Abstract
Optimal preparation conditions of Newcastle disease virus (NDV) F gene deoxyribonucleic acid (DNA) vaccine encapsulated in chitosan nanoparticles (pFNDV-CS-NPs) were determined. The pFNDV-CS-NPs were prepared according to a complex coacervation method. The pFNDV-CS-NPs were produced with good morphology, high stability, a mean diameter of 199.5 nm, encapsulation efficiency of 98.37%±0.87%, loading capacity of 36.12%±0.19%, and a zeta potential of +12.11 mV. The in vitro release assay showed that the plasmid DNA was sustainably released from the pFNDV-CS-NPs, up to 82.9%±2.9% of the total amount. Cell transfection test indicated that the vaccine expressed the F gene in cells and maintained good bioactivity. Additionally, the safety of mucosal immunity delivery system of the pFNDV-CS-NPs was also tested in vitro by cell cytotoxicity and in vivo by safety test in chickens. In vivo immunization showed that better immune responses of specific pathogen-free chickens immunized with the pFNDV-CS-NPs were induced, and prolonged release of the plasmid DNA was achieved compared to the chickens immunized with the control plasmid. This study lays the foundation for the further development of mucosal vaccines and drugs encapsulated in chitosan nanoparticles.
Keywords: Newcastle disease, DNA vaccine, chitosan nanoparticles, mucosal immunity delivery system, immune effectiveness
Introduction
Newcastle disease (ND) is a highly contagious viral disease of poultry that is characterized by nervous, respiratory, enteric, and reproductive infections. The causative agent of Newcastle disease is the virulent ND virus (vNDV), which belongs to the order Mononegavirales, family Paramyxoviridae, and genus Avulavirus.1 NDV is a single stranded, non-segmented, enveloped ribonucleic acid (RNA) virus with negative polarity and consists of six genes that encode six major proteins, including RNA polymerase (L gene), hemagglutinin-neuraminidase (HN gene), fusion (F gene), matrix (M gene), phosphoprotein (P gene), and nucleocapsid (NP gene) proteins, in order from the 5′ terminus to the 3′ terminus.2,3 Among these proteins, the F protein is an indispensable glycoprotein that allows the virus to bind and fuse to the host cells to initiate ND and induce vaccine immunity.
ND has been a devastating disease and still remains one of the major problems in existing and developing poultry industries in many countries.4 There are no treatments available for ND. Vaccination, however, is an effective method to control ND. The NDV inactivated vaccines and attenuated live vaccines are used universally to control ND. However, these conventional vaccines have some disadvantages.
Live vaccines have some limitations, including the need for biocontainment during production, cold chain requirements, and safety concerns due to the possibility of reversion, especially for RNA viruses.5,6 A major problem with the use of inactivated vaccines administered by the mucosal route is that they generally have poor immunogenicity and can cause disease if they are not completely inactivated.7 Due to the disadvantages of these vaccines, there is a need to improve and extend the impact of vaccination programs against NDV. Novel strategies, such as using deoxyribonucleic acid (DNA) vaccines, are being developed to produce a new formulation of vaccines, which can improve efficacy.8 DNA vaccines represent a promising technology due to their safety, genetic stability, ease of production, non-requirement for cold chain, and activation of innate immunity pathways.9,10 Until recently, intramuscular injection was the primary route for DNA vaccine administration. After intramuscular injection, it is difficult for the vaccines to move through cell membranes, so only a small amount reaches antigen-presenting cells (APCs) to induce immune responses.11–13 A number of clinical trials14 have shown that the magnitude of immune responses elicited by DNA vaccines is generally weaker, especially in large animals, so the amount of DNA required for effective immunization is much greater. Therefore, the development of DNA vaccine candidates has been limited due to their relatively modest immunogenicity.14 In recent years, several strategies have been proposed to improve the efficacy of DNA vaccines through optimizing the plasmid, improving delivery methods and routes of immunization, targeting for effective antigen presentation, and utilizing the powerful adjuvant to enhance immunogenicity.15,16
Among these antigen delivery systems, the nanoparticles prepared by biomaterials can offer several advantages over other antigen delivery systems. For instance, they can protect antigen against degradation in vitro and in vivo, limit systemic distribution, and thereby reduce the dose and the probable side effects.17 Chitosan is a natural biodegradable polysaccharide extracted from crustacean shells.18 It has been proven that chitosan is non-toxic in both experimental animals19 and humans.20 Chitosan nanoparticles have the potential to act as mediators of protein antigens or plasmid DNA, and to protect against biological degradation by nucleases.21,22 Recently, chitosan nanoparticles have also been utilized for sustained release of various drugs, including oligonucleotides.23–25
Studies have shown that chitosan is a promising DNA vector with sustained-releasing ability.26 Chitosan/DNA particle system prepared by this ionic gelation technique is suitable for mammalian cell transfection, and the expression level of the reporter gene with the chitosan particle system was comparable to that produced by the positive control.27 Therefore, chitosan nanoparticles as a novel and efficient gene transduction vector, by use of their targeted and sustained release,28,29 can greatly enhance transfection and expression efficiency of DNA vaccines, thereby increasing their bioavailability.
Zaharoff et al demonstrated that chitosan solution improved humoral and cell-mediated immune responses to a subcutaneous vaccination with a model protein antigen in the absence of additional adjuvant.30 Koppolu and Zaharoff demonstrated that chitosan nanoparticles were capable of efficiently delivering encapsulated antigens and enhancing the activation status of both macrophages and dendritic cells.31 These studies laid a foundation for the use of chitosan nanoparticles as a mucosal immune delivery system.
In this paper, the F gene plasmid DNA of NDV encapsulated in chitosan nanoparticles (pFNDV-CS-NPs) was prepared by a complex coacervation method to enhance the efficacy of a DNA vaccine against ND. The immune responses elicited in specific pathogen-free (SPF) chickens by the pFNDV-CS-NPs were evaluated. In addition, bioactivity and safety of the chitosan nanoparticles were studied by transfection and cytotoxicity analyses. This work has laid a foundation for future work on a wide range of mucosa immunity delivery systems including those for DNA vaccines and drugs.
