Abstract
Background
We investigated the changes in the values of carotid intima-media thickness (IMT) and Doppler index measurements in the autosomal dominant polycystic kidney disease (ADPKD), peritoneal dialysis (PD), and hemodialysis (HD) patients.
Material/Methods
Twenty outpatients on HD (mean age 46.1±16.4), 27 outpatients on PD (mean age 45±12.4), and 26 normotensive outpatients with ADPKD (mean age 52.4±16.7) as the case groups and 21 healthy subjects (mean age 48.4±7.2), as the control group, were included. The participants underwent ultrasonography of the common, right, and left carotid arteries for the IMT and Doppler flow measurements.
Results
Overall, compared to the normal group, in the study groups, the IMT and peak systolic velocity (PSV), end-diastolic velocity (EDV), resistive index (RI), and pulsatility index (PI) were significantly higher in common carotid arteries; however, their differences were not meaningful in internal carotid arteries (p<0.05).
Conclusions
Overall, ADPKD, PD, and HD increase the IMT, PSV, EDV, RI, and PI values of CCA; however, their effect considerable less on the study parameters of ICA. There is no considerable difference among the effects of ADPKD, HD, and PD on the study parameters. Of CKD patients during the first diagnostic and follow-up workups, the measurements of carotid IMT and Doppler indices may provide valuable data for improving success of the clinical management.
Keywords: carotid arteries, Doppler ultrasonography, intima-media thickness, autosomal dominant polycystic kidney disease, peritoneal dialysis, hemodialysis
Background
Several radiologic studies are used to evaluate the patient with renal disease. Ultrasonography with Doppler modality is one of the most frequently used technique to assess renal structure and systemic cardiovascular diseases in the diagnosis and management of renal conditions such as chronic kidney disease (CKD) and polycystic renal abnormalities.
CKD has been known as an independent risk factor for cardiovascular disease, including stroke [1,2]. In addition, patients with CKD often have numerous traditional and nontraditional risk factors for the development of cardiovascular disease. Cardiovascular diseases are a major cause of morbidity and mortality in patients with end-stage renal disease (ESRD) [3,4]. Traditional risk factors appear to be associated with much larger absolute increases in risk in the earlier stages of CKD [5]. Hemodialysis (HD) and peritoneal dialysis (PD) are dialysis options for patients with end-stage renal disease (ESRD) in whom preemptive kidney transplantation is not possible [6]. As contributors having a potential to cause cardiovascular disease, nontraditional risk factors, including perhaps hemodialysis procedure itself and the uremic milieu, may be contributory [7].
Polycystic kidney disease includes inherited diseases characterized by cyst formation and enlargement in the kidney, decreasing kidney function irreversible. PKD may have a autosomal dominant or recessive trait. The autosomal dominant PKD (ADPKD) is the most common genetic variant of CKD [8,9], at its final stage, requiring renal replacement commonly [8].
Carotid intima-media thickness (IMT) is known as a reliable marker of atherosclerosis and a predictor of cerebrovascular and cardiovascular events [10–15]. There is ongoing research to assess the value of carotid IMT and carotid pulsatility and resistive indices in patients with chronic kidney disease [16,17]. There are limited available study investigating the value of carotid IMT and Doppler indices in the same clinical settings in the ADPKD, PD, and HD patients [17]. In this study, we investigated the changes in the values of carotid IMT and Doppler index measurements in the ADPKD, PD and HD patients.
Material and Methods
Written informed consent was obtained from each subject, and the study protocol was approved by the Human Ethics Committee of our university. In this case-control setting, 20 outpatients on HD (8 female, 12 male; mean age 46.1±16.4), 27 outpatients on PD (20 female, 7 male; mean age 45±12.4), and 26 normotensive outpatients with ADPKD (11 female, 15 male; mean age 52.4±16.7) as the case groups and 21 healthy subjects (11 female, 10 male; mean age 48.4±7.2), as the control group, were recruited. All the ADPKD patients had family history. Obese patients (body mass index =30), cases with diabetes mellitus, or underlying coronary artery disease and cigarette smokers were excluded. The participants studied underwent ultrasonography of the common, right, and left carotid arteries for the IMT and Doppler flow measurements. One radiologist performed all ultrasound examinations by an ultrasonography device (General Electric Logic 9; USA) equipped with a 10 MHz linear probe as blind to the grouping of subjects.
