Abstract
Objective
To compare the relative hazard of muscle toxicity, renal dysfunction, and hepatic dysfunction associated with the drug interaction between statins and concomitant medications that inhibit the CYP3A4 isoenzyme.
Background
Although statins provide important clinical benefits related to mitigating the risk of cardiovascular events, this class of medications also has the potential for severe adverse reactions. The risk for adverse events may be potentiated by concomitant use of medications that interfere with statin metabolism.
Methods
Data from The Health Improvement Network (THIN) from 1990 to 2008 were used to conduct a retrospective cohort study. Cohorts were created to evaluate each outcome (muscle toxicity, renal dysfunction, and hepatic dysfunction) independently. Each cohort included new statin initiators and compared the relative hazard of the outcome. The interaction ratio (I*R) was the primary contrast of interest. The I*R represents the relative effect of each statin type (statin 3A4 substrate vs. statin non-3A4 substrate) with a CYP3A4 inhibitor, independent of the effect of the statin type without a CYP3A4 inhibitor. We adjusted for confounding variables using the multinomial propensity score.
Results
The median follow-up time per cohort was 1.5 years. There were 7889 muscle toxicity events among 362 809 patients and 792 665 person-years. The adjusted muscle toxicity I*R was 1.22 (95% confidence interval [CI] = 0.90–1.66). There were 1449 renal dysfunction events among 272,099 patients and 574 584 person-years. The adjusted renal dysfunction I*R was 0.91 (95%CI = 0.58–1.44). There were 1434 hepatic dysfunction events among 367 612 patients and 815 945 person-years. The adjusted hepatic dysfunction I*R was 0.78 (95%CI = 0.45–1.31).
Conclusions
Overall, this study found no difference in the relative hazard of muscle toxicity, renal dysfunction, or hepatic dysfunction for patients prescribed a statin 3A4 substrate versus a statin non-3A4 substrate with CYP3A4 inhibitor concomitancy.
INTRODUCTION
Statins, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, are effective in the treatment of dyslipidemia and have been shown to reduce the risk of major coronary outcomes and all-cause mortality.1,2 Although statins are well tolerated by the vast majority of patients, their use can lead to infrequent muscle, renal, and hepatic adverse events.3–9 Statin-associated muscle and renal toxicity occur on a continuum from minor myalgias and proteinuria to severe myositis, renal failure, and fatal rhabdomyolysis.10–12 Statin-associated hepatic toxicity is characterized by transaminitis and rarely, serious hepatic dysfunction or hepatic failure.13,14 These adverse events can occur with all marketed statins.9,15–17 Although the incidence of serious statin adverse events is low, muscle toxicity is a leading cause of statin discontinuation.18,19 It has been shown that statin-related adverse events occur in a potency-dependent manner and therefore may be exacerbated by pharmacokinetic (PK) statin–drug interactions that increase statin systemic exposure.8,15,20,17,21–25
Of particular importance is the drug interaction between statins and drugs that inhibit the CYP3A4 metabolic pathway. The CYP3A4 isoenzyme metabolizes more than 50% of marketed pharmaceuticals.26 Because of unique physiochemical properties, not all statins have the same drug interaction potential. Statins that undergo Phase I metabolism by the CYP3A4 isoenzyme are referred to as statin 3A4 substrates (atorvastatin and simvastatin). Statins that do not use the CYP3A4 isoenzyme metabolic pathway are referred to as statin non-3A4 substrates (pravastatin, fluvastatin, and rosuvastatin). CYP3A4 inhibitors prevent CYP3A4 isoenzymes from metabolizing other drugs (e.g., statin 3A4 substrates). As a result of this interaction, it is recognized that plasma levels of statins 3A4 substrates may increase with concomitant administration of CYP3A4 inhibitors.27 This may in turn increase the risk of significant statin toxicity. Because of the documented increased systemic statin exposure (demonstrated through PK studies and increased potential for adverse events), statin 3A4 substrate product labels warn against concomitant administration of these statins with CYP3A4 inhibitors. Despite these warnings, statin 3A4 substrates and CYP3A4 inhibitors are frequently coprescribed.28 Commonly prescribed CYP3A4 inhibitors include calcium channel blockers, histamine H2 receptor antagonists, antibiotics, antifungals, antidepressants, antiretrovirals, and immunosuppressants.29
Studies quantifying the relative hazard of statin adverse events for different statins (with different metabolism) with CYP3A4 inhibitor concomitancy are limited. The clinical importance of this drug interaction was described in an analysis of spontaneous adverse event reports associated with statin use.22 Using the US Food and Drug Administration Adverse Event Reporting System database, the adverse event reporting rate and ratio (AERR) of rhabdomyolysis reports were compared for simvastatin (a statin 3A4 substrate) and pravastatin (a statin non-3A4 substrate) with versus without a CYP3A4 inhibitor. This study showed a sixfold increase in the AERR for simvastatin (with vs. without a CYP3A4 inhibitor) and no increase for pravastatin (with vs. without a CYP3A4 inhibitor).22 Given substantial limitations of spontaneous report analyses (e.g., nonanalytic studies, lacking internal validity, estimated denominator, inadequate sample size, and potential reporting bias), further research was warranted to evaluate these findings in a well-designed study with internal validity, conducted in one database, and powered to detect a difference of adverse events with a multinomial (i.e., drug–drug interaction) exposure.
The purpose of the current investigation was to detect adverse clinical outcomes associated with statins and CYP3A4 inhibitors. Our specific aim was to measure the relative hazard of muscle toxicity, kidney dysfunction, and hepatic dysfunction associated with statin 3A4 substrates compared with statin non-3A4 substrates with and without CYP3A4 inhibitor concomitancy. We hypothesized an increased relative hazard for statin 3A4 substrates compared with statin non-3A4 substrates with CYP3A4 inhibitor concomitancy.
METHODS
Design and study population
We conducted a retrospective cohort study using The Health Improvement Network (THIN) from 1990 through October 2008. THIN is an anonymized electronic medical record database of primary care medical records from the UK.30 As of October 2008, THIN consisted of contributions from 415 general practices and data from more than three million actively registered patients. This study included only patients currently or once permanently registered with a general practice.31 Some of the general practices contributing data to THIN also contribute data to the General Practice Research Database (GPRD). Lewis et al. showed exposure-outcome estimates produced from THIN data, but collected outside of the GPRD contributing practices, appeared as valid as the data collected as part of the GPRD.32
Inclusion and exclusion criteria
We assembled statin-naïve cohorts with no history of the outcome event (renal dysfunction, hepatic dysfunction, or muscle injury). New statin initiators were eligible for cohort entry if they were at least 18 years old at first statin initiation and registered with a general practice for 12 consecutive months prior to the first statin drug prescription (the baseline period). The rationale for requiring a 12-month baseline period prior to statin initiation was to collect baseline medical, therapy, outcome, and confounder data.
Cerivastatin initiators were excluded given the associated idiosyncratic increased risk for serious adverse events.8,15 For the renal dysfunction cohort, we also excluded patients with a serum creatinine (sCr) above the upper limit of normal (ULN) during the baseline period. For the hepatic cohort, we excluded patients with a transaminase level (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) greater than three times the ULN during the baseline period.
We excluded patients with an organ transplant prior to statin initiation and patients with relevant chronic medical conditions. The excluded chronic medical conditions were history of dermatomyositis (for the muscle toxicity cohort), genetic kidney disease and chronic nephritis (for the renal dysfunction cohort), and a history of alcoholism and viral hepatitis (for the hepatic dysfunction cohort). We also excluded patients with these chronic medical conditions if they were identified during follow-up because of a concern that these conditions may have been present prior to the date of recording in the medical record. In a prespecified secondary analysis, we instead censored follow-up at documentation of these specific chronic medical conditions, rather than excluding the entire patient record.