Materials and methods
Materials
Eukaryotic expression plasmids (pVAX I-optiF), Escherichia coli, NDV F48E9 strain positive serum, and 293T cells were provided by State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute (HVRI), and the Chinese Academy of Agricultural Sciences (CAAS), Harbin, People’s Republic of China. SPF chickens were provided and raised by Harbin Veterinary Research Institute Laboratory Animal Center. Chitosan (with a molecular weight of 71.3 kDa and deacetylation degree of 80%), fluorescein isothiocyanate (FITC)-labeled goat-anti-chicken IgG, and horseradish peroxidase (HRP)-labeled goat-anti-chicken IgG were purchased from Sigma-Aldrich (St Louis, MO, USA); HRP-labeled goat-anti-chicken IgA was purchased from Bethly Ltd (Montgomery, TX, USA); Lipofectamine™ 2000 transfection reagent was purchased from Invitrogen Corporation (Carlsbad, CA, USA); Cell Counting Kit-8 (CCK-8) from Dojindo Ltd (Tokyo, Japan); Agarose and SDS from GIBCOBRL Ltd (New Delhi, India); Dulbecco’s Modified Eagle’s Medium (DMEM) and GIBCO serum from GIBCO Ltd (New Delhi, India); and DNA marker DL 15000 and DNase I enzyme were purchased from TaKaRa (Tokyo, Japan). Newcastle Disease Antibody Test Kit was purchased from Beijing Aideshi Yuanheng Biological Technology Co, Ltd (Beijing, People’s Republic of China). NDV vaccine strain LaSota was provided by State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute (HVRI), The Chinese Academy of Agricultural Sciences (CAAS), Harbin, People’s Republic of China.
Extraction and purification of eukaryotic expression plasmid pVAX I-optiF
The eukaryotic expression plasmid pVAX I-optiF that expressed the F gene of NDV was extracted by the alkaline lysis method.32 Extraction and purification of the large amount of the eukaryotic expression plasmid pVAX I-optiF were carried out as previously described.33
Preparation of chitosan solutions and plasmid DNA solutions
Chitosan solutions of 1.0% were prepared by slowly dissolving 1.0 g chitosan in an aqueous solution of 1.0% acetic acid and adjusted to different concentrations with 5.0 mmol/L acetate. The plasmid DNA solutions were diluted to different concentrations with Na2SO4 solution.
Preparation of the plasmid DNA chitosan nanoparticles
The plasmid DNA chitosan nanoparticles were prepared by a complex coacervation method as described previously.34 Briefly, 500 μL chitosan solutions with an equal volume of the plasmid DNA solutions were heated in a water bath of 55°C for 30 minutes. Subsequently, an equal volume of plasmid DNA solution was quickly transferred to the chitosan solutions and vortexed for 30 seconds at 2500 r/min. The plasmid DNA chitosan nanoparticles were collected by centrifugation at 2500 r/min for 10 minutes at 4°C, and the precipitate was resuspended in phosphate buffered saline (PBS). These nano-particles were simply named as the pFNDV-CS-NPs.
Optimization of the pFNDV-CS-NPs preparation conditions
Several factors that affected the characteristics of the pFNDV-CS-NPs, including the concentrations of chitosan and flocculating agent Na2SO4 solutions, N/P ratio (referring to the number of nitrogen residues per DNA phosphate; volume [v]/v), and pH, were tested. Single factor experiments were performed on the effect of preparation conditions of the pFNDV-CS-NPs. Based on the results obtained in single factor experiments, the three key factors were chitosan concentration, flocculating agent Na2SO4 concentration, and N/P ratio (v/v). The orthogonal experiments with three factors and four levels were designed. Considering the DNA vaccine carrier, keeping other factors unchanged, the more DNA vaccines loaded into the nanoparticles, the more DNA vaccine available for transfecting the target. Therefore, encapsulation efficiency (EE) was used as the evaluation indexes to optimize the preparation conditions. The orthogonal experimental scheme is shown in Table 1.
Table 1.
Optimization of pFNDV-CS-NPs preparation conditions by orthogonal experimental design
| Experiment number | Chitosan concentration (μg/mL) | Na2SO4 concentration (mmol/mL) | N/P ratio | Entrapment efficiency (%) |
|---|---|---|---|---|
| 1 | 100 | 5 | 1 | 74.87±0.92 |
| 2 | 100 | 10 | 2 | 83.03±1.91 |
| 3 | 100 | 15 | 3 | 92.46±1.65 |
| 4 | 100 | 20 | 4 | 87.97±1.47 |
| 5 | 150 | 5 | 2 | 84.57±1.04 |
| 6 | 150 | 10 | 1 | 73.43±0.49 |
| 7 | 150 | 15 | 4 | 89.46±1.17 |
| 8 | 150 | 20 | 3 | 93.10±1.40 |
| 9 | 200 | 5 | 3 | 98.37±0.87 |
| 10 | 200 | 10 | 4 | 93.43±1.00 |
| 11 | 200 | 15 | 1 | 76.37±1.10 |
| 12 | 200 | 20 | 2 | 87.47±0.49 |
| 13 | 250 | 5 | 4 | 91.43±0.50 |
| 14 | 250 | 10 | 3 | 83.40±0.67 |
| 15 | 250 | 15 | 2 | 87.70±0.98 |
| 16 | 250 | 20 | 1 | 71.33±1.80 |
| T1 | 84.58% | 87.31% | 74% | |
| T2 | 85.14% | 83.32% | 85.69% | |
| T3 | 88.91% | 86.50% | 91.83% | |
| T4 | 83.47% | 84.97% | 90.57% | |
| R | 5.44% | 3.99% | 17.83% |
Notes: T1, T2, T3 and T4 represent the mean value of factors at 1, 2, 3, and 4 levels, respectively; R represents range; data are shown as the mean ± standard deviation (n=3); N/P ratio refers to the number of nitrogen residues per deoxyribonucleic acid (DNA) phosphate.
Abbreviation: pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles.
Characterization of the pFNDV-CS-NPs
The pFNDV-CS-NPs were examined by JEM-200EX transmission electron microscopy (TEM) (Hitachi Ltd, Tokyo, Japan) to assess the morphological and surface characteristics. Chitosan nanoparticle colloidal suspension was sonicated for 2 minutes for better dispersion and to prevent particle agglomeration on the copper grid. One drop of colloidal suspension was spread onto a carbon-coated copper grid, which was then dried at room temperature for TEM analysis.
The particle size and zeta potentials of the pFNDV-CS-NPs were measured using a Zeta Sizer 2000 from Malvern Instruments (Malvern, UK). Samples were diluted with pure water, and the measurements were performed at a scattering angle of 90 degrees and a temperature of 25°C. The diameter was calculated from the autocorrelation function of the intensity of light scattered from particles, assuming a spherical form of the particles.
Evaluation of encapsulation efficiency and loading capacity
The encapsulation efficiency and loading capacity of the pFNDV-CS-NPs were determined by the separation of nano-particles from the aqueous medium containing free plasmid DNA by centrifugation at 16,000 r/min for 10 minutes at 4°C. The amount of free plasmid DNA was measured in the clear supernatant using ultraviolet (UV) spectrometry. The amount of free chitosan in the supernatant was measured with a spectrophotometer at 595 nm and calculated by the standard curve:
| [1] |
EE and loading capacity (LC) of the pFNDV-CS-NPs were calculated as follows:
| [2] |
| [3] |
where W0 is the total amount of the plasmid DNA added, W1 is the amount of the free plasmid DNA, and WCS is the amount of chitosan in the pFNDV-CS-NPs. All the measurements were performed five times.
