. 2002). Given the role of these regions in reward processing (see Diekhof et al. 2008 for review), it is believed that activation of these nAChRs on midbrain dopaminergic neurons participates in nicotine’s rewarding effects.
In addition to its rewarding/reinforcing effects, nicotine influences several higher cognitive processes across multiple species (e.g., Lawrence et al. 2002; Levin and Rezvani 2000; Levin et al. 2006; Rezvani and Levin 2001; Stolerman et al. 1995). In humans, the modulation of cognitive function by nicotine is evident between cognitive domains and cohorts. Indeed, postnicotine enhancement and withdrawal-induced attenuation of cognitive processes in smokers have both been observed (see Heishman et al. 1994 for a review). This divergent effect of acute nicotine vs. nicotine withdrawal1 has been demonstrated in tests of interference, such as smoking and nonsmoking variations of the Stroop test (Domier et al. 2007; Gross et al. 1993; Powell et al. 2002; Rusted et al. 2000), in measures of sensorimotor gating (Della Casa et al. 1998, 1999; Duncan et al. 2001; Kumari et al. 1996; Kumari and Gray 1999), and in psychomotor performance (Davranche and Audiffren 2002; Ernst et al. 2001; Le Houezec et al. 1994; Snyder et al. 1989). It has been suggested that nicotine exerts its most robust effects during tasks that have a high attentional requirement (Newhouse et al. 2004; Swan and Lessov-Schlaggar 2007; Warburton and Rusted 1993), and one review concluded that the available evidence supports a consistent nicotine-mediated effect on sustained attention and motor processing (i.e., response latency and/or reaction time (RT)), with modest effects on selective attention and sensorimotor gating and little or no impact of nicotine on verbal learning, working memory, or executive function (Sacco et al. 2004). However, more recently, London and colleagues have demonstrated an impact of both nicotine withdrawal and acute nicotine on working memory and associated brain function (e.g., dorsolateral prefrontal cortex; Mendrek et al. 2006; Xu et al. 2005, 2006).
The myriad of cognitive and affective functions amenable to nicotine may be related to the variety of brain regions activated by nicotine. Nicotine activates limbic and para-limbic regions, including the nucleus accumbens, amygdala, anterior cingulate cortex (ACC), and prefrontal cortex (Stein et al. 1998). It also enhances activity in thalamus, caudate, and parietal cortex and in regions mediating visual attention, arousal, and motor activity (Lawrence et al. 2002). Nicotine-induced alterations in regional cerebral blood flow (rCBF) have been noted in both smokers and nonsmokers, with intravenous (IV) nicotine causing rCBF decreases in the ACC and cerebellum and increases in the occipital cortex (Ghatan et al. 1998). Furthermore, nicotine also differentially and dose-dependently affects blood flow in regions associated with arousal and reward (Rose et al. 2003) and has been shown to lead to global reductions in the rate of cerebral glucose metabolism in the brain (rCMRglc; Stapleton et al. 2003). In a comparison of subjects who received a variable dose of nicotine (based upon individual usage), there was a dose-dependent response of the reticular system that correlated with measures of self-reported craving and addiction in smokers (Rose et al. 2003).
Both the cognitive and neuroimaging literature suggest a consistent nicotine-mediated effect on behavioral and functional measures of the cognitive stages of information processing input, i.e., attention. Since these studies either do not consider alternative processing stages or control for other mechanisms (e.g., motor preparation, psychomotor response), the impact that nicotine has on other aspects of information processing, e.g., output, is less clearly delineated. Thus, it would seem difficult to determine whether nicotine’s effects on information processing are attention-specific or whether alternative/additional cognitive processes are impacted. This investigation sought to determine whether nicotine mediates input functions only or whether it also influences other information processing stages.
Information processing models suggest two modes of response selection: (1) stimulus-set selection, which is input selective (i.e., selective attention), and (2) response-set selection, which is output selective (i.e., intention; Broadbent 1970, 1971). While selective attention is a form of orienting, intention can be defined as the strategic selection and planning of a response (Rushworth and Taylor 2006; Thoenissen et al. 2002; Verfaellie and Heilman 1990). The neural substrates of intention are found primarily in the left posterior parietal cortex (Rushworth et al. 2001a, b; Toni et al. 2001), the supramarginal gyrus, the occipital lobe, and the insula (Hesse et al. 2006), while selective attention is mediated by function in a network of areas including the pulvinar, superior colliculus, superior parietal lobe, temporoparietal junction, superior temporal lobe, and frontal eye fields (see Raz and Buhle 2006 for a review). Activity in the premotor and supplementary motor areas has also been noted during intention tasks (e.g., Lau et al. 2004), although it would appear that this activity is more closely associated with motor preparation than intention per se (Thoenissen et al. 2002; Toni et al. 2001).
The dissociation between attention and intention has been studied using the intention/attention task (IAT; Verfaellie et al. 1988a, b). This priming paradigm was originally designed to ascertain the nature of a stimulus-response compatibility (SRC) effect, known as the Simon effect (Simon 1969; Simon and Rudell 1967; Simon and Small 1969), i.e., the observation that RT is faster and responses more accurate when a target position and the response to that target occur in the same relative location (e.g., left or right), even when the target location is task irrelevant. While both intentional and attentional primes facilitate RT compared to nonprime stimuli, the authors of the original IAT concluded that SRC effects are attributable to intentional and not attentional factors (Verfaellie et al. 1988b). With regards to cortical processing of these prime stimuli, there appears to be a hemispheric bias for intentional primes only (Verfaellie et al. 1988a). This asymmetry was recently confirmed in an investigation that found that motor intention was mediated by activation in the left parietal cortex in healthy subjects (Hesse et al. 2006). Although right parietal activation was associated with spatial orienting, which was in accordance with other studies (e.g., Coull and Nobre 1998), activity in this region did not differ significantly from alerting alone.
Using the IAT as a measure of information processing during functional magnetic resonance imaging (MRI), we hypothesized that the acute administration of nicotine to dependent, adult smokers would facilitate performance on both attention and intention trials, via activation of those brain regions that support selective attention and response preparedness/intention.
Methods
Written, informed consent was obtained from all subjects in this National Institute on Drug Abuse Intramural Research Program Institutional Review Board approved study. Participants were recruited from the general population using print advertisements, flyers, and referrals. The experimental groups were right-handed (Edinburgh Handedness Inventory; Oldfield 1971) adult (>18 years) smokers (N=25) and nonsmoking controls (N=23), matched for age, IQ (Wechsler Abbreviated Scale of Intelligence; Wechsler 2007), and gender (see Table 1). Smokers smoked a minimum of 15 cigarettes per day for at least 1 year prior to participation. Control status was defined as never having smoked daily, having never met DSM-IV criteria for nicotine abuse or dependence, and no nicotine use in the 12 months preceding the study. Poststudy, one control participant was found to have a history of nicotine dependence. Given the extended duration of abstinence (i.e., >16 years), the lack of any nicotine use in the previous 12 months, and the lack of evidence to suggest persistent nicotine-mediated changes in cortical plasticity in regions associated with the cognitive processes of interest in this task, this individual was included as a control in both behavioral and imaging analyses. Exclusion criteria included, but were not limited to, significant neurological, medical, or psychiatric history (i.e., current or past axis I diagnosis as determined by a board certified psychiatrist), claustrophobia, pregnancy, current use/abuse of any drug other than nicotine or marijuana, and dependence on any substance other than nicotine.