Assessment of IMT
Ultrasonographic exam was performed during subjects in supine position with a slight extension of the neck. The CCA IMT was evaluated at the wall of artery 2 cm proximal to its bifurcation. The left and right CCA IMT was calculated as the mean value of three individual measurements at different points within the region of interest.
Assessment of carotid Doppler ultrasonography
Ultrasound exams were performed patients in a supine position. In all patients, routine carotid US exams including gray-scale and color and pulsed Doppler ultrasound examinations of the left and right CCAs and ICAs were conducted. All measurements were made by using angle correction. The peak systolic velocity (PSV), end-diastolic velocity (EDV), resistive index (RI), and pulsatility index (PI) were calculated [18].
Statistical analysis
Data were expressed as mean ±SD. The ultrasonographic and Doppler ultrasonographic findings were analyzed with ANOVA with post hoc Tukey test. Significance was determined at the p<0.05 level.
Results
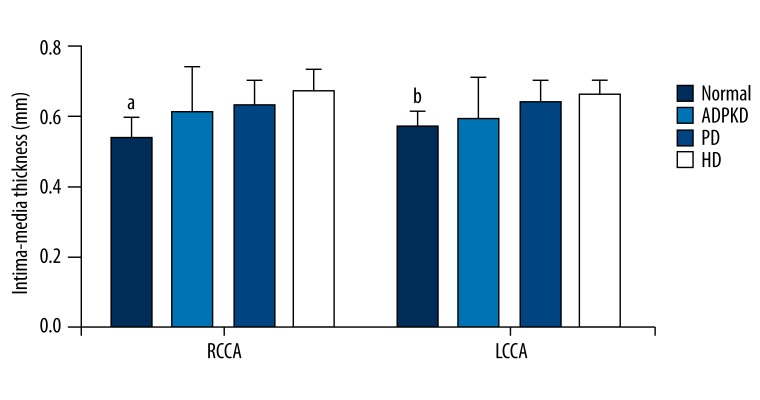
Figure 1 presents the IMTs of right and left CCAs of the normal, ADPKD, PD, and HD groups. Of left and right CCAs, the IMTs in ADPKD, PD, and HD groups were significantly higher than that of the normal group (p<0.05); however, the ADPKD, PD, and HD groups were found similar with regard to the IMTs (p>0.05).
Figure 1.
Intima-media thicknesses of right and left common carotid arteries of study groups. Data were expressed as mean ±SD. RCCA, right common carotid artery; LCCA, left common carotid artery; ADPKD, adult polycystic kidney disease; PD, peritoneal dialysis; HD, hemodialysis. a,bP<0.05 vs. ADPKD, PD, and HD groups.
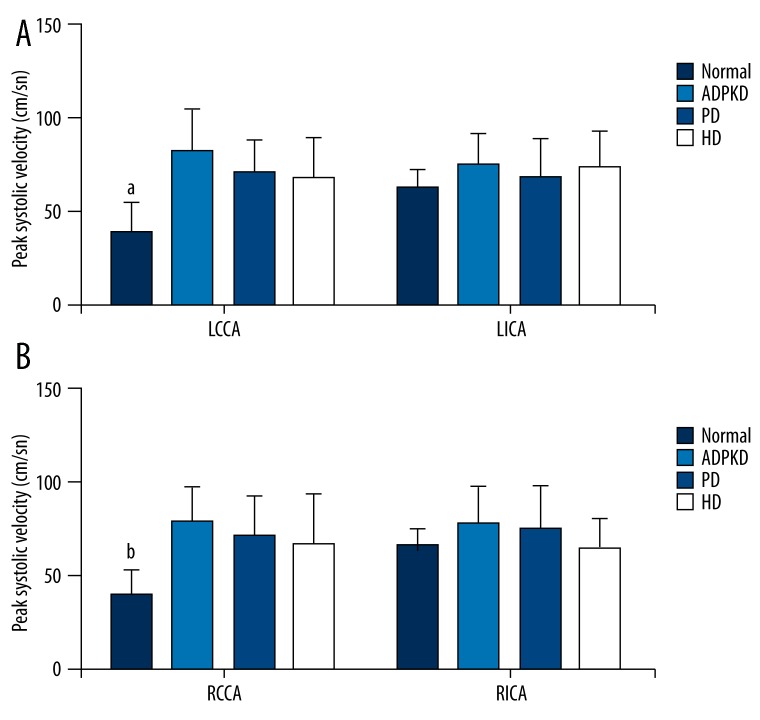
Figure 2 shows the PSVs of left (Figure 2A) and right (Figure 2B) CCAs and ICAs of normal, ADPKD, PD, and HD groups. Of left and right CCAs, the PSVs in ADPKD, PD, and HD groups were significantly higher than that of the normal group (p<0.05); however, the ADPKD, PD, and HD groups were found comparable with regard to the PSVs (p>0.05). The normal, ADPKD, PD, and HD groups were similar with regard to the PSVs of left and right ICAs (p>0.05).