Stability of the pFNDV-CS-NPs
Both 1.35 μg of naked plasmid DNA (5.0 mmol/L Na2SO4) and the pFNDV-CS-NPs suspension containing 1.35 μg of plasmid DNA were incubated with DNase I (1.0 U/mL) at 37°C for 30 minutes, respectively. The reaction was stopped by adding 100 μL of termination solutions (400 mmol/L NaCl, 100 mmol/L ethylenediaminetetraacetic acid [EDTA], pH 8.0) at 65°C for 10 minutes. Then 16 μL of chitosanase (0.2 U/mL) and 4.0 μL of lysozyme (0.2 U/mL) were added and incubated in a 37°C water bath for 4 hours. The pFNDV-CS-NPs suspension and the naked plasmid DNA were used as negative controls. The integrity of plasmid DNA was analyzed using 0.8% agarose gel electrophoresis.
In vitro release of pFNDV-CS-NPs
The pFNDV-CS-NPs suspension was centrifugalized at 16,000 r/min for 10 minutes at 4°C, and the precipitation was then resuspended with 1.0 mL PBS (pH 7.4) and stirred at 100 r/min at 4°C. At different time intervals (0, 6, 12, 18, 24, 36, 48, 72, 96, 120, 144, 168, 192, 216, and 240 hours), a sample was taken and centrifuged at 10,000 r/min for 10 minutes at 4°C. The concentration of released plasmid DNA in the supernatant was determined using UV spectrophotometry. The experiments were performed in triplicate. The plasmid DNA release curve of pFNDV-CS-NPs was plotted against the release time at the x-axis and the accumulative release amount at the y-axis.
In vitro expression of the pFNDV-CS-NPs
In vitro transfection of pFNDV-CS-NPs
293-T cell monolayers were grown in polylysine treated 6-well plates at 37°C in a CO2 incubator, and were used for in vitro transfection analysis when 80% confluence was reached. The following experimental groups were set up: 1) naked DNA control – 4 μg DNA was transfected into the cells according to the instructions from the Lipofectamine™ 2000 reagent kit; 2) pFNDV-CS-NPs – the amount of pFNDV-CS-NPs equivalent to 4 μg DNA was added and incubated with the cells for 5 hours; 3) blank chitosan particle control – the amount of chitosan equivalent to that in pFNDV-CS-NPs was added and the procedure was the same as in 2); and 4) cell free control – DMEM containing 1% serum was added instead of cells. After initial transfection, the cells were incubated for 72 hours in DMEM containing 1% of serum. The cells were fixed and an indirect immunofluorescent method was used to detect the plasmid DNA expression in transfected cells. The dilutions of NDV positive serum and FITC labeled goat-anti-chicken IgG were at 1:100 and 1:2,000, respectively. All transfection experiments were performed in triplicate.
In vitro expression of pFNDV-CS-NPS
293-T cells were grown in polylysine treated 6-well plates for 24 hours. DNA was extracted from pFNDV-CS-NPs, and 4 μg of extracted plasmids were transfected into the 293-T cells according to the instructions from the Lipofectamine™ 2000 reagent kit. After 72 hours, cells were lysed with radio-immunoprecipitation assay (RIPA) solution. The lysate was centrifuged at 14,000 r/min and 4°C for 15 minutes, and the supernatant aliquots were stored in a −80°C freezer. Western-blot analysis was carried out as previously described.35 Briefly, the supernatant was mixed with 2 × sodium dodecyl sulfate (SDS) buffer and boiled for 8 minutes. After cooling to room temperature, 20 μL of the samples were loaded on a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel. After electrophoresis, proteins were transferred to a nitrocellulose membrane using a Bio-Rad Laboratories Inc (Hercules, CA, USA) semi-dry unit. The membrane was washed with PBS and blocked with 5% skimmed milk in PBS overnight, followed by incubation with NDV positive serum (hemagglutination-inhibition [HI] antibody titer 8.0 log 2) at a 1:100 dilution for 1 hour. After washing with PBS, HRP-labeled goat-anti-chicken IgG was added at a dilution of 1:2,000 for 1 hour; enhanced chemiluminescent (ECL) was used to visualize the band and the image was acquired using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Evaluation of the safety of pFNDV-CS-NPs
In vitro cytotoxicity of the pFNDV-CS-NPs
In vitro cytotoxicity of the pFNDV-CS-NPs was evaluated as previously described.36 293-T cells were cultured in DMEM and then diluted to 2×106 cells/mL. Cells were transferred to 96-well plates at 200 μL per well and cultured at 37°C for 5 hours. Fifty microliters of pFNDV-CS-NPs (diluted in DMEM culture at 1 mg/mL) were added to the wells, followed by incubation at 37°C for 2 hours. Cell culture medium was used as a positive control for cell viability. Ten microliters of WST-8 reagent was added and incubated for 5 hours. Optical density (OD)450 was measured to determine the survival rate of the cells, which was calculated using the following formula:
| [4] |
where As represents the test wells (containing cell medium, WST-8, and chitosan nanoparticles); Ac represents the control wells (containing cell medium and WST-8); and Ab represents the blank wells (containing cell medium only).
Safety of the pFNDV-CS-NPs
Thirty 4-week old SPF chickens obtained from Harbin Veterinary Research Institute Laboratory Animal Center were randomly grouped into two groups: chickens in Group 1 were immunized intranasally with the pFNDV-CS-NPs containing 200 μg plasmid DNA; chickens in Group 2 were immunized intramuscularly with 200 μg naked plasmid DNA. Any abnormal changes in chickens were continuously observed and recorded for 3 weeks.
Immunization of SPF chickens
SPF chickens at 30 days old were randomly grouped into six groups; each group had 20 chickens. Chickens in Group 1 were immunized intramuscularly with PBS; chickens in Group 2 were immunized intramuscularly with the blank CS-NP; chickens in Group 3 were immunized intranasally with the blank CS-NP; chickens in Group 4 were immunized intramuscularly with naked DNA (200 μg); chickens in Group 5 were immunized intramuscularly with the pFNDV-CS-NPs (containing 200 μg plasmid DNA); and chickens in Group 6 were immunized intranasally with the pFNDV-CS-NPs (containing 200 μg plasmid DNA). Booster immunization was performed 14 days later with the same dose.
IgG antibody in serum
Blood samples were collected from wing veins every week post primary immunization and serum was subsequently separated. The IgG levels in immune serum were measured using NDV IgG enzyme-linked immunosorbent assay (ELISA) Kit from Rapidbio Co, Ltd (West Hills, CA, USA) according to the manufacturer’s instructions.
IgA antibody assay
To evaluate the mucosal immune response, serum, tears, bile, and tracheal fluid were collected from two chickens once a week post-primary immunization. Mucosal extracts were obtained by centrifugation to collect the supernatant. IgA antibody was detected by NDV IgA ELISA Kit from Rapidbio Co, Ltd.