Table 1.
Participant demographic data
| Smokers (N=25) | Controls (N=23) | Comparison | |
|---|---|---|---|
| Age (years; mean (SD)) | 31.28 (8.33) | 28.74 (7.53) | t(46)=1.11, N.S. |
| IQ (mean (SD)) | 107.71 (11.06) | 108.32 (11.16) | t(44)=−0.19, N.S. |
| Years of education (mean (SD)) | 13.18 (2.25) | 14.85 (2.96) | t(46)=∼2.21, p=0.03 |
| Ethnicity (African American/Caucasian/Asian American) | 8:17:0 | 13:6:4 | χ2= 10.39, df=2, p<0.01 |
| Gender (male/female) | 12:13 | 9:14 | χ2=0.38, df=1, N.S. |
| Number of cigarettes smoked/day (min/max (mean)) | 15:40 (21.90) | N/A | – |
| Age at first use (min/max (mean)) | 9:28 (14.88) | N/A | – |
| Years since first use (min/max (mean)) | 4:33 (16.40) | N/A | – |
| Fagerstrom Test for Nicotine Dependence (min/max (mean)) | 1:9 (5.04) | N/A | – |
Design
This study employed a mixed design, with a between-subjects factor of participant group (smokers vs. non-smokers) and within-subjects factors of prime (intentional vs. attentional primes), SRC, and congruency. The smoking cohort had an additional within-subjects factor of drug condition (nicotine vs. placebo). Dependent variables of interest were RT, accuracy, and the percentage change in blood oxygenation level-dependent (BOLD) response.
Procedure
Subjects made three study visits: task and procedural training in a mock scanner and two MRI sessions. The sessions were identical for all participants, with the exception that smokers had a 21-mg nicotine or placebo patch (Nicoderm®; GlaxoSmithKline Inc., Research Triangle Park, NC, USA) applied 2 h prior to MRI sessions (see details below). Session order (i.e., nicotine vs. placebo) for smokers was determined randomly and counter-balanced between participants (N=13 nicotine first; N=12 placebo first). Participants were not permitted to consume any alcohol or over-the-counter medications for 24 h prior to each visit and were limited to not more than half a cup of caffeinated beverages before each scan. Prior to MRI sessions, participants were tested for current drug use (TRIAGE® urine drug test), alcohol use (breathalyzer test; Alco-Sensor IV, Intoximeters Incorporated, St. Louis, MO, USA), pregnancy, and expired carbon monoxide (CO) levels (Vitalograph Breath CO monitor, Lenexa, KS, USA). Smokers also completed a detailed smoking history, including the Fagerstrom Test for Nicotine Dependence (Heatherton et al. 1991). While there was no specific requirement or CO cutoff value for participants in either group, expired CO was used to confirm smoking status. Typical CO values for individuals in the control group were less than 1, and a CO value of >5 for a control participant resulted in further investigation of nonsmoking status.
Patch administration
Placebo and nicotine patches were identical in appearance and were manufactured by the same pharmaceutical company. Patches were affixed on the participant’s upper back 30 min after their last cigarette and 2 h before the start of scanning. Participants were not informed of patch type prior to placement and were not debriefed regarding session order until they had completed the study. Withdrawal intensity, mood, and nicotine craving were queried before and after each scan session for both smokers and controls, using the Parrott mood assessment (Parrott et al. 1996) and a 12-item short-form of the Tobacco Craving Questionnaire (TCQ; Heishman et al. 2003, 2007).
The IAT paradigm
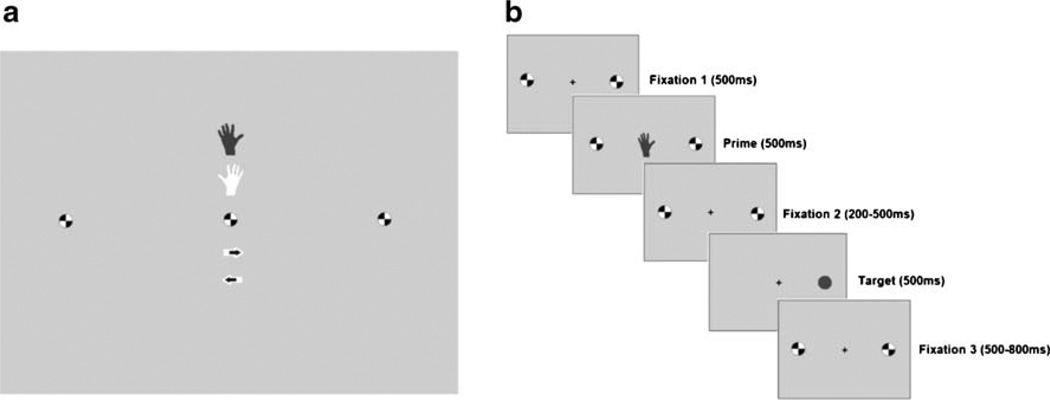
Participants responded to a circle target that appeared either to the left or right of a central fixation point with a button press; a dark circle required a left hand button press, while a light circle required a right hand button press (Fig. 1). Prior to target stimulus presentation, participants saw one of three priming stimuli—a hand, an arrow, or a circle. The hand stimulus was an intentional prime and indicated which hand participants should respond with but gave no information as to where on the screen the target would appear. The arrow prime provided attentional information and notified the participants of the target location but gave no information as to which hand would be required. These primes had an 80% probability of being accurate. The circle prime served as neutral comparison and gave no information regarding either response hand or target location. These task variations result in three potential factors of interest—prime type, SRC, and congruency (i.e., prime accuracy).
Fig. 1.
IAT stimuli and task procedure. a Prime stimuli, i.e., left hand, right hand, left arrow, right arrow, or circle. b Task procedure. Shown here is an example of an SR-/congruent trial, i.e., the prime accurately predicts that the target requires a left button press, but the target appears to the right of the screen
Participants completed five task blocks, each of which was separated by a 1-min rest period. Each block contained 52 randomly selected prime trials and 52 rest trials. All trials had a total duration of 2,300 ms and the interstimulus interval was jittered within trials, between stimuli (mean= 500 ms; range=200–800 ms). The intertrial interval was 2,300 ms. There were 12 neutral, 20 hand, and 20 arrow prime events, with eight incongruent and 32 congruent trials, per block.
Functional imaging
Whole brain echo planar images were acquired on a 3-T Siemens Allegra MRI scanner (Erlangen, Germany). Thirty-nine 4-mm slices were acquired in the sagittal plane with the following imaging parameters: repetition time= 2,300 ms, echo time=27 ms, field of view=220×220 mm at 64×64, and flip angle=80°. A T1-weighted MPRAGE structural image, with a voxel size of 1×1×1 mm, was also acquired in each session.