Definition of exposure
The cohort included subjects exposed to statins with and without a concomitant CYP3A4 inhibitor. We categorized statin exposure by the metabolic properties of each statin. Statin 3A4 substrates, metabolized by the CYP3A4 isoenzyme, included atorvastatin and simvastatin. Statin non-3A4 substrates, not metabolized by the CYP3A4 isoenzyme, included fluvastatin, pravastatin, and rosuvastatin. We identified CYP3A4 inhibitors from the University of Indiana’s cytochrome P450 table.29 We included concomitant exposure to the following CYP3A4 inhibitors: clarithromycin,33 erythromycin,34 telithromycin, norfloxacin, diltiazem,25 verapamil,34 mibefradil35, amiodarone, ketoconazole,36 itraconazole,37 voriconazole, fluconazole36, nefazodone,38 fluvoxamine,39 cyclosporine,40 cimetidine, ritonavir, saquinavir, nelfinavir, indinavir, lopinavir, imatinib, and aprepitant.
To evaluate the potential drug interaction by statin metabolism, we classified the following four exposure categories: (i) statin 3A4 substrates with a concomitant CYP3A4, (ii) statin 3A4 substrates without a concomitant CYP3A4 inhibitor, (iii) statin non-3A4 substrates with a concomitant CYP3A4 inhibitor, and (iv) statin non-3A4 substrates without a concomitant CYP3A4 inhibitor. Statin exposure with and without a concomitant CYP3A4 inhibitor was evaluated in a time-varying manner. That is, subjects could contribute person-time to both the statin with a concomitant CYP3A4 inhibitor category and the statin without a concomitant CYP3A4 inhibitor category.
Statin potency was evaluated as a categorical time-varying covariate. Statin potency categorization was based on percentage low-density lipoprotein cholesterol (LDL-C) reduction in that dose range.41 In the primary analyses, we did not account for different strengths of CYP3A4 metabolic inhibition for different CYP3A4 inhibitors. In planned secondary analyses, we stratified by the strength of CYP3A4 inhibition. A strong CYP3A4 inhibitor was defined as one that causes greater than a fivefold increase in plasma area under the curve (AUC) values or more than 80% decrease in clearance.29 A moderate inhibitor was defined as one that causes a greater than twofold increase in the plasma AUC values or 50%–80% decrease in clearance.29
Follow-up and censoring
Follow-up was measured in person-years on a statin, either with or without a concomitant CYP3A4 inhibitor beginning after the first day of the first statin drug prescription and continuing with subsequent statin prescriptions. We excluded outcomes occurring on the first day of statin exposure because of pharmacological data suggesting that a single day of statin exposure is not sufficient to cause an adverse event. Follow-up was censored at the first occurrence of (i) the end of the statin days supplied, (ii) prescription of a statin other than the one that triggered cohort entry, (iii) the outcome in question, or (iv) the end of the study (October 2008).
Outcome definitions
Outcome definitions were derived from recently published research on statin-related adverse events.3–7,42, 43 Each outcome was analyzed independently. We utilized medical diagnoses or laboratory evidence to identify incident outcomes. Medical diagnoses are recorded in THIN using READ codes (analogous to International Classification of Diseases, 9th Edition/Revision codes). The lists of specific READ codes are available from the corresponding author.
Muscle toxicity was defined by a READ code for muscle symptoms (e.g., myalgia, myopathy, myositis, and muscle pain) or a creatine kinase (CK) elevation greater than five times the ULN.
Renal dysfunction was defined by a READ code for acute kidney injury, chronic kidney disease, end-stage renal disease, dialysis, or a doubling of sCr (elevated to at least above the sCr ULN) over the baseline sCr or a single sCr value greater than twice the ULN. The baseline sCr measurement was the lowest sCr value occurring within 365 days before the elevated sCr measurement. A secondary analysis excluded patients with a READ code for chronic kidney disease.
Hepatic dysfunction was defined as the first READ code for hepatic failure, toxic liver disease, acute liver necrosis, acute hepatitis, jaundice, or an ALT/AST measurement greater than five times the ULN. We utilized the 5× ULN ALT/AST outcome threshold, consistent with the Drug-Induced Liver Injury Network criteria.44 In addition, we conducted a secondary analysis of severe transaminitis (using the ALT/AST threshold of 10× ULN).
Outcomes identified by laboratory evidence were considered confirmed. Records from patients with outcomes identified by READ codes but with no laboratory evidence were reviewed for additional supporting evidence. We searched physician comments associated with each READ code event. READ-code-based events—where additional physician comments supported the suspected event—were also considered confirmed. We conducted secondary analyses using confirmed outcomes only.
Outcome timing
To be classified as an outcome, the READ code or laboratory elevation must have occurred within 30 days following the end of follow-up time, consistent with the work of Graham and colleagues.8 The 30-day period following the end of statin exposure (with no subsequent statin exposure) accounts for imperfect patient adherence and delayed outcome recording. Outcomes occurring during follow-up time were attributed to the current exposure category. Outcomes occurring within 30 days following included follow-up time were attributed to the prior exposure category. Outcomes occurring more than 30 days following included follow-up time were not included in the analysis, and patient follow-up was censored.
Confounding variables
We identified potential confounding variables associated with each outcome from previous research; these variables are listed in Table 1.17,19,21,45 Patient demographics and medical history were collected during or prior to the baseline period prior to statin initiation. To depict each patient’s current health status, physician care, concomitant therapies, laboratory data, patient surveillance, and pharmacotherapy confounders were collected only during the baseline period.
Table 1.
Subject characteristics (at or prior to the first statin)
| Baseline characteristics | Muscle cohort
|
Renal cohort
|
Hepatic cohort
|
|||
|---|---|---|---|---|---|---|
| Statin 3A4 substrate | Statin non-3A4 substrate | Statin 3A4 substrate | Statin non-3A4 substrate | Statin 3A4 substrate | Statin non-3A4 substrate | |
| Statin initiators, n | 325 460 | 37 349 | 243 707 | 28 392 | 329 668 | 37 944 |
| Age (years), M | 63 | 64 | 62 | 62 | 64 | 63 |
| <54 | 22% | 22% | 26% | 27% | 21% | 22% |
| 55–64 | 29% | 30% | 32% | 32% | 29% | 30% |
| 65–74 | 30% | 32% | 28% | 29% | 30% | 32% |
| >75 | 20% | 17% | 14% | 13% | 20% | 17% |
| Male | 54% | 54% | 56% | 56% | 53% | 53% |
| BMI, M | 28 | 28 | 28 | 28 | 28 | 28 |
| Alcoholism | 1.6% | 1.3% | 1.9% | 1.5% | Excluded | Excluded |
| Current smoker | 11% | 6% | 12% | 6% | 11% | 6% |
| Medical diagnoses (any time prior to statin initiation) | ||||||
| Chronic heart failure | 4% | 5% | 2% | 3% | 4% | 5% |
| Previous myocardial infarction | 28% | 37% | 26% | 35% | 28% | 37% |
| Previous stroke | 4% | 5% | 4% | 4% | 4% | 5% |
| Diabetes | 21% | 19% | 19% | 16% | 21% | 19% |