Figure 2.
Peak systolic velocities of left (A) and right (B) common and internal carotid arteries of study groups. Data were expressed as mean ±SD. LCCA, left common carotid artery; LICA, left internal carotid artery; RCCA, Right common carotid artery; RICA, right internal carotid artery; ADPKD, adult polycystic kidney disease; PD, peritoneal dialysis; HD, hemodialysis. a,bP<0.05 vs. ADPKD, PD, and HD groups.
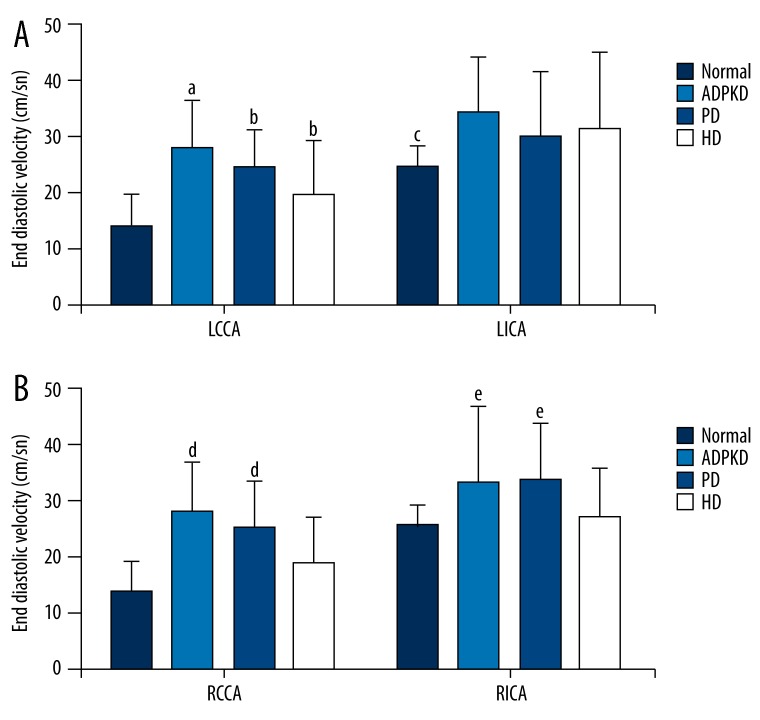
Figure 3 shows EDVs of left (Figure 3A) and right (Figure 3B) CCAs and ICAs of the normal, ADPKD, PD, and HD groups. Of left CCAs, the EDV in ADPKD group was significantly higher than those of the normal, PD, and HD groups (p<0.05); and the EDVs in PD and HD groups were significantly higher than that of the normal group (p<0.05); however, there was no significant difference between the PD and HD groups with regard to the EDV (p>0.05). The EDVs of left ICAs of ADPKD, PD, and HD groups were significantly higher than that of the normal group (p<0.05); however, there was no significant difference among the ADPKD, PD, and HD groups (p>0.05). Of right CCAs and ICAs, the EDV in ADPKD and PD groups were significantly higher than those of the normal and HD groups (p<0.05); however, there was no significant difference among the normal and HD groups (p>0.05).
Figure 3.
End-diastolic velocities of left (A) and right (B) common and internal carotid arteries of study groups. Data were expressed as mean ±SD. LCCA, left common carotid artery; LICA left internal carotid artery; RCCA, Right common carotid artery; RICA, right internal carotid artery; ADPKD, adult polycystic kidney disease; PD, peritoneal dialysis; HD, hemodialysis. aP<0.05 vs. normal, PD, and HD groups. bP<0.05 vs. normal group. cP<0.05 vs. ADPKD, PD, and HD groups. d,eP<0.05 vs. normal and HD groups.