Data analysis
Functional imaging data were analyzed using Analysis of Functional Neuroimages (Cox 1996). To correct for head motion, 3D data for each subject were coregistered to a base volume. The data time series were analyzed using a voxel-wise multiple regression in which regressors were expressed as a delta function time-locked to cue presentation convolved with a hemodynamic response function and its temporal derivative. There were 20 regressors representing the different trial types (i.e., see Supplementary Table S4 for complete list). In addition, six head motion parameters were included as regressors of no interest. A voxel-wise average amplitude change (β) equal to the percentage change from baseline was calculated for each participant for each trial type and session. The resultant activation maps were then registered to a higher resolution (1µl), standard stereotaxic space (Talairach and Tournoux 1988), and spatially blurred using a 4.2-mm full width at half maximum Gaussian isotropic kernel.
Within- and between-group repeated measures ANOVA were conducted using β values for accurate, congruent trials only2. These analyses considered prime type, SRC, and drug condition. A voxel-wise threshold correcting for multiple comparisons and controlling for family wise error was calculated using a Monte Carlo simulation. This deterministic sampling algorithm ascertains the frequency of significant clusters that would occur by chance under the null hypothesis (i.e., the false positive rate). Using this approach, cluster significance was determined as equaling or exceeding a given cluster extent criteria at pcorrected ≤ 0.05 (see Table S1). Post hoc region-of-interest analyses were conducted for those clusters exhibiting a significant interaction between drug condition or group and prime type (see Tables S3, S4, and S5).
Behavioral data were analyzed in SPSS (Release 14; SPSS Inc., Chicago, IL, USA). Initial ANOVA examining the effect of prime (hand, arrow, and neutral) and group on behavioral indices were conducted to determine whether the anticipated priming effects had occurred. Due to the unbalanced nature of the data, all other behavioral analyses utilized linear mixed effects (LME) model analyses. The LME analyses modeled fixed and random effects corresponding to the task, group, and drug variables and included IQ as a covariate. Since the contrast between attention and intention trials was of primary interest, these analyses included hand and arrow primes only and not neutral trials. Using only these levels allowed us to model other task-relevant factors (i.e., congruency and SRC).
Due to variability in the total number of each trial type, raw accuracy scores were not used in the LME analyses; instead, the number of correct items was converted to a percentage score. To normalize the distribution of these scores, a Box–Cox transformation (i.e., T(Y)=(Yλ−1)/λ was performed (Box and Cox 1964). Box–Cox adjusted accuracy scores were then included as the dependent variable in the LME model.
ANOVA comparisons of prescan and postscan ratings on the Parrott questionnaire between controls and smokers (across drug conditions) and between drug conditions for smokers only were also carried out. TCQ items can be grouped according to four craving factors— emotionality: smoking in anticipation of relief from withdrawal or negative mood; expectancy: anticipation of positive outcomes from smoking; compulsivity: an inability to control tobacco use; and purposefulness: intention and planning to smoke for positive outcomes. Thus, between-condition ANOVA analyses for the smokers focused on these craving indices rather than individual TCQ rating items.
Results
Behavioral results
Parrott and TCQ
Drug condition significantly affected indices of craving. In the placebo condition, smokers had lower ratings on two of the four TCQ factors, i.e., emotionality and purposefulness (F(1, 23)=10.66, p=0.003 and F(1, 23)=27.28, p<0.001, respectively), compared to the nicotine condition. This difference did not vary as a function of whether the measure was obtained pre- or postsession. In addition, participants were more likely to agree with statements pertaining to expectancy and purposefulness on the TCQ after scanning sessions than they were before (F(1, 23)=5.58, p=0.03 and F(1, 23)=14.16, p=0.001, respectively), irrespective of drug condition.
The Parrott ratings indicated that at the end of the session vs. the start, participants were less tired (F(1, 40)= 12.31, p=0.001), more alert (F(1, 43)=17.21, p<0.001), less irritated (F(1, 44)=5.29, p=0.03), more focused (F(1, 44)= 6.07, p=0.02), happier (F(1, 44)=4.66, p=0.04), and hungrier (F(1, 44)=34.66, p<0.001). This pattern of results did not vary as a function of participant group or session (1 vs. 2). Moreover, smokers in the placebo condition were more likely to rate themselves at less tired (F(1, 23)=4.58, p =0.04), more alert (F(1, 23)=7.44, p=0.01), and more focused (F(1, 23)=4.21, p=0.05) across the session, compared to the nicotine condition. There were no significant interactions between time (pre- vs. postsession) and drug condition for smokers on any of the Parrot items.
Accuracy
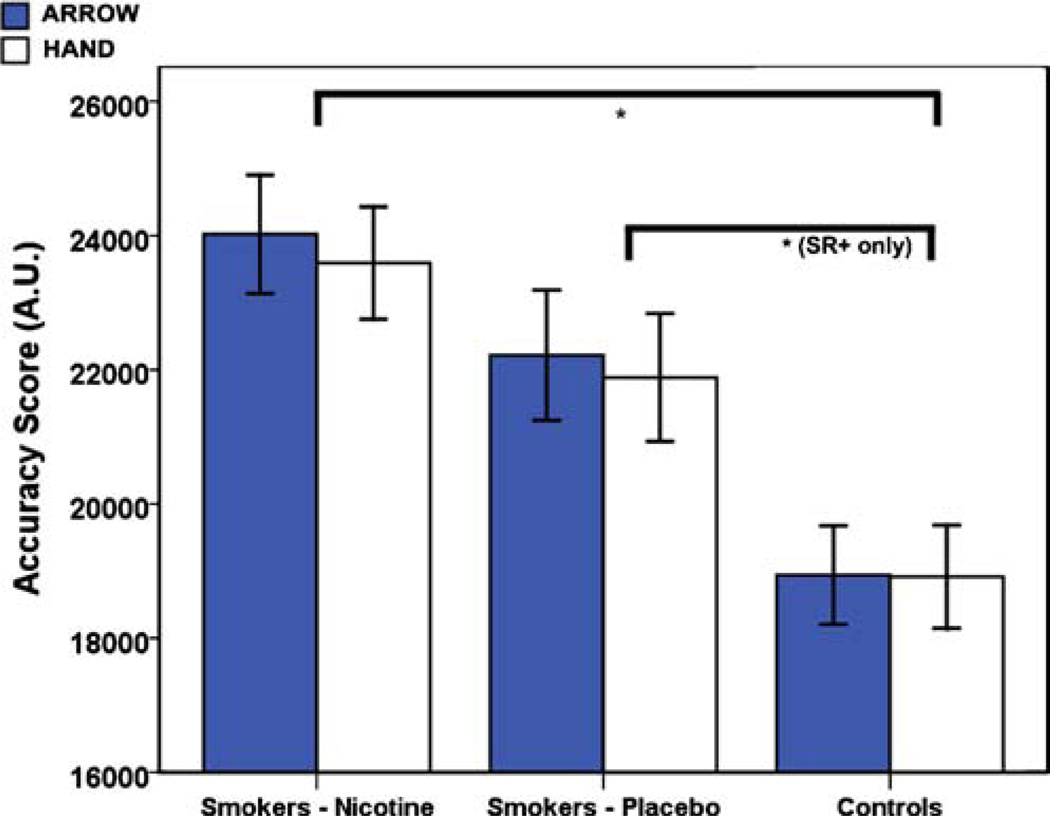
There was no significant main effect of prime type on accuracy. This was confirmed in both the initial ANOVA analysis (see Figure S1) and the LME analysis. However, accuracy was significantly affected by group (see Fig. 2), and this effect varied as a function of SRC. For example, smokers in the nicotine condition were more accurate than controls across all trial types (F(1, 44)=6.84, p=0.012), whereas in the placebo condition, smokers were significantly more accurate than controls on SR-compatible (SR +) trials only (t(282) =3.24, p=0.001). Furthermore, smokers and controls both demonstrated a significant reduction in accuracy between congruent and incongruent trials for all SR+trials (tARROW(140)=−3.19, p=0.02 and tHAND(140) =−3.59, p<0.001) and for arrow primes on SR-incompatible (SR-) trials only (t(140)=−2.87, p=0.005).