Lymphocyte proliferation test
Lymphocyte proliferation of the immunized chickens was conducted using 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric assay as previously described.26 All experiments were repeated three times and each was measured in triplicate. The OD570 was measured to determine the stimulation index using the following formula:
| [5] |
where OD570 T is the average value of the test group and OD570 C is the average value of the control group.
Protective efficacy
An experiment was carried out to evaluate the protective efficacy after inoculation with different vaccines. When the level of ND serum antibody of every immune group increased to 6.0 log 2, five chickens were selected at random from the six groups and infected intramuscularly with 0.1 mL of the highly virulent NDV strain F48E9 for challenge studies with a viral titer of 104.5 EID50/0.1 mL. The genotype of NDV strain F48E9 is IX, and the strain F48E9 is a highly virulent strain (mean death time [MDT] ≤60 h, intracerebral pathogenicity index [ICPI] >1.6). Clinical signs of disease and mortality were monitored on a daily basis, and continuously observed for 14 days. The infected chickens and corresponding negative control chickens were euthanized and the glandular stomach, duodenum, and bursa of Fabricius were collected for examination by histological staining.
Statistical analysis
All experiments were repeated three times and each value was measured in triplicate. Data were presented as mean values ± standard deviation (SD). Mean values were analyzed using the one-sided Student’s t-test. Differences were considered to be statistically significant at P<0.05.
Results
Optimization of the pFNDV-CS-NPs preparation conditions
The factors that affected the pFNDV-CS-NPs preparation are ranked from high to low impact: N/P ratio >chitosan concentration >Na2SO4 concentration. The optimal combination for the pFNDV-CS-NPs was a chitosan concentration of 200 μg/mL, a Na2SO4 concentration of 5.0 mmol/L, and an N/P ratio of 3:1 (Table 1). EE was 98.37%±0.87% and LC was 36.12%±0.19%. A validation test was performed in which chitosan nanoparticles containing the plasmid DNA were prepared according to the above optimal combination (n=5), and the results showed that there was no significant difference in EE (P>0.05; Table 2), indicating that the optimal combination was achieved.
Table 2.
Evaluation of entrapment efficiency and loading capacity
| Experiment number | Entrapment efficiency (%) | Loading capacity (%) |
|---|---|---|
| 1 | 99.5 | 36.8 |
| 2 | 98.2 | 35.2 |
| 3 | 97.4 | 37.1 |
| 4 | 98.7 | 38.5 |
| 5 | 99.1 | 36.7 |
| Mean value | 98.58±0.73 | 36.86±1.05 |
Note: Values are presented as mean ± standard deviation of five experiments in each group.
Characterization of the pFNDV-CS-NPs
Typical pFNDV-CS-NPs showed spherical and polydisperse nature as revealed by TEM (Figure 1A and B). The morphology of the pFNDV-CS-NPs had regular round shapes, smooth surfaces, and good dispersion, and did not have adhesion or subsidence damage. The average diameter was 199.5 nm, the particle-size dispersity was 0.336 (Figure 2A), and the zeta potential was +12.11 mV (Figure 2B).
Figure 1.
Transmission electron microscopy micrograph of the pFNDV-CS-NPs (magnification 30,000×). (A) pFNDV-CS-NPs at pH 5.5; (B) pFNDV-CS-NPs at pH 7.4.
Abbreviation: pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles.
Figure 2.
Size distribution and Zeta potential of the pFNDV-CS-NPs. (A) Measurement of these particles showed a narrow distribution of the pFNDV-CS-NPs, and the average diameter was 199.5 nm; (B) measurement of these particles showed a Zeta potential of +12.11 mV.
Abbreviation: pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles.
Stability of plasmid DNA in the pFNDV-CS-NPs
The naked plasmid DNA was degraded within 30 minutes of incubation with DNase I (Lane 3 of Figure 3). The plasmid DNA encapsulated in chitosan nanoparticles was protected from degradation by DNase I and chitosanase (Lanes 4–5 of Figure 3). The results demonstrated that chitosan encapsulation protected the DNA from DNase digestion.
Figure 3.
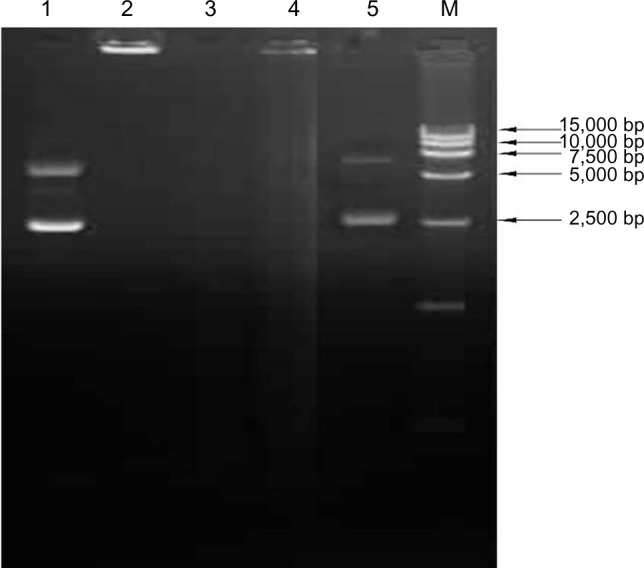
Stability analysis of the plasmid DNA after encapsulation in the chitosan nanoparticles.
Notes: Lane1: untreated naked plasmid pVAX I-optiF; Lane 2: untreated chitosan encapsulated plasmid DNA; Lane 3: naked plasmid pVAX I-optiF treated by DNase I; Lane 4: pFNDV-CS-NPs treated by DNase I; Lane 5: pFNDV-CS-NPs treated by DNase I and chitosanase; M: DNA marker DL 15000.
Abbreviations: DNA, deoxyribonucleic acid; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; pVAX I-optiF, eukaryotic expression plasmids.
In vitro release of the pFNDV-CS-NPs
The in vitro release profiles showed that the encapsulated plasmid DNA was burst released within the first 24 hours and the release amount reached 31.67% (Figure 4). From 24 to 120 hours, the release amount reached 71.73%; the plasmid DNA release amount reached 82.9% at 240 hours.
Figure 4.
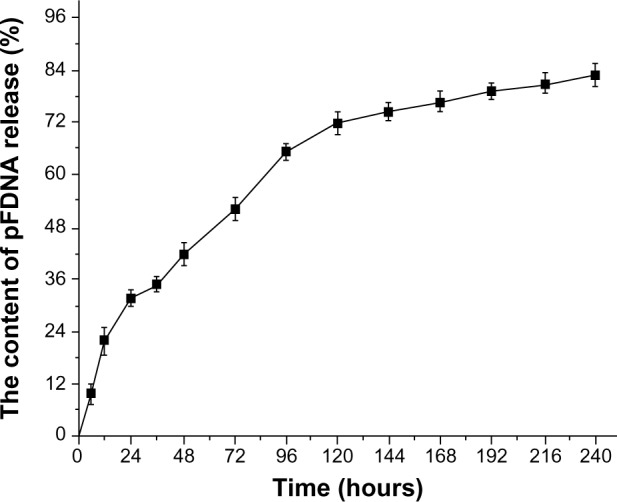
In vitro release profiles of the plasmid DNA pVAX I-optiF from pFNDV-CS-NPs.