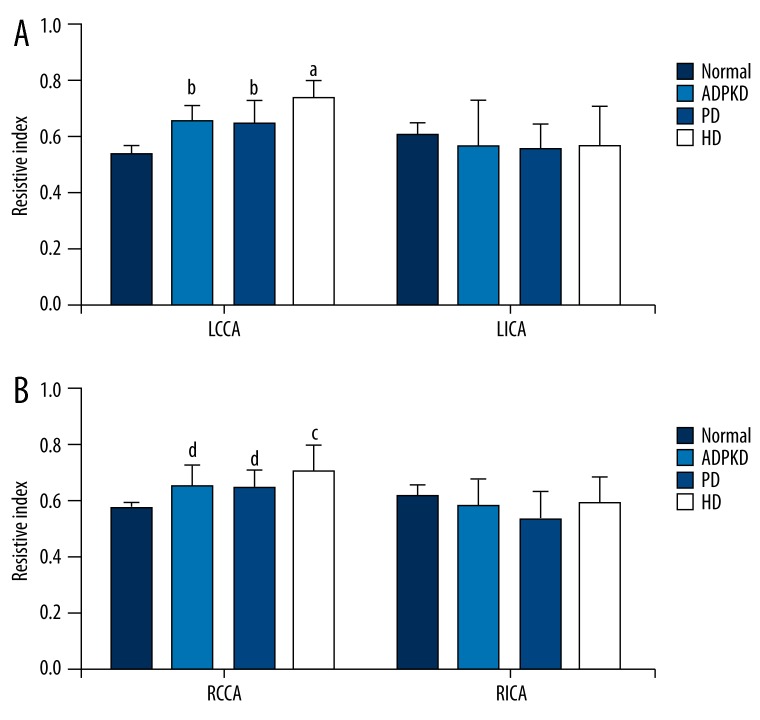
Figure 4 shows RIs of left (Figure 4A) and right (Figure 4B) CCAs and ICAs of the normal, ADPKD, PD, and HD groups. Of left and right CCAs, the RIs in the HD group were significantly higher than those of the other groups (p<0.05); the RIs of the ADPKD and PD groups were significantly higher than that of the normal group (p<0.05); however, the ADPKD and PD groups were similar with regard to the RIs (p>0.05). The normal, ADPKD, PD, and HD groups were similar with regard to the RIs of left and right ICAs (p>0.05).
Figure 4.
Resistive indices of left (A) and right (B) common and internal carotid arteries of study groups. Data were expressed as mean ±SD. LCCA, left common carotid artery; LICA left internal carotid artery; RCCA, Right common carotid artery; RICA, right internal carotid artery; ADPKD, adult polycystic kidney disease; PD, peritoneal dialysis; HD, hemodialysis. a,cP<0.05 vs. normal, ADPKD, and PD groups. b,dP<0.05 vs. normal group.
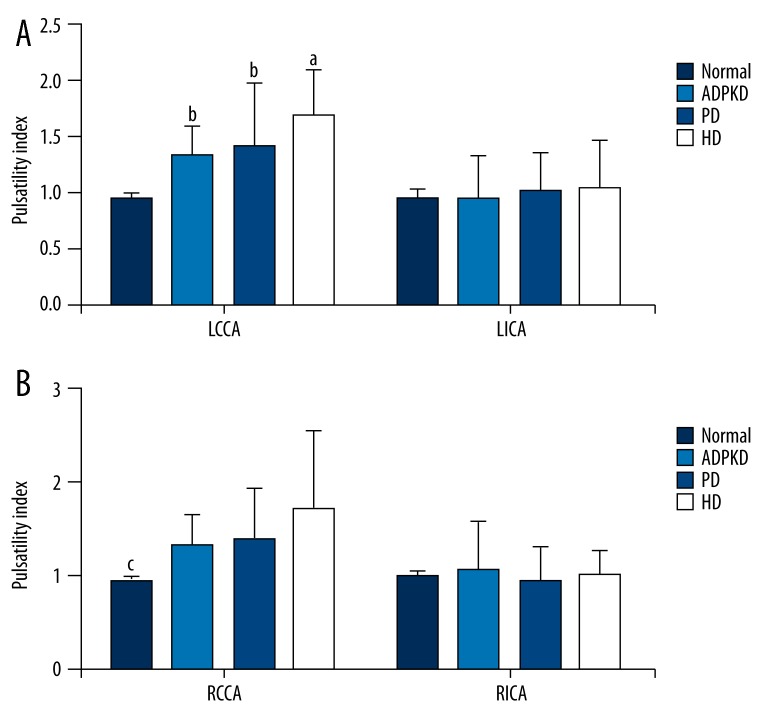
Figure 5 shows pulsatility indices of left (Figure 5A) and right (Figure 5B) CCAs and ICAs of the normal, ADPKD, PD, and HD groups. Of left CCAs, the PI in the HD group was significantly higher than those of the normal, ADPKD, and PD groups (p<0.05); and the PIs in the ADPKD and PD groups were significantly higher than that of the normal group (p<0.05); however, the ADPKD and PD groups were similar with regard to the PIs (p>0.05). Of right CCAs, the PIs in the ADPKD, PD, and HD groups were significantly higher than that of the normal group (p<0.05); however, the ADPKD, PD, and HD groups were similar with regard to the PIs (p>0.5). The study groups were similar with regard to the PIs of left and right ICAs (p>0.05).