Fig. 2.
Average accuracy on the IAT. Shown are the mean accuracy scores for smokers postnicotine, smokers postplacebo, and controls for each prime type (arrow vs. hand), averaged across SRC and congruency conditions. Error bars show ±1 standard error
For smokers, there was also a significant drug condition×SRC×congruency interaction (F(1, 264)=4.16, p= 0.043), resulting from a postnicotine reduction in accuracy on incongruent, SR+trials only (t(98)=−2.66, p=0.009; see Figure S2).
Reaction time
Mixed measure ANOVA confirmed that the priming effects seen in IAT studies were replicated (see Figure S1).
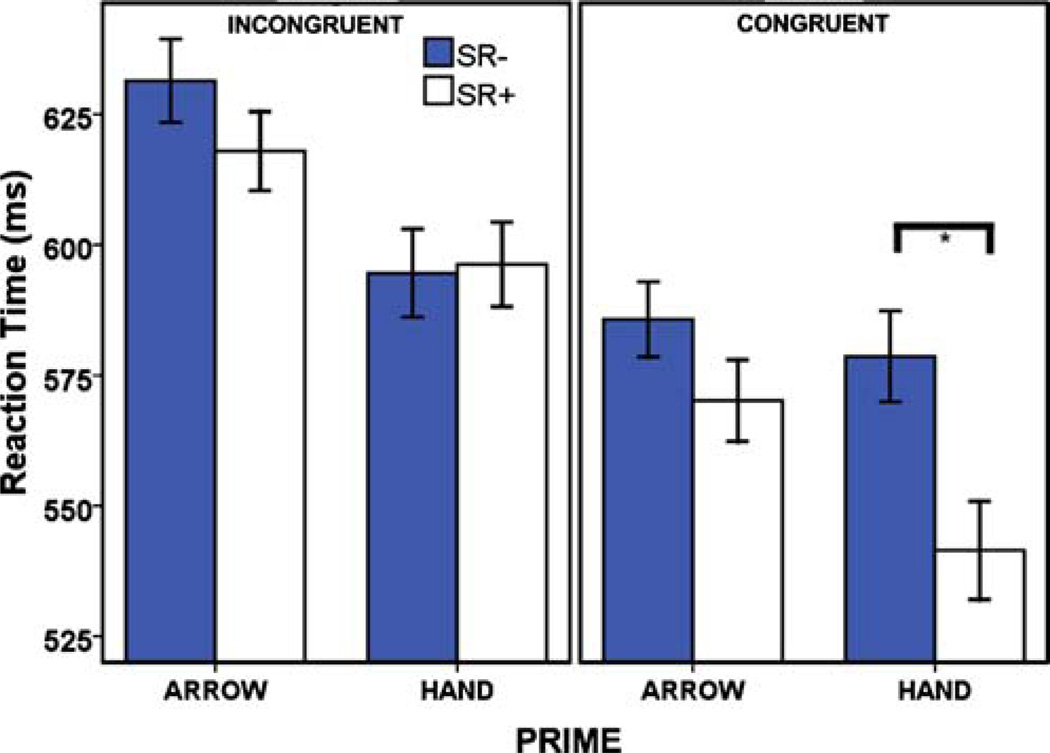
Participants were significantly faster to respond following either a hand or arrow stimuli compared to a neutral stimulus, but group did not alter this effect. The LME analysis confirmed this observation and suggested that RT was also unaffected by drug condition (see Table S1). In both within- and between-group comparisons, there were significant interactions between prime, SRC, and congruency (see Table S1). Post hoc analysis showed this to be the consequence of a significant interaction between congruency and SRC for hand primes only (F(1, 47)=30.18, p< 0.001), resulting from significant increase in RT between SR+and SR- trials only when hand primes were congruent (tCONGRUENT(47)=8.97, p<0.001 vs. tINCONGRUENT(47)= −0.42, NS; Fig. 3).
Fig. 3.
Average reaction time (milliseconds) on the IAT. Shown here are the effects of prime type (arrow vs. hand) upon RT, for each SRC condition and parsed by congruency condition. Errors bars show ±1 standard error
Functional imaging results
A complete list of regions of activity that varied as a function of each of the experimental factors can be found in Table S2. In the interests of relevance and clarity, only data pertaining to the neural substrates of drug condition and group and the impact of these factors upon attentional and intentional processing are highlighted here.
Smokers: nicotine vs. placebo
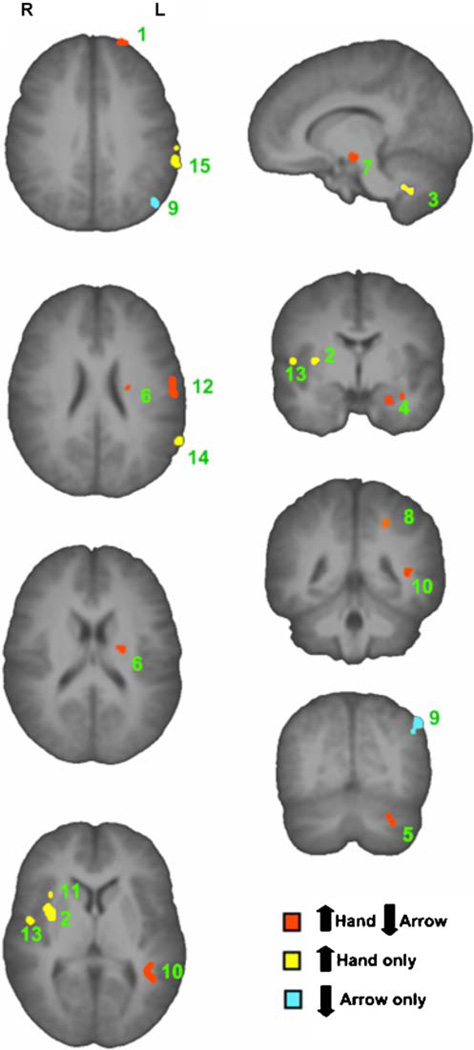
Drug condition and prime interacted significantly in multiple regions (see Fig. 4 and Table S2). In all clusters that exhibited this effect, BOLD signal increased in the nicotine condition in response to hand stimuli and decreased in response to arrow stimuli; controls exhibited no prime effect in any of these regions. There was a significant increase in activity in response to hand stimuli postnicotine in the left inferior parietal lobule and supramarginal gyrus, regions known to mediate intention (Hesse et al. 2006; Rushworth et al. 1997, 2001a, 2003; Toni et al. 2001), and in the right superior temporal gyrus, an area more commonly associated with attention function (Raz and Buhle 2006). In the left postcentral gyrus, a region linked to motor preparation (Eschen et al. 2007), there was a significant increase in response to hand primes postnicotine and a significant decrease following arrow stimuli.