Note: Data are presented as the mean ± standard deviation (n=3).
Abbreviations: DNA, deoxyribonucleic acid; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; pVAX I-optiF, eukaryotic expression plasmids; pFDNA, DNA in pFNDV-CS-NPs.
In vitro expression of the pFNDV-CS-NPs
As shown in Figure 5, specific fluorescence was observed in the plasmid DNA from the pFNDV-CS-NPs transfected group (Figure 5A) and the naked plasmid DNA group (Figure 5B), but the blank CS-NP group (Figure 5C) and the negative cell control group (Figure 5D) had no observable fluorescence.
Figure 5.
In vitro expression of the pFNDV-CS-NPs in 293T cells by the indirect immunofluorescence analysis (×40) and Western blot. (A) The naked plasmid DNA pVAX I-optiF group; (B) pFNDV-CS-NPs transfected group; (C) blank CS-NP group; (D) 293T cell group as the negative control; (E) Lane 1: pFNDV-CS-NPs transfected group; Lane 2: naked plasmid DNA groups; M: Protein marker; Lane 3: 293T cells as the negative control; Lane 4: blank CS-NP group.
Abbreviations: CS, chitosan; DNA, deoxyribonucleic acid; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; pVAX I-optiF, eukaryotic expression plasmids.
The antigen expression was further proven by Western blot (Figure 5E), which showed that the plasmid DNA encapsulated in the pFNDV-CS-NPs and the naked plasmid DNA expressed the expected 58 kDa antigen in 293T cells, and the blank CS-NP and the cell control group had no expressed antigen. The above results prove that the plasmid DNA encapsulated in the pFNDV-CS-NPs expressed the antigen in vitro.
Evaluation of the pFNDV-CS-NPs safety
In vitro cytotoxicity analysis of the pFNDV-CS-NPs
To test the safety of the pFNDV-CS-NPs as a DNA vaccine carrier for mucosal immune delivery system, cytotoxico-logical analysis was performed. The survival rate of chicken embryo kidney cells (CEK cells) in Group 1 (pFNDV-CS-NPs) was 84.36%±4.21%, and no significant changes in cell morphology were observed compared to control cells. The result showed that the pFNDV-CS-NPs had little cytotoxicity, but had a high safety level.
In vivo safety analysis of the pFNDV-CS-NPs
No nervous signs, clinical symptoms, or necropsy lesions were observed in chickens immunized either with the pFNDV-CS-NPs or with the naked plasmid DNA within 3 weeks post inoculation. No obvious abnormal changes were observed in these immunized chickens. These revealed that the pFNDV-CS-NPs were safe by the administration routes.
Immune efficacy of the pFNDV-CS-NPs
IgG antibody in serum
As shown in Figure 6, the antibody titers quickly increased at the second week post immunization in chickens immunized with the naked plasmid DNA injected intramuscularly (IM). The titers peaked at the fourth week post immunization, which was significantly different (P<0.05) compared to the immunized groups. Conversely, the antibody titers of chickens immunized with the pFNDV-CS-NPs IM and intranasally (IN), peaked at the fifth week post immunization and maintained a higher IgG level up to the seventh week. In addition, they were different from those immunized with the naked plasmid DNA IM (P<0.05), suggesting a sustained release profile.
Figure 6.
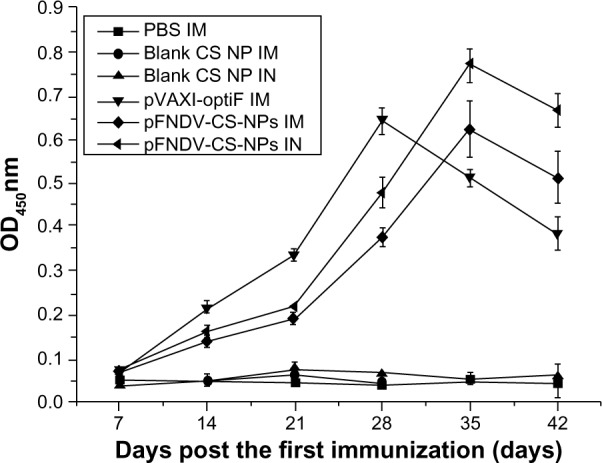
IgG antibody titers in serum of SPF chickens immunized with PBS (IM), blank CS-NP (IM), blank CS-NP (IN), and the naked plasmid DNA (IM), pFNDV-CS-NPs (IM), pFNDV-CS-NPs (IN).
Note: Data are presented as the mean ± standard deviation (n=5).
Abbreviations: CS, chitosan; DNA, deoxyribonucleic acid; IM, intramuscularly; IN, intranasally; OD, optical density; PBS, phosphate buffered saline; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; SPF, specific pathogen free; IgG, immunoglobulin G.
IgA antibody assay
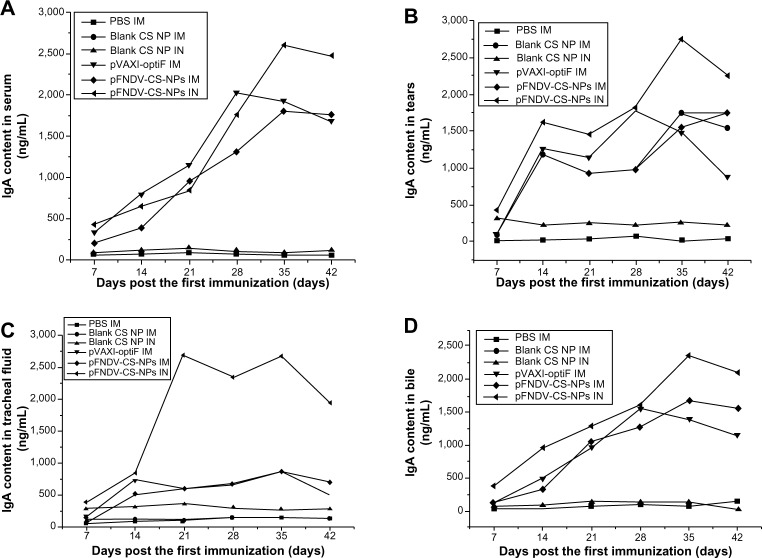
The changes of IgA content in serum, tears, tracheal fluid, and bile are shown in Figure 7. The chickens immunized with the pFNDV-CS-NPs injected IN had significantly higher IgA antibody titers (P<0.05) and a longer IgA antibody secretion period in tears (Figure 7B), tracheal fluid (Figure 7C), and bile (Figure 7D) compared to other groups. In serum, although there was no difference among the immunization groups, the IgA antibody titers were significantly higher than the blank CS-NP and PBS control groups (Figure 7A). These findings indicated that the pFNDV-CS-NPs induced quicker and better mucosal immune responses than the naked plasmid DNA vaccine.