| Hypertension | 52% | 49% | 47% | 45% | 52% | 49% |
| Hypothyroidism | 4% | 4% | 4% | 3% | 5% | 4% |
| Acute kidney disease | 0.5% | 0.4% | Excluded | Excluded | 0.5% | 0.4% |
| Chronic kidney disease | 3.4% | 1.2% | Excluded | Excluded | 3.4% | 1.2% |
| Acute liver disease | 0.4% | 0.3% | 0.3% | 0.3% | Excluded | Excluded |
| Chronic liver disease | 0.3% | 0.2% | 0.3% | 0.3% | Excluded | Excluded |
| Average surveillance rate in the 12 months prior to statin initiation | ||||||
| Office visits | 2.0 | 1.7 | 1.8 | 1.6 | 2.0 | 1.7 |
| Serum creatinine | 1.0 | 0.6 | 0.9 | 0.6 | 1.0 | 0.6 |
| ALT or AST | 0.7 | 0.4 | 0.7 | 0.4 | 0.7 | 0.4 |
| Baseline laboratories (within12 months prior to statin initiation) | ||||||
| Total cholesterol (mmol/l) | ||||||
| n | 273 245 | 26 734 | 202 169 | 19 707 | 276 993 | 27 150 |
| % w/measurement | 84.0 | 71.6 | 83.0 | 69.4 | 84.0 | 71.6 |
| Mean cholesterol sCr (μmol/l) | 6.3 | 6.4 | 6.3 | 6.5 | 6.3 | 6.4 |
| n | 235,183 | 18,122 | 166,387 | 12,124 | 238,169 | 18,395 |
| % w/measurement | 72.3 | 48.5 | 68.3 | 42.7 | 72.2 | 48.5 |
| Mean sCr ALT or AST (U/l) | 93.1 | 95.1 | 83.9 | 84.9 | 93.1 | 95.1 |
| n | 150,614 | 8,827 | 109,144 | 6,195 | 151,670 | 8,887 |
| % w/measurement | 46.3 | 23.6 | 44.8 | 21.8 | 46.0 | 23.4 |
| Mean ALT CK (U/l) | 28.8 | 29.1 | 30.1 | 30.1 | 27.3 | 27.3 |
| n | 16,090 | 1,120 | 11,625 | 800 | 17,012 | 1,172 |
| % w/measurement | 4.9 | 3.0 | 4.8 | 2.8 | 5.2 | 3.1 |
| Mean CK | 112.1 | 112.0 | 126.0 | 131.2 | 122.8 | 124.3 |
| First statin | ||||||
| Atorvastatin | 26% | – | 25% | – | 26% | – |
| Simvastatin | 74% | – | 75% | – | 74% | – |
| Fluvastatin | – | 17% | – | 17% | – | 17% |
| Pravastatin | – | 64% | – | 63% | – | 64% |
| Rosuvastatin | – | 19% | – | 20% | – | 19% |
| Standardized statin potency category (at statin initiation) | ||||||
| Low | 20% | 59% | 20% | 59% | 20% | 59% |
| Medium | 49% | 23% | 49% | 22% | 49% | 23% |
| High | 31% | 18% | 31% | 19% | 31% | 18% |
| Pharmacotherapy (at statin initiation) | ||||||
| CYP3A4 inhibitor | 6% | 8% | 5% | 7% | 6% | 8% |
| Diabetes drug | 11% | 10% | 10% | 8% | 11% | 10% |
| Hypertension drug | 63% | 64% | 57% | 60% | 63% | 65% |
| Thyroid drug | 7% | 7% | 6% | 6% | 7% | 7% |
| Gemfibrozil | 0.1% | 0.1% | 0.1% | 0.2% | 0.1% | 0.1% |
| Other fibrate | 1.1% | 1.8% | 1.0% | 1.8% | 1% | 2% |
| Niacin | 0.0% | 0.0% | 0.0% | 0.0% | 0.01% | 0.01% |
| Vitamin D | 2.0% | 1.4% | 1.6% | 1.1% | 0% | 0% |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; sCr, serum creatinine; CK, creatine kinase.
Because of incomplete baseline laboratory data (e.g., cholesterol, CK, sCr, and ALT/AST), only baseline cholesterol was evaluated as a potential confounder. The number of normal (below the threshold for outcome/exclusion from each specific cohort) laboratory measurements during the baseline period were used to evaluate the intensity of patient surveillance as a potential confounder.
Analysis
Stata version 11.1 (Stata Corporation, College Station, Texas, USA) was used to perform all analyses. Continuous variables were described using means, and categorical variables were described using percentages.
Primary analysis
The primary effect estimates (for each outcome independently) were derived through Cox proportional hazards regression.46 The contrast of interest is the interaction ratio (I*R). The I*R is a ratio of two hazard ratios. It represents the relative hazard of each statin type (statin 3A4 substrate vs. statin non-3A4 substrate) with a concomitant CYP3A4 inhibitor adjusted for the hazard of each statin type without a CYP3A4 inhibitor. This method controls for the hazard of the outcome associated with each statin type alone, thus focusing on the effect on the differential hazard due to the statin–CYP3A4 inhibitor interaction.
Secondary analyses
All secondary analyses used the same analytic method as described in the primary analysis. We conducted secondary analyses restricted to confirmed outcomes. Confirmed outcomes were determined by obtaining additional outcome evidence in the electronic physician notes. In addition, we evaluated the effect (I*R) of statin potency (low, medium, and high potency) and duration of response at specific time intervals (0–6, 6–12, 12–24, >24 months). We evaluated different potencies of CYP3A4 inhibition using the categorization from the University of Indiana’s cytochrome P450 table. These analyses restricted concomitant exposure to CYP3A4 inhibitors exhibiting moderate and strong inhibitory characteristics. We also conducted secondary analyses stratified by chronic (e.g., calcium channel blockers) and acute (e.g., antibiotics and antifungals) concomitant CYP3A4 inhibitors. In addition, we describe statin and CYP3A4 inhibitor concomitant person-years of exposure and events for each CYP3A4 inhibitor evaluated by statin type (statin 3A4 substrate and statin non-3A4 substrate).
Propensity score adjustment
To adjust for confounding, we used the multinomial propensity score methodology. The multinomial propensity score is the probability of being in each exposure category given baseline covariates.47–49 Given four exposure categories, we modeled three (of the four) propensity scores in each analytic model. Using the propensity score variable selection method described by Brookhart et al.,50 we included only baseline variables associated (p <0.1) with the outcome. This confounder selection procedure was conducted independently for each outcome.
To assess baseline covariate balance, we graphically evaluated the distribution of propensity scores for each of the four exposure categories. Graphic representation of propensity score distributions showed ample overlap to permit valid comparison among the four exposure categories (data not shown).
Missing data
For statins or CYP3A4 inhibitors missing the prescribed quantity or dosing instructions, we used median value imputation based on the median prescription duration for statins or CYP3A4 inhibitors with available prescribed quantity and dosing instructions. The proportion of statin and CYP3A4 inhibitor drug codes missing either the prescribed quantity or dosage instructions was 0.1 for statins and 0.2 for CYP3A4 inhibitors. Baseline body mass index (BMI) and cholesterol values were imputed using multiple imputation.51 We determined the average propensity score adjusted I*R from 10 imputed datasets. Variance determination accounted for the within- and between-dataset variation.51,52 This study was approved by the institutional review board of the University of Pennsylvania and registered with the National Health Service-Central Office for Research Ethics Committees, UK.
RESULTS
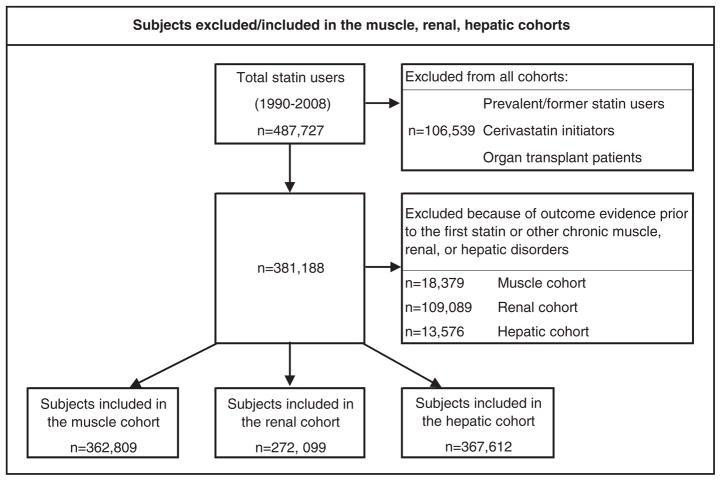
Figure 1 displays the subjects in the cohort who were included or excluded in each analysis. The median follow-up time in each analysis was 1.5 years (Table 1). Approximately 88% of patients initiated a statin 3A4 substrate. Mean age, gender, and BMI were balanced for statin 3A4 substrate and statin non-3A4 substrate initiators. Baseline variables associated with each outcome and therefore included in the propensity score adjusted model (for that specific analysis) are listed at the bottom of each results table (Tables 2a, 2b, and 2c).
Figure 1.
Subjects excluded or included in the muscle, renal, and hepatic
Table 2a.