Figure 5.
Pulsatility indices of left (A) and right (B) common and internal carotid arteries of study groups. Data were expressed as mean ±SD. LCCA, left common carotid artery; LICA left internal carotid artery; RCCA, Right common carotid artery; RICA, right internal carotid artery; ADPKD, adult polycystic kidney disease; PD, peritoneal dialysis; HD, hemodialysis. aP<0.05 vs. normal, ADPKD, and PD groups. bP<0.05 vs. normal group. cP<0.05 vs. ADPKD, PD, and HD groups.
Discussion
In this study, we assessed the changes in the carotid IMT and Doppler index measurements in the ADPKD, PD and HD patients as compared to the healthy controls. Of left and right CCAs, the IMT and PSV values were found increased due to ADPKD, PD and HD; however, the IMTs and PSVs of ADPKD, PD and HD were found comparable. Of left and right ICAs, the PSVs of ICAs were found similar. Of left CCAs, the EDV in ADPKD group was found more compared to the other groups; and the EDVs in PD and HD groups were found more compared to the normal group; however, the PD and HD groups were found comparable with regard to the EDV. The EDVs of left ICAs of CKD groups were found increased compared to the normal group; however, the EDVs of CKD groups were found similar. Of right CCAs and ICAs, the EDV in ADPKD and PD groups were found increased compared to the normal and HD groups; however, the normal and HD groups were found comparable with regard the EDV. Of left and right CCAs, the RIs in the HD group were found higher compared to the normal, ADPKD, and PD groups; the RIs of the ADPKD and PD groups were found higher compared to the normal group; however, the ADPKD and PD groups were similar with regard to the RIs. All the study groups were similar with regard to the RIs of left and right ICAs. Of left CCAs, the PI in the HD group was found higher compared to the other groups; and the PIs in the ADPKD and PD groups were found higher compared to the normal group; however, the ADPKD and PD groups were similar with regard to the PIs. Of right CCAs, the PIs in the CKD groups were found higher compared to the normal group; however, the CKD groups were similar with regard to the PIs. The study groups were comparable with regard to the PIs of left and right ICAs.
In ESRD patients, cardiovascular disease is the major cause of death, and the incidence of atherosclerosis-related complications is significantly higher if dialysis required. The serious cause of morbidity and mortality is atherosclerotic vascular damage in ESRD patients [17,19]. ESRD may induce endothelial dysfunction that is an important contributor of the development of atherosclerosis [20] and may result in an increase in the incidence of important atherosclerotic events such as acute myocardial infarction and stroke [21] Identification of risk factors for PD and HD patients is important in order to establish preventive and interventional strategies. In general, it is suitable to assume that the traditional cardiovascular risk factors for the general population are also applicable to the HD patients, and that HD patients suffer more cardiovascular diseases because these risk factors are present more commonly [7].