Figure 7.
IgA antibody content in serum (A), tears (B), tracheal fluid (C), and bile (D) of SPF chickens immunized with PBS (IM), blank CS-NP (IM), blank CS-NPs (IN), and the naked plasmid DNA (IM), pFNDV-CS-NPs (IM), pFNDV-CS-NPs (IN).
Note: Data are presented as the mean ± standard deviation (n=5).
Abbreviations: CS, chitosan; DNA, deoxyribonucleic acid; IM, intramuscular; IN, intranasal; PBS, phosphate buffered saline; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles; SPF, specific pathogen free; IgA, immunoglobulin A.
Lymphocyte proliferation assay
The cell-mediated immune responses of immunized chickens were assessed by the stimulating index (SI) in the lymphocyte proliferation test at 2, 4, and 6 weeks post immunization (Table 3). The stimulation indices of chickens immunized with the pFNDV-CS-NPs either IM or IN were significantly higher than those of chickens immunized with the plasmid DNA, or with blank CS-NP (P<0.01) at the fourth and sixth weeks post immunization. The group of chickens immunized with the pFNDV-CS-NPs IN were significantly higher than the chickens immunized with the pFNDV-CS-NP IM (P<0.05). These findings indicate that the pFNDV-CS-NPs significantly enhanced immunity function of T lymphocytes in immunized chickens.
Table 3.
The stimulating index of T lymphocyte proliferation in specific pathogen free chickens after immunization
| Group | Stimulating index
|
||
|---|---|---|---|
| 14 days post primary immunization | 28 days post primary immunization | 42 days post primary immunization | |
| PBS | 1.0345±0.0115c | 1.0805±0.0065c | 1.1055±0.0035d |
| Blank CS-NPs IM | 1.0275±0.0155c | 1.2385±0.0075c | 1.3240±0.003d |
| Blank CS-NPs IN | 1.1660±0.048c | 1.3065±0.0175c | 1.4170±0.004d |
| pVAXI-opti F IM | 2.0645±0.0785a | 3.3300±0.0090a | 2.8165±0.0305c |
| pFNDV-CS-NPs IM | 1.8865±0.1005b | 2.9245±0.0515b | 3.3490±0.008b |
| pFNDV-CS-NPs IN | 2.1850±0.0400a | 3.4895±0.0585a | 4.1755±0.0375a |
Notes: Values are presented as mean ± standard deviation of five experiments in each group; values within the same column with the different lower case letter (a–d) in the superscript are significantly different (P<0.05; Student’s t-test).
Abbreviations: CS, chitosan; IM, intramuscularly; IN, intranasally; PBS, phosphate buffered saline; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles.
Protective efficacy of the pFNDV-CS-NPs
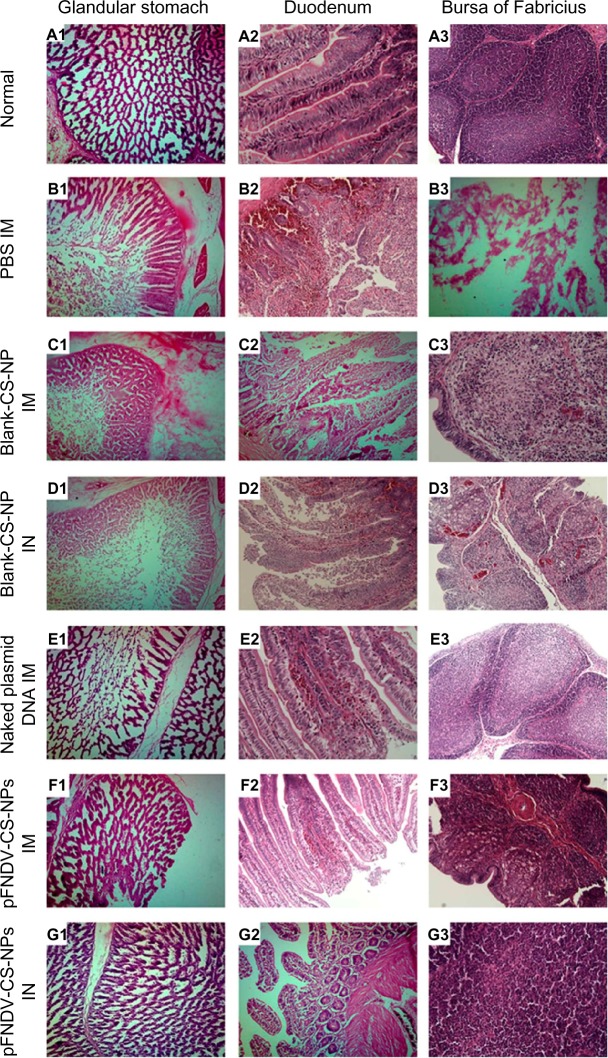
There were no clinical symptoms and no mortality in chickens immunized with the pFNDV-CS-NPs IN after challenge with the highly virulent NDV strain F48E9. The protective efficacy was 100% (Table 4). Feeding, drinking water, and mental state were normal. Pathoanatomical results showed that there were no obvious pathological changes of ND; histopathology slides of the glandular stomach, duodenum, and bursa of Fabricius showed that there were no histopathological changes (Figure 8).
Table 4.
Protective efficacy of the immunized specific pathogen free chickens after challenge with the highly virulent NDV strain F48E9
| Group | Mortality/total | Morbidity(%) | Protective efficacy (%) |
|---|---|---|---|
| PBS | 5/5 | 100 | 0 |
| Blank CS-NPs IM | 5/5 | 100 | 0 |
| Blank CS-NPs IN | 5/5 | 100 | 0 |
| pVAXI-opti F IM | 2/5 | 40 | 60 |
| pFNDV-CS-NPs IM | 1/5 | 20 | 80 |
| pFNDV-CS-NPs IN | 0/5 | 0 | 100 |
Abbreviations: CS, chitosan; IM, intramuscularly; IN, intranasally; NDV, Newcastle disease virus; PBS, phosphate buffered saline; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles.
Figure 8.
Histopathology slides of normal glandular stomach, duodenum, and bursa of Fabricius and the same organs challenged with the highly virulent NDV strain F48E9.
Notes: (A1–A3) normal tissues of the glandular stomach, duodenum, and bursa of Fabricius; (B1, C1, D1, E1, F1 and G1) tissues of the glandular stomach PBS (IM), blank CS-NP (IM), blank CS-NPs (IN), and the naked plasmid DNA (IM), pFNDV-CS-NPs (IM), pFNDV-CS-NPs (IM); (B2, C2, D2, E2, F2 and G2) tissues of the duodenum PBS (IM), blank CS-NP (IM), blank CS-NPs (IN), and the naked plasmid DNA (IM), pFNDV-CS-NPs (IM), pFNDV-CS-NPs (IN); (B3, C3, D3, E3, F3 and G3) tissues of the bursa of Fabricius PBS (IM), blank CS-NP (IM), blank CS-NPs (IN), and the naked plasmid DNA (IM), pFNDV-CS-NPs (IM), pFNDV-CS-NPs (IN).