Muscle toxicity analyses: number of events (events), person-years (p-y), incidence rates per 1000 person-years (IR), unadjusted and adjusted hazard ratios (HR), and unadjusted and adjusted interaction ratios (I* R)
| Events | p-y | IR | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| HR (95%CI) | I*R (95%CI) | ||||||
| Primary analysis | |||||||
| Statin 3A4 substrate + CYP3A4X | 446 | 50 608 | 8.81 | 0.93 | 0.97 | 1.20 (0.89–1.63) | 1.22 (0.90–1.66) |
| Statin 3A4 substrate | 6688 | 657 276 | 10.18 | (0.85–1.03) | (0.88–1.07) | ||
| Statin non-3A4 substrate + CYP3A4X | 49 | 7227 | 6.78 | 0.76 | 0.75 | ||
| Statin non-3A4 substrate | 706 | 77 555 | 9.10 | (0.57–1.01) | (0.56–1.00) | ||
| Total | 7889 | 792 665 | 9.95 | ||||
| Confirmed outcomes | |||||||
| Statin 3A4 substrate + CYP3A4X‡ | 131 | 50 608 | 2.59 | 0.79 | 0.88 | 0.87 (0.52–1.48) | 0.90 (0.53–1.52) |
| Statin 3A4 substrate | 2358 | 657 276 | 3.59 | (0.66–0.94) | (0.74–1.06) | ||
| Statin non-3A4 substrate + CYP3A4X | 17 | 7227 | 2.35 | 0.89 | 0.94 | ||
| Statin non-3A4 substrate | 212 | 77 555 | 2.73 | (0.54–1.46) | (0.57–1.55) | ||
| Total | 2718 | 792 665 | 3.43 | ||||
Model adjusted for the following baseline variables (i.e., at or prior to statin initiation): age, sex, cholesterol, year at statin initiation, chronic heart failure, stroke, diabetes, hypothyroidism, fluoroquinolone antibiotics, diabetes drugs, thyroid drugs, number of office visits, serum creatinine measurements, alanine aminotransferase/aspartate aminotransferase measurements during the baseline period, and statin potency (as a time-varying covariate).
Table 2b.
Renal dysfunction analyses: number of events (events), person-years (p-y), incidence rates per 1000 person-years (IR), unadjusted and adjusted hazard ratios (HR), and unadjusted and adjusted interaction ratios (I*R)
| Events | p-y | IR | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95%CI) | I*R (95%CI) | ||||||
| Primary analysis | |||||||
| Statin 3A4 substrate + CYP3A4X | 175 | 33 543 | 5.22 | 2.10 | 1.69 | 0.95 (0.60–1.50) | 0.91 (0.57–1.43) |
| Statin 3A4 substrate | 1119 | 478 830 | 2.34 | (1.79–2.46) | (1.43–1.99) | ||
| Statin non-3A4 substrate + CYP3A4X | 25 | 4872 | 5.13 | 2.21 | 1.80 | ||
| Statin non-3A4 substrate | 130 | 57 339 | 2.27 | (1.44–3.39) | (1.16–2.79) | ||
| Total | 1449 | 574 584 | 2.52 | ||||
| Confirmed outcomes | |||||||
| Statin 3A4 substrate + CYP3A4X | 131 | 33 543 | 3.91 | 2.53 | 2.15 | 0.90 (0.51–1.46) | 0.86 (0.50–1.45) |
| Statin 3A4 Substrate | 701 | 478 830 | 1.46 | (2.09–3.05) | (1.77–2.60) | ||
| Statin non-3A4 substrate + CYP3A4X | 20 | 4872 | 4.10 | 2.80 | 2.23 | ||
| Statin non-3A4 substrate | 82 | 57 339 | 1.43 | (1.71–4.56) | (1.35–3.69) | ||
| Total | 934 | 574 584 | 1.63 | ||||
| Excluding chronic kidney disease outcomes | |||||||
| Statin 3A4 substrate + CYP3A4X | 152 | 33 543 | 4.53 | 2.20 | 1.75 | 0.96 (0.59–1.57) | 0.91 (0.55–1.49) |
| Statin 3A4 substrate | 935 | 478 847 | 1.95 | (1.85–2.62) | (1.46–2.08) | ||
| Statin non-3A4 substrate + CYP3A4X | 22 | 4872 | 4.52 | 2.27 | 1.79 | ||
| Statin non-3A4 substrate | 111 | 57 339 | 1.94 | (1.44–3.60) | (1.12–2.86) | ||
| Total | 1220 | 574 601 | 2.12 | ||||
Model adjusted for the following baseline variables (i.e., at or prior to statin initiation): age, sex, body mass index, cholesterol, alcoholism, year at statin initiation, chronic heart failure, myocardial infarction, stroke, diabetes, hypertension, vitamin D, diabetes drug use, hypertension drug use, number of office visits during the baseline, period, and statin potency (as a time varying covariate).
Table 2c.
Hepatic dysfunction analyses: number of events (events), person-years (p-y), incidence rates per 1000 person-years (IR), unadjusted and adjusted hazard ratios (HR), and unadjusted and adjusted interaction ratios (I* R)
| Events | p-y | IR | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR (95%CI) | I*R (95%CI) | ||||||
| Primary analysis | |||||||
| Statin 3A4 substrate + CYP3A4X | 116 | 52 957 | 2.19 | 1.25 | 1.19 | 0.78 (0.46–1.32) | 0.78 (0.46–1.33) |
| Statin 3A4 substrate | 1183 | 675 312 | 1.75 | (1.03–1.52) | (0.97–1.44) | ||
| Statin non-3A4 substrate + CYP3A4X | 18 | 7624 | 2.36 | 1.62 | 1.64 | ||
| Statin non-3A4 substrate | 117 | 80 052 | 1.46 | (0.99–2.66) | (0.98–2.72) | ||
| Total | 1434 | 815 945 | 1.76 | ||||
| Confirmed outcomes | |||||||
| Statin 3A4 substrate + CYP3A4X | 97 | 52 957 | 1.83 | 1.21 | 1.20 | 0.65 (0.37–1.11) | 0.66 (0.38–1.14) |
| Statin 3A4 substrate | 1024 | 675 312 | 1.52 | (0.98–1.50) | (0.97–1.49) | ||
| Statin non-3A4 substrate + CYP3A4X | 18 | 7624 | 2.36 | 1.86 | 2.01 | ||
| Statin non-3A4 substrate | 102 | 80 052 | 1.27 | (1.12–3.07) | (1.20–3.36) | ||
| Total | 1241 | 815 945 | 1.52 | ||||
| ALT/AST 10× ULN OR med codes | |||||||
| Statin 3A4 substrate + CYP3A4X | 62 | 33 543 | 4.53 | 1.27 | 1.14 | 0.86 (0.39–1.88) | 0.85 (0.39–1.87) |
| Statin 3A4 substrate | 627 | 478 847 | 1.95 | (0.97–1.65) | (0.87–1.49) | ||
| Statin non-3A4 substrate + CYP3A4X | 8 | 7625 | 1.05 | 1.47 | 1.34 | ||
| Statin non-3A4 substrate | 57 | 80 056 | 0.71 | (0.70–3.09) | (0.63–2.86) | ||
| Total | 754 | 816 000 | 0.92 | ||||
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Model adjusted for the following baseline variables (i.e., at or prior to statin initiation): age, sex, cholesterol, year at statin initiation, chronic heart failure, myocardial infarction, stroke, diabetes, hypertension, hypothyroidism, diabetes drugs, hypertension drugs, number of office visits, serum creatinine measurements, ALT/AST, measurements in the 12 months prior to statin initiation, and statin potency (as a time-varying covariate).
Muscle toxicity results
Table 2a shows results for muscle toxicity (primary and confirmed outcome analyses). The adjusted relative hazard of muscle toxicity for each statin type with a concomitant CYP3A4 inhibitor, adjusted for the effect of each statin type without a CYP3A4 inhibitor, was 1.22 (95% confidence interval [CI] = 0.90–1.66).