In patients having no clinical complaints, atherosclerotic changes can be detected as three main manifestations on vessel walls: increasing IMT, plaque formation and alterations in arterial hemodynamics; however, the most applicable of them is the measurement of IMT due to its simplicity and reproducibility in the clinical practice [22]. Tanaka et al. [16] to assess whether carotid IMT is associated with CKD, and whether renal dysfunction is associated with calcified plaque in carotid arteries. Their study included 1003 patients aged ≥50 years who underwent carotid ultrasonography. In their study, kidney function was evaluated based on the estimated glomerular filtration rate (eGFR) and the presence of proteinuria. They excluded ESRD patients. They assessed carotid IMTs, and examined the characteristics of the maximal plaques by carotid ultrasonography. They found that eGFR was significantly correlated with carotid IMT values. They noted that reduced eGFR, proteinuria, age, male sex, cardiovascular disease, hypertension, diabetes, and smoking were independently associated with carotid IMT findings. They concluded that there was an association between the degrees of carotid atherosclerosis and CKD. In our study, we evaluated carotid atherosclerosis in patients with CKD requiring hemodialysis and continuous ambulatory peritoneal dialysis.
Karaman et al. [17] investigated that the relationship between carotid IMT and PI or RI values in hemodialysis patients. They found that the carotid IMT of hemodialysis patients were increased but PI and RI decreased in hemodialysis patients; however, they determined a weak but negative correlation between only right IMT and right PI. They concluded that PI–RI values lower than expected in hemodialysis patients. They suggested that peripheral vasodilatation related to anemia might be the cause of those lower values. According to our knowledge there is no other study investigating carotid IMT and Doppler indices in the PD and HD patients.
Cheung et al. [7] have conducted a study determining the prevalence and severity of atherosclerotic cardiovascular diseases in a representative chronic hemodialysis population. In their study, they have assessed the relationship between several traditional cardiovascular disease risk factors and the presence or history of cardiovascular events in 936 hemodialysis patients. They have concluded that there was a high prevalence of atherosclerotic cardiovascular diseases in the chronic hemodialysis patients and that several of the traditional coronary risk factors in the general population appeared to be applicable to the hemodialysis population.
Helal et al. [4] have evaluated the effects of HD and PD on the cardiovascular risk. They found the marked tendency of patients with impaired renal function for developing cardiovascular diseases, irrespectively of the type of dialysis. They suggested that the inclusion of uremia-related risk factors in the panel for evaluation of cardiovascular risk in dialysis patients was required.
Nakayama et al. [23] have evaluated whether the carotid artery calcification at the initiation of hemodialysis was a risk factor for cardiovascular events in 133 patients with end-stage renal disease. They have confirmed the carotid artery calcification at the initiation of hemodialysis as an independent risk factor for subsequent cardiovascular events in patients with ESRD.
Turkmen et al. [24] have investigated the carotid IMT and coronary flow velocity reserve in ADPKD patients. Normotensive patients with ADPKD with well-preserved renal function have significantly increased carotid IMT and significantly decreased coronary flow velocity reserve compared with healthy participants. They have suggested that atherosclerosis starts at an early stage in the course of their disease in ADPKD patients. Our findings related to the IMT in ADPKD patients are in accordance with those findings.
Conclusions
Overall, according to our findings, ADPKD, PD, and HD increase the IMT, PSV, EDV, RI, and PI values of CCA; however, they cause no meaningful increase in the IMT, PSV, EDV, RI, and PI values of ICA. There is no considerable difference among the effects of ADPKD, HD, and PD on the IMT, PSV, EDV, RI, and PI values. Of CKD patients during the first diagnostic workup and at the regular follow-ups, the measurement of carotid IMT and Doppler indices may provide valuable data helpful for determining success of the clinical management. Future studies including carotid artery imaging with color Doppler ultrasound in a higher number of ADPKD, PD and HD patients are necessary to determine the impact of risk factors, especially related to the type of dialysis, on atherosclerotic cardiovascular disease and to reduce these risk factor as a strategy for preventing cardiovascular disease in ADPKD, PD and HD patients.