Abbreviations: CS, chitosan; DNA, deoxyribonucleic acid; IM, intramuscularly; IN, intranasally; NP, nanoparticles; NDV, Newcastle disease virus; PBS, phosphate buffered saline; pFNDV-CS-NPs, Newcastle disease virus F gene encapsulated in chitosan nanoparticles.
Feeding, drinking water, and mental state were normal in chickens immunized with the pFNDV-CS-NPs IM after challenge with the highly virulent NDV strain F48E9, but one chicken died and the protective efficacy was 80%. Histopathology slide results showed that there were a small amount of necrocytosis in the glandular stomach, a miniscule amount of hemorrhage in the mucous layer of duodenum but with intact intestinal wall and intestinal villus, and some myelomonocytic thanatosis in the bursa of Fabricius showing vacuole-shapes.
Chickens immunized with the naked plasmid DNA group had mild clinical symptoms of lassitude, discharged yellow and green stools, and had empurpled cockscombs; the protective efficacy was 60% in chickens immunized with naked plasmid DNA. Histopathology slide results showed that there was a small amount of necrocytosis in the glandular stomach showing network structures, a miniscule amount of hemorrhage in the mucous layer of duodenum, and some follicular cell thanatosis but without noticeable reduction.
Chickens immunized with PBS and blank CS-NPs were all dead in 2–5 days after the challenge; the protective efficacy was 0% (Table 3). The dead chickens had typical pathological changes of ND, such as mucosal hemorrhages in proventriculus papillae, duodenum, heart fat, and intestine. Histopathology slide results showed that there was a large amount of necrocytosis in the glandular stomach, non-existent network structures, serious hemorrhage in the mucous layer of duodenum with incomplete intestinal wall and intestinal villus, and some follicular cell thanatosis in the bursa of Fabricius showing vacuole-shapes.
These results showed that the pFNDV-CS-NPs by intranasal route quickly induced effective mucosal immune response.
Discussion
DNA vaccines have received much attention because they offer several advantages over classical antigen vaccines. The potential for DNA vaccines to overcome maternal immunity, stability issues, costs, and the non-requirement of cold chain has highlighted the promise of DNA vaccines. The main purpose of this study was to develop an NDV DNA nanoparticle vaccine that could express the plasmid DNA of the DNA vaccine and enhance mucosal immune response and antibody immune responses compared to the naked DNA vaccine. Microencapsulation of vaccine antigens and adjuvants has been studied for years as a strategy for mucosal immunization. In this regard, biodegradable polymer microparticles have been investigated as potential carriers for more than 20 years for their role in peptide and protein antigen delivery for human diseases therapy. However, there are few reports on microencapsulation of veterinary vaccines as mucosal delivery systems. Hence, in order to study biodegradable polymers as delivery vectors for veterinary virus vaccines, by using a low-virulence live-virus vaccine against ND as a model vaccine, we prepared chitosan nanoparticles containing the low-virulence live-virus vaccine against ND (NDV-CS-NPs) using an ionic crosslinking method. The results showed that there was no damage to NDV proteins after encapsulation; better immune responses in SPF chickens immunized with NDV-CS-NPs were induced; and the release of the NDV was prolonged compared to chickens immunized with the live NDV vaccine strain La Sota.37 To further prove whether biodegradable polymers could also be used as a delivery vector for veterinary antigen genes and the prepared biodegradable polymer microparticles could realize sustained release and induce desired mucosal immunity, we prepared F gene plasmid DNA of NDV encapsulated in chitosan nanoparticles (pFNDV-CS-NPs) using a complex coacervation method. We chose chitosan as a carrier for DNA encapsulation because DNA plasmids are negatively charged and chitosan (cationic polypeptides) are positively charged, which enhances bioadhesivity and site-specific applications in controlled delivery systems.25,38 Therefore, microspheres/nanoparticles can increase the residence time of drugs in the nasal mucosa compared with solutions, and exert a direct effect on the nasal mucosa, resulting in the opening of tight junctions between the epithelial cells.39 It should be noted that microspheres/nanoparticles have been proven as an effective carrier for the delivery of drugs in nasal administration.35,40–42
The pFNDV-CS-NPs prepared using a complex coacervation method have the advantages of mild preparation conditions, no organic solvents, protection of plasmid DNA from degradation, remaining solvent in the course of nanoparticles preparation, and ease of obtaining high quality chitosan nanoparticles.43 We discussed the different concentrations of chitosan and Na2SO4 solutions, N/P ratio, and pH in the process of nanoparticle preparation. Finally, we standardized the optimal preparation conditions of the nanoparticles with a chitosan concentration of 200 μg/mL, a Na2SO4 concentration of 5.0 mmol/L, and an N/P ratio of 3:1. Under the optimal preparation conditions, the nanoparticles were produced in the desired size and the bioactivity of loaded DNA plasmid remained. The prepared pFNDV-CS-NPs showed a smooth surface, good dispersity, and no adherence or collapse phenomenon. The average size of the pFNDV-CS-NPs was 199.5 nm, which is in accordance with previously published results on the use of chitosan to form DNA complexes.44–46 The polydispersity index (PDI) was 0.336 and the Zeta potential was +12.11 mV.
It is important to determine the encapsulation efficiency for incorporating DNA into nanoparticles because it determines the effectiveness of the gene delivery and subsequent expression of encoded genes in vitro and in vivo.44 Encapsulation of DNA plasmids with chitosan to form nano-particles is dependent on factors such as molecular weight of the chitosan, N/P ratio, polymer charge density, pH, polymer structure, and degree of deacetylation.47–49 In this study, we used the complex coacervation method, which yielded a high encapsulation efficiency of 98.37%±0.87%. The loading capacity of the nanoparticles was 36.12%±0.19%, which is comparable to previously reported work.47 The pFNDV-CS-NPs also protected the plasmid DNA encapsulated in the chitosan nanoparticles from DNase digestion, as shown by electrophoresis after the enzyme digestion. These results indicate that the prepared pFNDV-CS-NPs achieved the desired objectives and provided a theoretical basis for DNA vaccine nanoparticle preparation. The reason why plasmid DNA from chitosan nanoparticles was partially digested after treatment with chitosanase and DNase I might be due to the short incubation time (30 minutes) for the two enzymes, or because the first step, digestion of chitosan nanoparticles by chitosanase, subsequently inhibited the digestion of the released plasmid DNA by DNase I, for unknown reasons.