Fig. 4.
Regions demonstrating an interaction between drug condition and prime type in smokers. This figure shows those clusters that exhibited a significant, i.e., F>10.70, p (corrected) <0.05 interaction between drug condition and prime type. Post hoc analysis of nicotine vs. placebo for each prime type (hand or arrow) revealed three interaction patterns, i.e., (1) increase response to hand and decrease response to arrow, postnicotine (shown in red); (2) increase response to hand postnicotine, no effect on arrow (shown in yellow); and (3) decrease response to arrow postnicotine, no effect on hand (shown in blue). The regions of interest are numbered 1–15 in descending order of cluster extent (see Table 2 for region descriptions)
Controls vs. smokers—placebo
There was a significant interaction of group and prime in the right superior temporal gyrus, resulting from higher signal intensity in smokers for arrow stimuli only (see Table S3). This is consistent with the role of this brain region in selective attention (Raz and Buhle 2006). There was also a significant main effect of group (i.e., smokers>controls) in the left superior temporal and inferior frontal gyri and a main effect of prime (i.e., hand>arrow) in the left postcentral and right angular gyri (see Table S2).
Controls vs. smokers—nicotine
Activation in the bilateral inferior frontal, middle frontal, and superior temporal gyri, ACC (BA32), insula, and right postcentral gyrus was significantly greater in smokers. Furthermore, in the left inferior parietal lobule, postcentral gyrus, superior and middle temporal gyri, and bilateral precuneus, smokers in the nicotine condition increased activity following hand (vs. arrow) stimuli, whereas controls showed the opposite effect. This pattern is not only similar to that seen in the comparison of drug conditions in smokers but also includes additional areas linked to information processing. Smokers also showed significant decreases in response to arrow stimuli in bilateral occipital gyri and the left middle temporal gyrus (see Table S2).
Discussion
Nicotine administration had a facilitatory effect on IAT accuracy. Smokers in the placebo condition performed more similarly to controls; indeed, they were only more accurate on SR+trials. Conversely, in the nicotine condition, smokers were more accurate than controls across all task levels. The lack of a significant effect of prime upon accuracy suggests that the nicotinic effect was not input/ output specific. There was, however, an effect of prime upon psychomotor performance (i.e., RT). In accordance with the original investigations of the IAT and recent imaging studies using this task, participants were significantly faster to respond when given an intentional or attentional prime, compared to a neutral stimulus, and RT was the lowest on intention trials, irrespective of the other experimental manipulations.
Since other studies have shown an equivalent effect of nicotine on both of these factors, the contrasting effect of nicotine on accuracy and RT seen here is of note. Indeed, nicotine has been shown to improve performance in both smokers and nonsmokers on a range of attentional tasks (Levin et al. 1998; Mancuso et al. 1999; Parrott and Craig 1992; Petrie and Deary 1989; Powell et al. 2002), with improvements normally seen in measures of both accuracy and psychomotor function (Foulds et al. 1996; Lawrence et al. 2002; Parrott and Craig 1992) or in RT alone (Ernst et al. 2001; Le Houezec et al. 1994). This suggests that nicotine improves either RT only or RT and accuracy concurrently, yet our data indicate a third, nonpredicted outcome, i.e., improved accuracy only.
The cognitive effects of nicotine in smokers may be the consequence of withdrawal amelioration (Bell et al. 1999; Heishman et al. 2002). If so, the lack of a significant difference in RT between nicotine and placebo conditions would be partially explained by an absence of acute withdrawal in the placebo condition during scanning. However, in an investigation with a smoking cohort demographically similar to our study, the effects of nicotine withdrawal were evident as rapidly as 30 min after the last cigarette (Hendricks et al. 2006). Since scanning commenced 120 min after patch placement, subjective feelings of withdrawal should have been notable before and during task performance in the placebo patch condition. The TCQ data support this assertion and suggest greater subjective feelings of nicotine withdrawal in the placebo condition, both before and after scanning. Therefore, the lack of an RT effect was probably not due to the absence of withdrawal in the placebo condition. Yet, the current experimental model does not allow us to fully determine whether the effects seen in the nicotine condition were partially or wholly attributable to a reversal or prevention of withdrawal symptoms. An examination of nicotine’s impact upon performance of the task in nonsmoking controls is necessary to elucidate whether nicotine’s effects on performance were the result of a reversal/prevention of withdrawal or were caused by nicotine per se.
Understanding the difference in accuracy between smokers and controls on the IAT is dependent upon the delineation of cognitive processes underlying the different processing stages. For example, although it has been asserted that the two trials types (i.e., attention and intention) involve different cognitive processes (Verfaellie et al. 1988a, b; Verfaellie and Heilman 1990), it is possible that both prime types involve a common cognitive component. The presentation of the intentional prime may have not only engaged response preparedness processes but also heighted participant vigilance in monitoring the screen for the upcoming target. Since nicotine has been widely shown to impact positively upon attentional processes (Sacco et al. 2004), the effect of nicotine on accuracy may be the consequence of a nicotine-mediated enhancement of attentional processes common to both trial types. The observed difference in accuracy between smokers and controls and the lack of a main effect of prime on accuracy scores supports the notion that intention and attention trials shared processes that were similarly affected by nicotine.
It was hypothesized that nicotine would modulate the function of those regions associated with attentional processing and would have unspecified effects upon intention. Collectively, the within-group analyses of smokers suggest that IAT performance postnicotine was associated with an increase in activity in those regions of the brain shown to support motor intention/preparedness (e.g., Eschen et al. 2007; Hesse et al. 2006; Quian et al. 2006; Rushworth et al. 2001a, b; Toni et al. 2001), but not selective attention. Data suggesting a hemispheric asymmetry in intentional processing (Hesse et al. 2006; Verfaellie et al. 1988a; Verfaellie and Heilman 1990) were also supported by the predominantly left lateralization of areas associated with intention (see Fig. 4).