Renal dysfunction results
Table 2b shows results for renal dysfunction (primary, confirmed outcomes, and the analysis excluding CKD outcomes). For the primary renal dysfunction analysis, the adjusted I*R was 0.91 (95%CI = 0.57–1.43).
Hepatic dysfunction results
Table 2c shows results for hepatic dysfunction (primary, confirmed outcomes, and ALT/AST >10× ULN). For the primary analysis, the adjusted I*R for renal dysfunction was 0.78 (95%CI = 0.45–1.33). The confirmed hepatic dysfunction outcome (adjusted) I*R was 0.66 (95%CI = 0.38–1.14). The adjusted I*R for the ALT/AST 10X ULN was 0.85 (95%CI = 0.39–1.87).
Statin potency results
Table 3 shows the results for the statin potency analyses. The test for trend among the muscle toxicity potency strata was not significant (p = 0.46). For renal dysfunction, because of sparse events and person-years in the statin non-3A4 substrate with a CYP3A4 inhibitor exposure category, we could not obtain an I*R in the high potency strata.
Table 3.
Standardized potency† analysis
| Outcome | Statin potency* | No. of events | p-y | IR/1000 p-y | Adjusted† I* R | 95%CI |
|---|---|---|---|---|---|---|
| Muscle toxicity | Low‡ | 1436 | 166 470 | 8.63 | 1.06 | 0.87–1.12 |
| Medium§ | 3405 | 348 824 | 9.76 | 1.28 | 0.77–2.11 | |
| High¶ | 3048 | 277 371 | 10.99 | 2.85 | 0.70–11.62 | |
| Renal dysfunction | Low | 291 | 120 934 | 2.41 | 0.84 | 0.39–1.83 |
| Medium | 620 | 251 108 | 2.47 | 0.78 | 0.42–1.45 | |
| High | 538 | 202 542 | 2.66 | – | – | |
| Hepatic dysfunction | Low | 257 | 171 580 | 1.50 | 0.51 | 0.22–1.15 |
| Medium | 609 | 359 195 | 1.70 | 1.27 | 0.97–1.67 | |
| High | 568 | 284 086 | 2.00 | 0.97 | 0.13–7.45 |
p-y, person-years; IR, incidence rates per 1000 person-years; I*R, adjusted interaction ratios; LDL-C, low-density lipoprotein cholesterol.
Statin potency standardization
Models adjusted for the same variables in the primary analysis. See Tables 2a, 2b, and 2c for specific variables.
Low potency: <25% LDL-C reduction (atorvastatin ≤5 mg, simvastatin ≤10 mg, fluvastatin ≤20 mg, pravastatin ≤20)
Medium potency: 25%–30% LDL-C reduction (atorvastatin 10 mg, simvastatin 20 mg, fluvastatin 80 mg, pravastatin 40 mg)
High potency: >30% LDL-C reduction (atorvastatin ≥20 mg, simvastatin gle;40 mg, fluvastatin 160 mg, pravastatin ≥80 mg, rosuvastatin ≥5 mg)
Duration of response results
Duration-of-response analyses are presented in Table 4. Because of sparse events in the statin non-3A4 substrate with a CYP3A4 inhibitor exposure category, we could not obtain stable I*Rs earlier than 6 months following statin initiation. We also attempted to determine the I*R during the first course of statin therapy, but there were insufficient person-years and events to obtain I*R estimates. Given this, we stratified the duration of follow-up as follows: 0–6, 6–12, 12–24, and >24 months. We found a nonsignificant increased hazard of muscle toxicity for statin 3A4 substrates with a CYP3A4 inhibitor compared with statin non-3A4 substrates with a CYP3A4 inhibitor in the 0–6 months strata (adjusted I*R = 2.07 [95%CI = 0.95–4.49]).
Table 4.
Duration of response analyses for muscle toxicity, renal dysfunction, and hepatic dysfunction stratified by statin 3A4 substrates and statin non-3A4 substrates with and without a CYP3A4 inhibitor (cyp).
| Outcome | Months | Events and p-y | Statin 3A4 substrate
|
Statin non-3A4 substrate
|
Totals | IR/1000 p-y | Adjusted* I*R | 95%CI | ||
|---|---|---|---|---|---|---|---|---|---|---|
| cyp + | cyp − | cyp + | cyp − | |||||||
| Muscle toxicity | 0–6 | Events | 122 | 2520 | 7 | 211 | 2860 | 23.12 | 2.07 | 0.95–4.49 |
| p-y | 6509 | 10 4377 | 1019 | 11 781 | 123 685 | |||||
| 6–12 | Events | 63 | 1082 | 10 | 89 | 1244 | 9.89 | 0.72 | 0.36–1.44 | |
| p-y | 6678 | 106 370 | 1018 | 11 772 | 125 838 | |||||
| 12–24 | Events | 78 | 1264 | 8 | 137 | 1487 | 7.77 | 1.37 | 0.65–2.89 | |
| p-y | 10 988 | 160 601 | 1629 | 18 213 | 191 431 | |||||
| >24 | Events | 183 | 1822 | 24 | 269 | 2298 | 6.54 | 1.20 | 0.79–1.87 | |
| p-y | 26 433 | 285 624 | 3561 | 35 789 | 351 408 | |||||
| Renal dysfunction | 0–6 | Events | 22 | 198 | 3 | 19 | 242 | 2.63 | 0.96 | 0.26–3.51 |
| p-y | 4363 | 78 150 | 710 | 8959 | 92 183 | |||||
| 6–12 | Events | 17 | 153 | 3 | 12 | 185 | 2.00 | 0.60 | 0.15–2.33 | |
| p-y | 4454 | 78 550 | 699 | 8821 | 92 524 | |||||
| 12–24 | Events | 30 | 210 | 5 | 25 | 270 | 1.94 | 0.89 | 0.32–2.51 | |
| p-y | 7236 | 117 080 | 1097 | 13 524 | 138 937 | |||||
| >24 | Events | 106 | 558 | 14 | 74 | 752 | 3.00 | 0.99 | 0.54–1.82 | |
| p-y | 17 489 | 205 049 | 2366 | 26 035 | 250 939 | |||||
| Hepatic dysfunction | 0–6 | Events | 17 | 296 | 4 | 23 | 340 | 2.71 | 0.43 | 0.13–1.38 |
| p-y | 6702 | 105 868 | 1061 | 11 982 | 125 614 | |||||
| 6–12 | Events | 11 | 172 | 2 | 15 | 200 | 1.56 | 0.65 | 0.13–3.19 | |
| p-y | 6904 | 108 304 | 1063 | 12 035 | 128 305 | |||||
| 12–24 | Events | 21 | 259 | 4 | 25 | 309 | 1.58 | 0.67 | 0.21–2.09 | |
| p-y | 11 387 | 164 342 | 1704 | 18 696 | 196 130 | |||||
| >24 | Events | 67 | 456 | 8 | 54 | 585 | 1.60 | 1.08 | 0.49–2.36 | |
| p-y | 27 964 | 296 798 | 3796 | 37 339 | 365 897 | |||||
Specific CYP3A4 inhibitor results
Table 5 describes the person-years and events for specific CYP3A4 inhibitors jointly prescribed with statins. Overall, the concomitant statin–CYP3A4 inhibitor person-years and events were similarly distributed for patients exposed to statin 3A4 substrates and statin non-3A4 substrates. For each cohort, diltiazem, verapamil, and amiodarone made up nearly 85% of all CYP3A4 inhibitor concomitancy among statin users.
Table 5.