Footnotes
Source of support: Departmental sources
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–95. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.Yilmaz FM, Akay H, Duranay M, et al. Carotid atherosclerosis and cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Clin Biochem. 2007;40(18):1361–66. doi: 10.1016/j.clinbiochem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Helal I, Smaoui W, Hamida FB, et al. Cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Saudi J Kidney Dis Transpl. 2010;21(1):59–62. [PubMed] [Google Scholar]
- 5.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293(14):1737–45. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 6.Sinnakirouchenan R, Holley JL. Peritoneal dialysis versus hemodialysis: risks, benefits, and access issues. Adv Chronic Kidney Dis. 2011;18(6):428–32. doi: 10.1053/j.ackd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58(1):353–62. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 8.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18(2):99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barua M, Cil O, Paterson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol. 2009;20(8):1833–38. doi: 10.1681/ASN.2009020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desbien AM, Chonchol M, Gnahn H, Sander D. Kidney function and progression of carotid intima-media thickness in a community study. Am J Kidney Dis. 2008;51(4):584–93. doi: 10.1053/j.ajkd.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Zhao F, Yang Y, et al. Association between carotid artery intima-media thickness and early-stage CKD in a Chinese population. Am J Kidney Dis. 2007;49(6):786–92. doi: 10.1053/j.ajkd.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Hueb JC, Bazan R, Pereira Braga G, et al. Carotid artery atherosclerotic profile as a predictor of the aorta atherosclerotic profile in patients with cerebrovascular events. Cerebrovasc Dis. 2013;36(1):26–32. doi: 10.1159/000351150. [DOI] [PubMed] [Google Scholar]
- 13.Mieczkowska J, Mosiewicz J, Sak J, et al. Effects of cigarette smoking, metabolic syndrome and dehydroepiandrosterone deficiency on intima-media thickness and endothelial function in hypertensive postmenopausal women. Med Sci Monit. 2012;18(4):CR225–34. doi: 10.12659/MSM.882622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzierwa K, Pieniazek P, Musialek P, et al. Treatment strategies in severe symptomatic carotid and coronary artery disease. Med Sci Monit. 2011;17(8):RA191–97. doi: 10.12659/MSM.881896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musialek P, Pieniazek P, Tracz W, et al. Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions. Med Sci Monit. 2012;18(2):MT7–18. doi: 10.12659/MSM.882452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Abe Y, Furukado S, et al. Chronic kidney disease and carotid atherosclerosis. J Stroke Cerebrovasc Dis. 2012;21(1):47–51. doi: 10.1016/j.jstrokecerebrovasdis.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Karaman O, Albayrak R, Colbay M, et al. Carotid hemodynamic parameters in hemodialysis patients. Int Urol Nephrol. 2008;40(3):779–84. doi: 10.1007/s11255-007-9314-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee VS, Hertzberg BS, Workman MJ, et al. Variability of Doppler US measurements along the common carotid artery: effects on estimates of internal carotid arterial stenosis in patients with angiographically proved disease. Radiology. 2000;214(2):387–92. doi: 10.1148/radiology.214.2.r00fe25387. [DOI] [PubMed] [Google Scholar]
- 19.Pascazio L, Bianco F, Giorgini A, et al. Echo color Doppler imaging of carotid vessels in hemodialysis patients: evidence of high levels of atherosclerotic lesions. Am J Kidney Dis. 1996;28(5):713–20. doi: 10.1016/s0272-6386(96)90253-x. [DOI] [PubMed] [Google Scholar]
- 20.Thambyrajah J, Landray MJ, McGlynn FJ, et al. Abnormalities of endothelial function in patients with predialysis renal failure. Heart. 2000;83(2):205–9. doi: 10.1136/heart.83.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iseki K, Fukiyama K. Long-term prognosis and incidence of acute myocardial infarction in patients on chronic hemodialysis. The Okinawa Dialysis Study Group. Am J Kidney Dis. 2000;36(4):820–25. doi: 10.1053/ajkd.2000.17676. [DOI] [PubMed] [Google Scholar]
- 22.Jurasic MJ, Lovrencic-Huzjan A, Bedekovic MR, Demarin V. How to monitor vascular aging with an ultrasound. J Neurol Sci. 2007;257(1–2):139–42. doi: 10.1016/j.jns.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama M, Ura Y, Nagata M, et al. Carotid artery calcification at the initiation of hemodialysis is a risk factor for cardiovascular events in patients with end-stage renal disease: a cohort study. BMC Nephrol. 2011;12:56. doi: 10.1186/1471-2369-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turkmen K, Oflaz H, Uslu B, et al. Coronary flow velocity reserve and carotid intima media thickness in patients with autosomal dominant polycystic kidney disease: from impaired tubules to impaired carotid and coronary arteries. Clin J Am Soc Nephrol. 2008;3(4):986–91. doi: 10.2215/CJN.02330607. [DOI] [PMC free article] [PubMed] [Google Scholar]