There was a significant interaction between drug condition and prime type in a number of regions shown to be involved in intention, such as the left postcentral gyrus, supramarginal gyrus, and inferior parietal lobe and the right insula (Hesse et al. 2006; Rushworth et al. 2001a, b; Toni et al. 2001). Post hoc analysis revealed a relative increase in these areas between hand and arrow trials in the nicotine condition and a relative decrease in the placebo condition. The comparison of smokers in the nicotine condition and controls also revealed interactions of group and prime in a reasonably similar network of areas, including the left inferior parietal lobe and postcentral gyrus, the right supramarginal gyrus, and the occipital gyrus bilaterally. With the exception of those clusters in the occipital gyrus, all of these regions were significantly more active following hand primes.
In the occipital gyrus, there was a relative increase in activity in this area following arrow primes only in control participants, compared to smokers postnicotine. The middle occipital gyrus is involved in motor responding (Astafiev et al. 2004; Hesse et al. 2006) and in mediating the attentional demands in tests of visual attention (e.g., Di Russo et al. 2002; Fu et al. 2005; Macaluso et al. 2003; Mangun et al. 1997). Therefore, the differences seen in response to attentional and intentional primes in the middle occipital gyri in controls may not be indicative of increased motor demands on attention trials but rather may be a function of the attentional demands of arrow primes. Moreover, the increased BOLD signal in response to attention, rather than intention trials, may suggest an equivalency of motor demands across trial types.
There were far fewer differences in activity between smokers in the placebo condition and controls. The only significant interaction effect noted in this analysis was a significant interaction between group and prime in the right superior temporal gyrus, which was mediated by a significant increase in smokers in response to arrow primes. The superior temporal gyrus has been shown to support selective attention (Raz and Buhle 2006), and since it is well known that nicotine facilitates attention (Sacco et al. 2004), this difference may be the consequence of the effects of chronic smoking or residual nicotine on attentional processes in abstinent smokers.
Nicotine-mediated alteration of function in intention-related areas suggests that nicotine’s influence on information processing may not be limited to cognitive input processes but may also generalize to information processing output. Despite a pattern of decreased BOLD signal between drug conditions and between smokers postnicotine and controls following arrow primes, overall there was no statistically significant change in these same areas during attention trials. In conjunction with the lack of a nicotinic modulation of function in areas typically associated with selective attention, this suggests that the effect of nicotine upon brain function associated with the IAT was intention specific. In light of the extensively documented nicotinic effects on attention (Newhouse et al. 2004; Swan and Lessov-Schlaggar 2007; Warburton and Rusted 1993) and the lack of a differential effect of nicotine on psychomotor performance on attention vs. intention trials, this is a rather interesting observation, which may encourage a reappraisal of past study interpretations.
The absence of an effect of nicotine on the neural substrates of attention may be the consequence of a ceiling effect of attentional priming in regions associated with selective attention. For example, if areas mediating selective attention (e.g., superior parietal cortex) were maximally activated following arrow primes in the placebo condition and in controls, then there may have been little room for improvement following nicotine. However, even if this was the case, one would still expect to see a dissociation in the regions of activation associated with each of the different prime types, e.g., between superior and inferior parietal cortex for attention and intention, and this was not the case. Alternatively, the response to both prime types may include common attentional components. If this conjecture is true, areas that support selective attention would be active during performance of both intention and attention trials, and our BOLD data would not have been able to differentiate this type of shared activation. This theory of shared activation in regions associated with selective attention would further support the facilitation of accuracy for both trial types. To confirm this assertion, it would be necessary to parametrically manipulate shared aspects of information processing, while holding others constant, something not tested in this study. Nonetheless, given the extensive literature supporting a role for nicotine in attentional processing (Newhouse et al. 2004; Swan and Lessov-Schlaggar 2007; Warburton and Rusted 1993), it seems more plausible that there is some sort of common activity between the trials, as opposed to there being a complete absence of an effect by nicotine upon selective attention.
The suggestion that intention and attention share common neural circuitry is further supported by the “premotor theory of attention”, which postulates that attention is not derived from a dedicated neural control mechanism but rather from activation of the same parietofrontal circuits that determine spatially specific motor behavior (Rizzolatti and Craighero 1998). Evidence from a multitude of sources, including psychological (Craighero et al. 2001, 2004; Deubel and Schneider 1996; Sheliga et al. 1995), neuroimaging (Corbetta et al. 1998; Garg et al. 2007; Nobre et al. 2000), and electrophysiological (Kustov and Robinson 1996; Moore and Armstrong 2003; Moore and Fallah 2001; Ruff et al. 2006) studies support the validity of the central tenet of this theory. Our data also seem to support the notion that there is some common processing of spatial attention and motor preparedness, which is more closely aligned to current theories of the cognitive influence of nicotine.
The notion of shared cognitive processes and activation associated with the intention and attention trials is further supported by the lack of a significant difference between trial types in activation in areas normally associated with selective attention. While others have seen significantly greater activation in the right parietal cortex in the attention trials, activation in this area did not differ from neutral trials, suggesting that its function was not specific to selective attention (Hesse et al. 2006). This seems contradictory to the notion of common attentional processes during attention and intention trials since it is presumed that the neutral trials involve neither attention nor intention. However, there may be a set of general attentional processes common to all task trial types, and while the IAT differentiates between trial types behaviorally, it may lack the sensitivity to dissociate the subtle differences in brain function related to attentional processing between attention, intention, and neutral trials.
There are certain limitations in this study that should be taken into consideration. Firstly, while self-reported race was monitored in order to determine equitable participant recruitment, our experimental groups were not well matched for these criteria. However, available data suggest that ethnicity alone would not impact upon the measures under investigation here. Secondly, given the debate as to whether nicotine leads to a true facilitation of performance or whether observed effects are merely a reversal of nicotine withdrawal, it seems important that future studies examine the performance of the IAT in nonsmokers before and after nicotine. Nonetheless, it is important to note that in a study of dependent smokers, it is impossible to disambiguate the effects of withdrawal prevention/reversal and the effects of nicotine, per se. Repeat testing of this paradigm in smoking participants at different times from last cigarette or the consideration of the impact of nicotine on IAT performance in controls may aid in the elucidation of the effects of nicotine with respect to withdrawal amelioration. Finally, a more parametric manipulation of the component processes that underlie different stages of information processing will facilitate our understanding not only of the nature of the task at hand, behaviorally and functionally, but will also allow for a better understanding of the impact of nicotine upon behavior and brain function.
In conclusion, our data support the notion that nicotine administered to regular smokers has a facilitatory effect upon accuracy, but not reaction time, for both information processing input (i.e., selective attention) and information processing output (i.e., motor intention). With respect to intention, this facilitation may be mediated by an increase in activity in regions of parietal cortex that are known to be active during motor intention. However, our data are inconclusive as to the functional changes leading to nicotinic facilitation of accuracy on attention trials.
Supplementary Material
Table 2.