Descriptive analysis of statin person-years and events with and without specific concomitant CYP3A4 inhibitors for muscle toxicity, renal dysfunction, and hepatic dysfunction stratified by statin 3A4 substrates and statin non-3A4 substrates
| Muscle toxicity
|
Renal dysfunction
|
Hepatic dysfunction
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statin 3A4 substrate
|
Statin non-3A4 substrate
|
Statin 3A substrate
|
Statin non-3A4 substrate
|
Statin 3A4 substrate
|
Statin non-3A4 substrate
|
|||||||||||||
| p-y | %* | Events | p-y | %* | Events | p-y | %* | Events | p-y | %* | Events | p-y | %* | Events | p-y | %* | Events | |
| Statin without CYP3A4 inhibitor | 657 726 | – | 6688 | 77 555 | – | 706 | 478 830 | – | 1119 | 57 339 | – | 130 | 675 312 | – | 1183 | 80 052 | – | 117 |
| Statin with CYP3A4 inhibitor | 50 608 | – | 446 | 7227 | – | 49 | 33 543 | – | 175 | 4872 | – | 25 | 52 957 | – | 116 | 7624 | – | 18 |
| Specific concomitant CYP3A4 inhibitors | ||||||||||||||||||
| Diltiazem | 36 770 | 72.75 | 330 | 5083 | 70.65 | 36 | 25 835 | 77.04 | 85 | 3644 | 74.88 | 9 | 38 558 | 73.05 | 57 | 5318 | 70.89 | 11 |
| Amiodarone | 7644 | 15.11 | 57 | 1214 | 16.78 | 7 | 3548 | 10.58 | 67 | 623 | 12.81 | 11 | 7807 | 14.79 | 42 | 1243 | 16.57 | 7 |
| Cimetidine | 3218 | 6.37 | 22 | 483 | 6.71 | 1 | 2048 | 6.11 | 10 | 309 | 6.35 | 1 | 3361 | 6.37 | 9 | 500 | 6.66 | 1 |
| Verapamil | 2111 | 4.18 | 29 | 283 | 3.93 | 2 | 1481 | 4.42 | 7 | 198 | 4.08 | 1 | 2211 | 4.19 | 4 | 288 | 3.84 | – |
| Erythromycin | 777 | 1.54 | 9 | 98 | 1.36 | – | 545 | 1.63 | 2 | 72 | 1.48 | – | 816 | 1.55 | 2 | 104 | 1.39 | – |
| Clarithromycin | 447 | 0.88 | 4 | 70 | 0.98 | – | 321 | 0.96 | 6 | 51 | 1.06 | 2 | 474 | 0.90 | 1 | 74 | 0.99 | – |
| Cyclosporine | 183 | 0.36 | 2 | 66 | 0.92 | 3 | 63 | 0.19 | 2 | 29 | 0.59 | 1 | 179 | 0.34 | 1 | 65 | 0.87 | – |
| Fluconazole | 101 | 0.20 | 1 | 13 | 0.18 | – | 76 | 0.23 | 1 | 8 | 0.17 | – | 106 | 0.20 | 1 | 13 | 0.18 | – |
| Fluvoxamine | 80 | 0.16 | 1 | 9 | 0.13 | – | 68 | 0.20 | – | 8 | 0.17 | – | 75 | 0.14 | – | 9 | 0.13 | – |
| Nefazadone | 42 | 0.08 | – | 16 | 0.22 | – | 33 | 0.10 | – | 13 | 0.26 | – | 48 | 0.09 | – | 15 | 0.19 | – |
| Itraconazole | 34 | 0.07 | – | 5 | 0.07 | – | 27 | 0.08 | – | 3 | 0.06 | – | 34 | 0.07 | – | 5 | 0.07 | – |
| Norfloxacin | 23 | 0.05 | – | 3 | 0.04 | – | 18 | 0.05 | – | 2 | 0.04 | – | 27 | 0.05 | – | 3 | 0.04 | – |
| Ketoconazole | 3 | 0.01 | – | 1 | 0.01 | – | 3 | 0.01 | – | 0 | 0.00 | – | 3 | 0.01 | – | 1 | 0.01 | – |
| Mibefradil | 2 | 0.00 | – | 4 | 0.05 | – | 1 | 0.00 | – | 3 | 0.06 | – | 2 | 0.00 | – | 4 | 0.05 | – |
| Imatinib | 0 | 0.00 | – | – | – | – | 0 | 0.00 | – | – | – | – | 0 | 0.00 | – | – | – | – |
| Voriconazole | 0 | 0.00 | – | 0 | 0.00 | – | 0 | 0.00 | – | – | – | – | 0 | 0.00 | – | 0 | 0.00 | – |
Percentage of total concomitant statin plus CYP3A4 inhibitor person-years.
Other secondary analysis results
The results from the secondary analysis censoring follow-up for patients with specific chronic medical conditions identified after statin initiation rather than excluding the entire patient record were consistent with the primary findings (data not shown). Results from the moderate or strong CYP3A4 inhibitor analyses and the short or long duration use CYP3A4 inhibitor analyses showed no increased hazard for statin 3A4 substrates compared with statin non-3A4 substrates (data not shown).
DISCUSSION
Our analyses showed no overall significant increased hazard associated with statin 3A4 substrates compared with statin non-3A4 substrates with a concomitant CYP3A4 inhibitor, adjusted for the hazard of each statin type without a concomitant CYP3A4 inhibitor. Unlike previous research of the statin–CYP3A4 inhibitor interaction, these primary analyses were well powered, had detailed information on comorbidities and potential confounders, used propensity score adjustment, and used the I*R to control for the hazard associated with each statin type alone, thus focusing on the effect on the differential hazard due to the statin–CYP3A4 inhibitor interaction. The I*R is an appropriate effect estimate for evaluating the clinical importance of drug interactions, provided a suitable comparator group is available. For the primary and confirmed outcome analyses, statin person-time in each of the four exposure categories was sufficient to allow I*R estimation. The results from this investigation indicate that the clinical implications of this well-documented drug interaction may be of less importance than suggested by PK studies, case reports, and analyses of spontaneous reports. However, due to the limited power of important secondary analyses, further research is warranted to evaluate the muscle toxicity I*R for high potency statins and in the first 6 months after statin initiation.
Pharmacokinetic studies consistently show higher systemic statin exposure with coadministration of statin 3A4 substrates and a CYP3A4 inhibitor compared with statin 3A4 substrates alone.35,53–55 However, the long-term effect and clinical importance of elevated statin exposure are not well characterized. The results of this study suggest that the increased systemic statin exposure may not translate into increased hazard for statin-related adverse events. However, in the duration-response analysis for muscle toxicity, the I*R showed a nonsignificant increased hazard in the first 6 months following statin initiation (I*R = 2.07; 95% CI = 0.95–4.48) and for the first statin course with and without a concomitant CYP3A4 inhibitor (data not shown). Further investigation of muscle toxicity is warranted to evaluate the early effect of joint exposure to statins and CYP3A4 inhibitors.
Previous research suggests that statin potency is associated with muscle toxicity.19,21 Consistent with previous findings, we saw a significant increased hazard of all three outcomes for each successive increase in statin potency; however, it was not quite statistically significant for renal dysfunction (data not shown). Although the continuous statin potency analyses—not accounting for the potential interaction with CYP3A4 inhibitor concomitancy—depicts the relationship between statin potency and the outcome, they do not reveal the differential effect for each statin type with a CYP3A4 inhibitor, compared with each statin type without a CYP3A4 inhibitor at each potency level. This contrast (i.e., the I*R) is depicted in the stratified potency analyses, where the I*Rs show a nonsignificant increasing hazard of muscle toxicity with increasing statin potency, but no difference for renal or hepatic dysfunction with increasing statin potency. Further evaluation of the muscle toxicity I*R for highly potent statin doses with CYP3A4 inhibitor concomitancy may be warranted. That said, the muscle toxicity I*R (I*R 2.85; 95%CI = 0.70–11.62) for highly potent statins was derived from a large sample size (277 371 person-years of statin exposure) and many muscle toxicity events (3048). It would take a much larger sample size to improve I*R precision.
Our results must be placed into the context of other observational studies showing an increased risk of statin-associated adverse events with concomitant CYP3A4 inhibitors. Cziraky and colleagues reported a sixfold relative risk (6.01; 95%CI = 2.08–17.38) of muscle toxicity for statins with CYP3A4 inhibitors compared with atorvastatin alone.9 However, statin plus concomitant CYP3A4 inhibitor exposure was aggregated among all person-years attributed to cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin with a concomitant CYP3A4 inhibitor. Stratification of statin exposure by oxidative metabolism was not evaluated, so they could not disaggregate the independent risk of the concomitant CYP3A4 inhibitor (or the indication for the concomitant CYP3A4 inhibitor) from the risk from the drug interaction. In the present study, the I*R separates the effect associated with each statin type with a CYP3A4 inhibitor from the effect associated with each statin type without a CYP3A4 inhibitor.
The results of the present study are also discordant from a spontaneous report study in which a sixfold AERR for simvastatin reports with a concomitant CYP3A4 inhibitor compared with simvastatin reports without a concomitant CYP3A4 inhibitor was reported.22 The current study, however, has substantial advantages in design and execution. The present study included only new statin initiators, excluded patients with prior outcomes, excluded organ transplant patients, used a validated electronic medical record database, adjusted for potential confounding variables, had a true denominator of statin person-years with and without CYP3A4 inhibitor concomitancy, was not dependent on external outcome reporting, and used Cox proportional hazards regression to estimate the I*R with 95%CIs. Spontaneous report analyses are critical for signal generation. However, the conclusiveness of their findings is limited.56 The present study is the largest observational study specifically designed to evaluate the clinical importance of the statin–CYP3A4 inhibitor drug interaction.
The Health Improvement Network has been used in many epidemiologic studies and has been validated for numerous medical conditions including studies of statin-related side effects.31,57,58 THIN is a powerful tool for studying drug interactions because the population included is large, diverse, and well characterized. Despite this, practice patterns, patient populations, prescribing patterns, and patient surveillance may be systematically different in the UK from other countries. We compared the baseline patient characteristics in this study with those in other recent statin safety investigations.3–7,9,42,45,59,60 These baseline patient characteristics were consistent with the baseline patient characteristics from other US, Canadian, and European statin safety cohorts (data not shown).
Regarding confounding, we could not control for variables which we could not identify or could not measure. However, we captured important variables previously shown to be risk factors for each outcome. We also separately controlled for confounding by chronic diseases, whether they were diagnosed before or after the initiation of the statin; the results were the same.
To minimize exposure misclassification, we defined precise exposure criteria for each exposure category, used up-to-date drug codes, and carefully constructed exposure episodes. These methods, of course, do not eliminate the possibility of poor medication adherence. In addition, THIN would not capture statins obtained over the counter nor would it capture dietary exposures as grapefruit juice. However, it has been shown that very high levels of grapefruit juice consumption are needed to inhibit the CYP3A4 isoenzyme. However, we would not expect medication adherence or dietary exposure to be different for users of statin 3A4 substrates compared with statin non-3A4 substrates.
One noteworthy class of CYP3A4 inhibitors not represented in this investigation is antiretroviral therapy (e.g., ritonavir, saquinavir, nelfinavir, indinavir, and lopinavir). This investigation included person-years of concomitant exposure to statins and antiretrovirals, but there was negligible use included in THIN. In the UK, antiretroviral treatment is given mainly by specialized genitourinary medical clinics, not by physicians in general practice. The results from this investigation may or may not extrapolate to statins with concomitant antiretroviral therapy.
Outcome misclassification threatens the validity of all retrospective cohort studies. To evaluate potential outcome misclassification, we conducted secondary analyses restricted to confirmed outcomes. These secondary analyses were consistent with our primary results.
CONCLUSION
This large retrospective cohort study showed no overall increased hazard for muscle toxicity, renal dysfunction, or hepatic dysfunction associated with statin 3A4 substrates compared with statin non-3A4 substrates with versus without a concomitant CYP3A4 inhibitor. Additional research could further evaluate the nonsignificant yet increased muscle toxicity I*R we observed for highly potent statin dosages and within 6 months following statin initiation. However, it is clear that the drug interaction between statins and CYP3A4 inhibitors does not represent an important public health concern.
KEY POINTS.
Studies warn of the potential increased risk of adverse events resulting from higher systemic statin exposure with coadministration of statin 3A4 substrates and a CYP3A4 inhibitor.
We found no overall difference in muscle toxicity, renal dysfunction, and hepatic dysfunction associated with statin 3A4 substrates compared with statin non-3A4 substrates with a concomitant CYP3A4 inhibitor.
The stratified potency analyses showed a nonsignificant yet increasing hazard of muscle toxicity for successive statin 3A4 substrate potency levels.
The duration of response analysis showed a non-significant increased hazard of muscle toxicity in the first 6 months for statin 3A4 substrates.
In this large drug interaction study of statins and CYP3A4 inhibitors, the overall results show no evidence of increased hazard of statin-related adverse events based on statin metabolism.
Footnotes
CONFLICT OF INTEREST
Brian L. Strom provided consulting services for AstraZeneca, Bristol-Myers-Squib, Pfizer, and Novartis and received educational support from Novatis and Pfizer. Daniel Mines was employed by Pfizer, maker of Lipitor (atorvastatin), from October 2009 through April 2010. None of his work then involved atorvastatin, and he does not own Pfizer stock. James D. Lewis served as a consultant to GlaxoSmithKline. Sean Hennessy received research funding from Bristol-Meyers Squibb and Astra Zeneca and have consulted for Astra-Zeneca (related to statins, unrelated to drug–drug interactions) and Pfizer (unrelated to statins).
References
- 1.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease - A meta-analysis of randomized controlled trials. JAMA. 1999;282(24):2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 3.McAfee AT, Ming EE, Seeger JD, et al. The comparative safety of rosuvastatin: a retrospective matched cohort study in over 48 000 initiators of statin therapy. Pharmacoepidemiol Drug Saf. 2006;15(7):444–453. doi: 10.1002/pds.1281. [DOI] [PubMed] [Google Scholar]
- 4.Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther. 2007;29(8):1761–1770. doi: 10.1016/j.clinthera.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 5.McClure DL, Valuck RJ, Glanz M, Murphy JR, Hokanson JE. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. J Clin Epidemiol. 2007;60(8):812–818. doi: 10.1016/j.jclinepi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Goettsch WG, Heintjes EM, Kastelein JJP, Rabelink TJ, Johansson S, Herings RMC. Results from a rosuvastatin historical cohort study in more than 45 000 Dutch statin users, a PHARMO study. Pharmacoepidemiol Drug Saf. 2006;15(7):435–443. doi: 10.1002/pds.1278. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Rodriguez LA, Masso-Gonzalez EL, Wallander MA, Johansson S. The safety of rosuvastatin in comparison with other statins in over 100 000 statin users in UK primary care. Pharmacoepidemiol Drug Saf. 2008;17(10):943–952. doi: 10.1002/pds.1603. [DOI] [PubMed] [Google Scholar]
- 8.Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292(21):2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 9.Cziraky MJ, Willey VJ, McKenney JM, et al. Statin safety. An assessment using an administrative claims database. 2006;97(8A):61 C–68 C. doi: 10.1016/j.amjcard.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Ucar M, Mjorndal T, Dahlqvist R. HMG-CoA reductase inhibitors and myotoxicity. Drug Saf. 2000;22(6):441–457. doi: 10.2165/00002018-200022060-00003. [DOI] [PubMed] [Google Scholar]
- 11.Deslypere JP, Delanghe J, Vermeulen A. Proteinuria as Complication of Simvastatin Treatment. Lancet. 1990;336(8728):1453–1453. doi: 10.1016/0140-6736(90)93164-k. [DOI] [PubMed] [Google Scholar]
- 12.Kostapanos MS, Milionis HJ, Gazi I, Kostara C, Bairaktari ET, Elisaf M. Rosuvastatin increases alpha-1 micrloglobulin urinary excretion in patients with primary dyslipidemia. J Clin Pharmacol. 2006;46(11):1337–1343. doi: 10.1177/0091270006292629. [DOI] [PubMed] [Google Scholar]