Results of paired samples t test comparison of the mean signal change between nicotine and placebo conditions for each prime type in smokers
| Region of interest | Arrow vs. hand |
Nicotine vs. placebo |
||||||
|---|---|---|---|---|---|---|---|---|
| Smokers—placebo |
Smokers—nicotine |
Arrow |
Hand |
|||||
| t | p | T | p | t | p | t | p | |
| (1) Left superior frontal gyrus | 3.530 | 0.002 | −3.936 | 0.001 | −3.554 | 0.002 | 2.049 | 0.052 |
| (2) Right claustrum/insula | 2.981 | 0.006 | −3.601 | 0.001 | −1.649 | NS | 3.612 | 0.001 |
| (3) Right cerebellar tonsil | 4.256 | <0.001 | −4.019 | 0.001 | −1.621 | NS | 4.357 | 0.000 |
| (4) Left uncus | 3.305 | 0.003 | −4.311 | <0.001 | −2.832 | 0.009 | 2.493 | 0.020 |
| (5) Left cerebellar tonsil | 2.112 | 0.045 | −6.140 | <0.001 | −1.912 | NS | 3.706 | 0.001 |
| (6) Left putamen | 3.870 | 0.001 | −4.141 | <0.001 | −2.493 | 0.020 | 3.954 | 0.001 |
| (7) Right parahippocampal gyrus | 4.005 | 0.001 | −4.482 | <0.001 | −3.016 | 0.006 | 2.902 | 0.008 |
| (8) Left precentral gyrus | 3.288 | 0.003 | −5.220 | <0.001 | −1.976 | NS | 1.420 | NS |
| (9) Left angular gyrus | 3.216 | 0.003 | −3.863 | 0.001 | −3.167 | 0.004 | 1.949 | NS |
| (10) Left middle temporal gyrus | 4.891 | <0.001 | −3.565 | 0.002 | −2.889 | 0.008 | 3.021 | 0.006 |
| (11) Right precentral gyrus and BA39 | 3.872 | 0.001 | −4.543 | <0.001 | −0.595 | NS | 3.693 | 0.001 |
| (12) Left postcentral gyrus and BA3 | 2.582 | 0.016 | −4.314 | <0.001 | −2.554 | 0.017 | 3.095 | 0.005 |
| (13) Right superior temporal gyrus and BA22 | 2.718 | 0.012 | −3.855 | 0.001 | −1.945 | NS | 2.558 | 0.017 |
| (14) Left supramarginal gyrus | 3.563 | 0.002 | −3.339 | 0.003 | −1.753 | NS | 3.150 | 0.004 |
| (15) Left inferior parietal lobule | 3.420 | 0.002 | −2.956 | 0.007 | −1.567 | NS | 2.361 | 0.027 |
The regions of interest are those regions that exhibited a significant interaction between drug condition and prime type on the change in β. Note: p values correspond to a two-tailed comparison, and results have not been corrected for multiple comparisons. Positive t values indicate arrow> hand and nicotine>placebo, while negative t values indicate activation to hand cue>arrow and placebo>nicotine, respectively
Acknowledgments
We would like to thank Loretta Spurgeon, Kimberley Modo, NIDA nursing staff, and Dr. Betty Jo Salmeron for their contributions in running this study and Dr. Frank Wolkenberg for experiment design assistance. This study was supported by the National Institute on Drug Abuse—Intramural Research Program.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-010-1788-9) contains supplementary material, which is available to authorized users.
In interpreting these observations, it is important to note that the effects of acute nicotine and nicotine withdrawal upon measures of cognition are not necessarily of equal magnitude, and the impact of acute nicotine in smokers may differ considerably from the effects seen in nonsmoking controls.
Given the ratio of congruent to incongruent trials and the number of other task manipulations, there were insufficient incongruent trials to allow for this level to be accurately modeled in the imaging analysis.
Contributor Information
Emma J. Rose, Neuroimaging Research Branch, National Institute on Drug Abuse—Intramural Research Program, 251 Bayview Blvd. Suite 200 (NIDA), Baltimore, MD 21224, USA Neuropsychiatric Genetics Group, Department of Psychiatry, Trinity College Dublin, Dublin, Ireland.
Thomas J. Ross, Neuroimaging Research Branch, National Institute on Drug Abuse—Intramural Research Program, 251 Bayview Blvd. Suite 200 (NIDA), Baltimore, MD 21224, USA
Pradeep K. Kurup, Neuroimaging Research Branch, National Institute on Drug Abuse—Intramural Research Program, 251 Bayview Blvd. Suite 200 (NIDA), Baltimore, MD 21224, USA
Elliot A. Stein, Email: estein@intra.nida.nih.gov, Neuroimaging Research Branch, National Institute on Drug Abuse—Intramural Research Program, 251 Bayview Blvd. Suite 200 (NIDA), Baltimore, MD 21224, USA.
References
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci. 2004;7:542–548. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob Res. 1999;1:45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B Stat Methodol. 1964;26:211–252. [Google Scholar]
- Broadbent DE. Stimulus set and response set: two kinds of selective attention. In: Mostofsky DI, editor. Attention: contemporary theory and analysis. New York: Appleton; 1970. [Google Scholar]
- Broadbent DE. Decsion and stress. London: Academic; 1971. [Google Scholar]
- Clementi F, Fornasari D, Gotti C. Neuronal nicotinic acetylcholine receptors: from structure to therapeutics. Trends Pharmacol Sci. 2000;21:35–37. doi: 10.1016/s0165-6147(99)01423-6. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craighero L, Carta A, Fadiga L. Peripheral oculomotor palsy affects orienting of visuospatial attention. NeuroReport. 2001;12:3283–3286. doi: 10.1097/00001756-200110290-00027. [DOI] [PubMed] [Google Scholar]
- Craighero L, Nascimben M, Fadiga L. Eye position affects orienting of visuospatial attention. Curr Biol. 2004;14:331–333. doi: 10.1016/j.cub.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Davranche K, Audiffren M. Effects of a low dose of transdermal nicotine on information processing. Nicotine Tob Res. 2002;4:275–285. doi: 10.1080/14622200210141635. [DOI] [PubMed] [Google Scholar]
- Della Casa V, Hofer I, Weiner I, Feldon J. The effects of smoking on acoustic prepulse inhibition in healthy men and women. Psychopharmacology (Berl) 1998;137:362–368. doi: 10.1007/s002130050631. [DOI] [PubMed] [Google Scholar]
- Della Casa V, Hofer I, Weiner I, Feldon J. Effects of smoking status and schizotypy on latent inhibition. J Psychopharmacol. 1999;13:45–57. doi: 10.1177/026988119901300106. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vis Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2002;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Domier CP, Monterosso JR, Brody AL, Simon SL, Mendrek A, Olmstead R, Jarvik ME, Cohen MS, London ED. Effects of cigarette smoking and abstinence on stroop task performance. Psychopharmacology. 2007;195:1–9. doi: 10.1007/s00213-007-0869-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, Gonzenbach S, Angrist B, Rotrosen J. Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacology (Berl) 2001;156:266–272. doi: 10.1007/s002130100719. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Eschen A, Freeman J, Dietrich T, Martin M, Ellis J, Martin E, Kliegel M. Motor brain regions are involved in the encoding of delayed intentions: a fMRI study. Int J Psychophysiol. 2007;64:259–268. doi: 10.1016/j.ijpsycho.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl) 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Fu SM, Greenwood PM, Parasuraman R. Brain mechanisms of involuntary visuospatial attention: an event-related potential study. Hum Brain Mapp. 2005;25:378–390. doi: 10.1002/hbm.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Schwartz D, Stevens AA. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;45:2307–2321. doi: 10.1016/j.neuropsychologia.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, Wahren J. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology. 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific stroop interference. Psychopharmacology (Berl) 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence—a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of effects on human performance. [Article] Exp Clin Psychopharmacol. 1994;2:345–395. [Google Scholar]