- 13.Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97(8A):77 C–81 C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: A meta-analysis. Pharmacotherapy. 2004;24(5):584–591. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 15.Chang JT, Staffa JA, Parks M, Green L. Rhabdomyolysis with HMG-CoA reductase inhibitors and gemfibrozil combination therapy. Pharmacoepidemiol Drug Saf. 2004;13(7):417–426. doi: 10.1002/pds.977. [DOI] [PubMed] [Google Scholar]
- 16.Evans M, Rees A. Effects of HMG-CoA reductase inhibitors on skeletal muscle: are all statins the same? Drug Saf. 2002;25(9):649–663. doi: 10.2165/00002018-200225090-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hedenmalm K, Alvan G, Ohagen P, Dahl ML. Muscle toxicity with statins. Pharmacoepidemiol Drug Saf. 2010;19(3):223–231. doi: 10.1002/pds.1895. [DOI] [PubMed] [Google Scholar]
- 18.Kasliwal R, Wilton LV, Cornelius V, Aurich-Barrera B, Shakir SAW. Safety profile of rosuvastatin - Results of a prescription-event monitoring study of 11680 patients. Drug Saf. 2007;30(2):157–170. doi: 10.2165/00002018-200730020-00005. [DOI] [PubMed] [Google Scholar]
- 19.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients - The PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 20.Holtzman CW, Wiggins BS, Spinler SA. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26(11):1601–1607. doi: 10.1592/phco.26.11.1601. [DOI] [PubMed] [Google Scholar]
- 21.Schech S, Graham D, Staffa J, et al. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16:352–358. doi: 10.1002/pds.1287. [DOI] [PubMed] [Google Scholar]
- 22.Rowan CG, Brinker AD, Nourjah P, Chang J, Moshholder A, Avigan M. Rhabdomyolysis reports show interaction between simvastatin and CYP3A4 inhibitors. Pharmacoepidemiol Drug Saf. 2008;17:526. doi: 10.1002/pds.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346(7):539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 24.Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients [see comment] Arch Intern Med. 2003;163(5):553–564. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 25.Azie NE, Brater DC, Becker PA, Jones DR, Hall SD. The interaction of diltiazem with lovastatin and pravastatin. Clin Pharmacol Ther. 1998;64(4):369–377. doi: 10.1016/S0009-9236(98)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez F, Tukey R. The CYPS. In: Brunton LLLJ, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11. McGraw-Hill; New York: 2006. [Google Scholar]
- 27.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112(1):71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Davidson MH, Gandhi SK, Ming EE, et al. Concomitant prescribing rates of statins and Cytochrome P450 3A4 inhibitors and inducers in real-world clinical practice. Pharmacoepidemiol Drug Saf. 2006;15:S289–S289. [Google Scholar]
- 29.Flockhart D. Drug Interactions: Cytochrome P450 Drug interaction table. Indiana University School of Medicine; 2007. [Google Scholar]
- 30.Bourke A, Dattani H, Robinson M. Feasibility Study and Methodology to create a Quality Evaluated Database of Primary Care Data. Inform Prim Care. 2004;12(3):171–177. doi: 10.14236/jhi.v12i3.124. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JD, SR, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 32.Lewis J, Schinnar R, Bilker W, Wang X, Strom B. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 33.Lee AJ, Maddix DS. Rhabdomyolysis secondary to a drug interaction between simvastatin and clarithromycin. Ann Pharmacother. 2001;35(1):26–31. doi: 10.1345/aph.10177. [DOI] [PubMed] [Google Scholar]
- 34.Kantola T, Kivisto KT, Neuvonen PJ. Erythromycin and verapamil considerably increase serum simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther. 1998;64(2):177–182. doi: 10.1016/S0009-9236(98)90151-5. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94(9):1140–1146. doi: 10.1016/j.amjcard.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 36.Venkatakrishnan K, von Moltke LL, Greenblatt DJ. Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet. 2000;38(2):111–180. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- 37.Neuvonen PJ, Jalava KM. Itraconazole drastically increases plasma concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1996;60(1):54–61. doi: 10.1016/S0009-9236(96)90167-8. [DOI] [PubMed] [Google Scholar]
- 38.DeVane CL, Donovan JL, Liston HL, et al. Comparative CYP3A4 inhibitory effects of venlafaxine, fluoxetine, sertraline, and nefazodone in healthy volunteers. J Clin Psychopharmacol. 2004;24(1):4–10. doi: 10.1097/01.jcp.0000104908.75206.26. [DOI] [PubMed] [Google Scholar]
- 39.Fleishaker JC, Hulst LK. A pharmacokinetic and pharmacodynamic evaluation of the combined administration of alprazolam and fluvoxamine. Eur J Clin Pharmacol. 1994;46(1):35–39. doi: 10.1007/BF00195913. [DOI] [PubMed] [Google Scholar]
- 40.Shitara Y, Itoh T, Sato H, Li AP, Sugiyama Y. Inhibition of transporter-mediated hepatic uptake as a mechanism for drug-drug interaction between cerivastatin and cyclosporin A. J Pharmacol Exp Ther. 2003;304(2):610–616. doi: 10.1124/jpet.102.041921. [DOI] [PubMed] [Google Scholar]
- 41.Weng TC, Yang YHK, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35(2):139–151. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Rodriguez LA, Gonzalez-Perez A, Stang MR, Wallander MA, Johansson S. The safety of rosuvastatin in comparison with other statins in over 25 000 statin users in the Saskatchewan Health Databases. Pharmacoepidemiol Drug Saf. 2008;17(10):953–961. doi: 10.1002/pds.1602. [DOI] [PubMed] [Google Scholar]
- 43.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106(8):1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 44.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, Clinical Features, and Outcomes From a Prospective Study of Drug-induced Liver Injury in the United States. Gastroenterology. 2008;135(6):1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. Br Med J. 2010;340:12. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34(2):187–220. [Google Scholar]
- 47.Imai K, van Dyk DA. Causal inference with general treatment regimes: Generalizing the propensity score. J Am Stat Assoc. 2004;99(467):854–866. [Google Scholar]
- 48.Huang IC, Frangakis C, Dominici F, Diette GB, Wu AW. Application of a propensity score approach for risk adjustment in profiling multiple physician groups on asthma care. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imbens GW. The role of the propensity score in estimating dose–response functions. Biometrika. 2000;87(3):706–710. [Google Scholar]
- 50.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 52.Rubin DB. Estimating Causal Effects of Treatments in Randomized and Nonrandomized Studies. J Educ Psychol. 1974;66(5):688–701. [Google Scholar]
- 53.Azie NE, Brater DC, Becker PA, Jones DR, Hall SD. The interaction of diltiazem with lovastatin and pravastatin. Clin Pharmacol Ther. 1998;64:369–377. doi: 10.1016/S0009-9236(98)90067-4. [DOI] [PubMed] [Google Scholar]
- 54.Neuvonen PJ, Kantola T, Kivisto KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther. 1998;63(3):332–341. doi: 10.1016/S0009-9236(98)90165-5. [DOI] [PubMed] [Google Scholar]
- 55.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16(4):569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 56.Ahmad SR, Goetsch RA, Marks NS. Spontaneous Reporting in the United States. In: Strom BL, editor. Pharmacoepidemiology. 4. Wiley; West Sussex, England: 2005. [Google Scholar]
- 57.Hammad TA, McAdams MA, Feight A, Iyasu S, Dal Pan GJ. Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2008;17(12):1197–1201. doi: 10.1002/pds.1672. [DOI] [PubMed] [Google Scholar]
- 58.Lo Re V, Haynes K, Forde KA, Localio AR, Schinnar R, Lewis JD. Validity of The Health Improvement Network (THIN) for epidemiologic studies of hepatitis C virus infection. Pharmacoepidemiol Drug Saf. 2009;18(9):807–814. doi: 10.1002/pds.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devold HM, Molden E, Skurtveit S, Furu K. Co-medication of statins and CYP3A4 inhibitors before and after introduction of new reimbursement policy. Br J Clin Pharmacol. 2009;67(2):234–241. doi: 10.1111/j.1365-2125.2008.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tirkkonen T, Ryynanen A, Vahlberg T, et al. Frequency and clinical relevance of drug interactions with lovastatin and simvastatin - An observational database study. Drug Saf. 2008;31(3):231–240. doi: 10.2165/00002018-200831030-00004. [DOI] [PubMed] [Google Scholar]