- Heishman SJ, Henningfield JE, Singleton EG. Tobacco, nicotine, and human cognition. Nicotine Tob Res. 2002;4:3–4. doi: 10.1080/14622200110101955. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Moolchan ET. Tobacco craving questionnaire: reliability and validity of a new multifactorial instrument. Nicotine Tob Res. 2003;5:645–654. doi: 10.1080/1462220031000158681. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Pickworth WB. Reliability and validity of a short form of the Tobacco Craving Questionnaire. Nicotine Tob Res. 2007;10:643–651. doi: 10.1080/14622200801908174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TJ. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;V187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hesse MD, Thiel CM, Stephan KE, Fink GR. The left parietal cortex and motor intention: an event-related functional magnetic resonance imaging study. Neuroscience. 2006;140:1209–1221. doi: 10.1016/j.neuroscience.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA. Smoking withdrawal, nicotine dependence and prepulse inhibition of the acoustic startle reflex. Psycho-pharmacology (Berl) 1999;141:11–15. doi: 10.1007/s002130050800. [DOI] [PubMed] [Google Scholar]
- Kumari V, Checkley SA, Gray JA. Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology (Berl) 1996;128:54–60. doi: 10.1007/s002130050109. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Le Houezec J, Halliday R, Benowitz NL, Callaway E, Naylor H, Herzig K. A low dose of subcutaneous nicotine improves information processing in non-smokers. Psychopharmacology (Berl) 1994;114:628–634. doi: 10.1007/BF02244994. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. Eur J Pharmacol. 2000;393:141–146. doi: 10.1016/s0014-2999(99)00885-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin E, McClernon F, Rezvani A. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Eimer M, Frith CD, Driver J. Preparatory states in crossmodal spatial attention: spatial specificity and possible control mechanisms. Exp Brain Res. 2003;149:62–74. doi: 10.1007/s00221-002-1335-y. [DOI] [PubMed] [Google Scholar]
- Mancuso G, Warburton DM, Melen M, Sherwood N, Tirelli E. Selective effects of nicotine on attentional processes. Psycho-pharmacology (Berl) 1999;146:199–204. doi: 10.1007/s002130051107. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher EM, Heinze HJ. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum Brain Mapp. 1997;5:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychology. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Craig D. Cigarette smoking and nicotine gum (0, 2 and 4 mg): effects upon four visual attention tasks. Neuropsychobiology. 1992;25:34–43. doi: 10.1159/000118807. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol Clin Exp. 1996;11:391–400. [Google Scholar]
- Petrie RX, Deary IJ. Smoking and human information processing. Psychopharmacology (Berl) 1989;99:393–396. doi: 10.1007/BF00445565. [DOI] [PubMed] [Google Scholar]
- Powell J, Tait S, Lessiter J. Cigarette smoking and attention to signals of reward and threat in the Stroop paradigm. Addiction. 2002;97:1163–1170. doi: 10.1046/j.1360-0443.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- Quian QR, Snyder LH, Batista AP, Cui H, Andersen RA. Movement intention is better predicted than attention in the posterior parietal cortex. J Neurosci. 2006;26:3615–3620. doi: 10.1523/JNEUROSCI.3468-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nat Rev Neurosci. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. Spatial attention: mechanisms and theories. In: Sabourin M, Craik F, Roberts AC, editors. Advances in psychological sciences: vol 2. Biological and cognitive aspects. Psychology, East Sussex. 1998. pp. 171–198. [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, Turkington TG, Coleman RE. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Taylor PCJ. TMS in the parietal cortex: updating representations for attention and action. Neuropsychologia. 2006;44:2700–2716. doi: 10.1016/j.neuropsychologia.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Renowden S, Wade DT, Passingham RE. The left parietal cortex and motor attention. Neuropsychologia. 1997;35:1261–1273. doi: 10.1016/s0028-3932(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Ellison A, Walsh V. Complementary localization and lateralization of orienting and motor attention. Nat Neurosci. 2001a;4:656–661. doi: 10.1038/88492. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Krams M, Passingham RE. The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001b;13:698–710. doi: 10.1162/089892901750363244. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Johansen-Berg H, Gobel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. Neuroimage. 2003;20:S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Caulfield D, King L, Goode A. Moving out of the laboratory: does nicotine improve everyday attention? Behav Pharmacol. 2000;11:621–629. doi: 10.1097/00008877-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Spatial attention and eye-movements. Exp Brain Res. 1995;105:261–275. doi: 10.1007/BF00240962. [DOI] [PubMed] [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81:174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Simon JR, Rudell AP. Auditory S-R compatibility: the effect of an irrelevant cue on information processing. J Appl Psychol. 1967;51:300–304. doi: 10.1037/h0020586. [DOI] [PubMed] [Google Scholar]
- Simon JR, Small AM., Jr Processing auditory information: interference from an irrelevant cue. J Appl Psychol. 1969;53:433–435. doi: 10.1037/h0028034. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RF, Henningfield JE, London ED. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003;28:765–772. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, Hawkins M, Rao SM, Bandettini PA, Bloom AS. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Mirza NR, Shoaib M. Nicotine psychoparmacology: addiction, cognition and neuroadaptation. Med Res Rev. 1995;15:47–72. doi: 10.1002/med.2610150105. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci. 2002;22:9024–9034. doi: 10.1523/JNEUROSCI.22-20-09024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Thoenissen D, Zilles K. Movement preparation and motor intention. Neuroimage. 2001;14:S110–S117. doi: 10.1006/nimg.2001.0841. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Heilman KM. Hemispheric asymmetries in attentional control—implications for hand preference in sensorimotor tasks. Brain Cogn. 1990;14:70–80. doi: 10.1016/0278-2626(90)90061-r. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Bowers D, Heilman KM. Hemispheric asymmetries in mediating intention, but not selective attention. Neuropsychologia. 1988a;26:521–531. doi: 10.1016/0028-3932(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Bowers D, Heilman KM. Attentional factors in the occurrence of stimulus-response compatibility effects. Neuropsychologia. 1988b;26:435–444. doi: 10.1016/0028-3932(88)90096-6. [DOI] [PubMed] [Google Scholar]
- Warburton DM, Rusted JM. Cholinergic control of cognitive resources. Neuropsychobiology. 1993;28:43–46. doi: 10.1159/000118998. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: PsychCorp; 2007. [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-Back task: a preliminary study. Psychiatry Res. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.