We also studied the in vitro release of plasmid DNA from chitosan nanoparticles. Chitosan can form salts with inorganic and organic acids such as hydrochloric acid, acetic acid, and glutamic acid, but it is insoluble at neutral and alkaline pH values.50 Hu et al51 showed that the swelling of the nanoparticles and pH values of the released medium influence the release of the gene from nanoparticles and at pH 7.0–7.4 the nanoparticles are swollen to a great extent compared to the nanoparticles at pH,4.0. Therefore, the release analysis study was conducted by using pFNDV-CS-NPs swollen in PBS (pH 7.4) solution and the degree of release was observed at different time intervals. There was a burst release of the plasmid DNA between 0 and 24 hours due to solubilization of the plasmid DNA that adhered to the nanoparticles surface. From 24 to 120 hours, the plasmid DNA was continuously released, which was the main and stable release stage of the nanoparticles. After 120 hours, the release curve of pFNDV-CS-NPs flattened out, and the plasmid DNA was released almost completely (up to 82.9%±2.9%).
The in vitro cell toxicity studies showed that the pFNDV-CS-NPs had low cytotoxicity and high safety. The cells transfected with the plasmid DNA from the pFNDV-CS-NPs showed NDV specific antigen expression as detected by indirect immunofluorescence staining, and a specific 58 kDa antigen expression was detected by Western blot analysis. In vivo cytotoxicity test by oral administration and IN and IM injection immunization showed that the pFNDV-CS-NPs were safe for use, as no pathological changes were observed in immunized chickens. All the results showed that the nanoparticle production procedure was safe, and the bioactivity of the plasmid DNA remained after the production of the nanoparticles.
Analyses of IgG and IgA antibody responses from the naked plasmid DNA, pFNDV-CS-NPs, and blank chitosan nanoparticles revealed that intranasal immunization with pFNDV-CS-NPs induced stronger antibody responses than immunization with naked plasmid DNA. Intranasal immunization is very effective in eliciting mucosal and systemic immune responses.52,53 Previous studies have suggested that chitosan enhances the immunity of the vaccine by providing longer residence times in the nasal cavity and by transiently opening the tight junctions among the mucosal cells, which allows the vaccine to better access the lymphoid tissue52 and results in increased IgG production.54 Similarly, chitosan has also been shown to increase membrane permeability when used as a nasal vaccine delivery system.55 Therefore, more B cells differentiate into immunoglobulin A (IgA) plasma cells and produce more IgA antibody.56 Our results revealed that intranasal immunization of pFNDV-CS-NPs induced stronger humoral and mucosal immunity and reached the sustained release effect.
Despite recent progress, the following challenges will need to be addressed in the future: 1) trace amounts of initiator, toxic organics, and other impurities in the polymer; 2) toxic solvent left in the natural polymer during nanoparticle preparation; 3) high cost; and 4) controlled and targeted release of nanoparticles. The solutions are in sight with technological advancements in the biomedical sciences and material sciences.
Conclusion
In this study, we successfully prepared pFNDV-CS-NPs that induced significantly higher mucosal and humoral immune responses. Moreover, low-viscosity biodegradable polymer nanoparticles such as chitosan can protect the plasmid DNA from degradation and help the expression of the plasmid DNA encapsulated. These results show that the CS-NPs encapsulated plasmid DNA is a safe and efficient drug release carrier system with immense potential for medical application in the future.
Acknowledgments
We gratefully acknowledge Key Laboratory of Functional Inorganic Material Chemistry (Heilongjiang University), the Ministry of Education and Engineering Research Center of Agricultural Microbiology Technology, and the Ministry of Education for providing the facilities to carry out this work. This work was supported in part by the National Natural Science Foundation of China (31072119), Program for International Science and Technology Cooperation Projects of China (2012DFA30250), Key Project of Chinese Ministry of Education (212048), Program for New Century Excellent Talents in University (NCET-12-0707), Innovative Research Team for Agricultural Microbiology Fermentation Technology in Heilongjiang Provincial University (2012td009), Chang Jiang Scholar Candidates Program for Provincial Universities in Heilongjiang, Scientific and Technological Key Project of Heilongjiang Province (GC13B403), Early Research and Development Cultivation Project of Scientific and Technological Achievements Industrialization for Provincial Universities in Heilongjiang (1253CGZH10), Program for New Century Excellent Talents in Heilongjiang Provincial University (1251-NCET-005), Innovation Foundation of Harbin (2013RFQXJ030 and 2011RFQXN039), and Education Committee Science and Technology Research General Projects of Heilongjiang Province (12521408).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alexander DJ. Newcastle disease virus – an Avian paramyxovirus. Newcastle Disease. Developments in Veterinary Virology. 1988;8:11–22. [Google Scholar]
- 2.Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147(8):1655–1663. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 3.Steel J, Burmakina SV, Thomas C, et al. A combination in-ovo vaccine for avian influenza virus and Newcastle disease virus. Vaccine. 2008;26(4):522–531. doi: 10.1016/j.vaccine.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arifin MA, Mel M, Abdul Karim MI, Ideris A. Production of Newcastle disease virus by Vero cells grown on cytodex 1 microcarriers in a 2-litre stirred tank bioreactor. J Biomed Biotechnol. 2010;2010:586363. doi: 10.1155/2010/586363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao F, Wu Y, Zhang X, et al. Enhanced immune response and protective efficacy of a Treponema pallidum Tp92 DNA vaccine vectored by chitosan nanoparticles and adjuvanted with IL-2. Hum Vaccin. 2011;7(10):1083–1089. doi: 10.4161/hv.7.10.16541. [DOI] [PubMed] [Google Scholar]
- 6.Tretyakova I, Lukashevich IS, Glass P, Wang E, Weaver S, Pushko P. Novel vaccine against Venezuelan equine encephalitis combines advantages of DNA immunization and a live attenuated vaccine. Vaccine. 2013;31(7):1019–1025. doi: 10.1016/j.vaccine.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng LP, Liang HJ, Deng MC, et al. The influence of liposomal adjuvant on intranasal vaccination of chickens against Newcastle disease. Vet J. 2010;185(2):204–210. doi: 10.1016/j.tvjl.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Loke CF, Omar AR, Raha AR, Yusoff K. Improved protection from velogenic Newcastle disease virus challenge following multiple immunizations with plasmid DNA encoding for F and HN genes. Vet Immunol Immunopathol. 2005;106(3–4):259–267. doi: 10.1016/j.vetimm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy LC, Schmaljohn CS. DNA vaccines for biodefense. Expert Rev Vaccines. 2009;8(12):1739–1754. doi: 10.1586/erv.09.132. [DOI] [PubMed] [Google Scholar]
- 10.Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther. 2006;17(11):1051–1061. doi: 10.1089/hum.2006.17.1051. [DOI] [PubMed] [Google Scholar]
- 11.Pachuk CJ, McCallus DE, Weiner DB, Satishchandran C. DNA vaccines – challenges in delivery. Curr Opin Mol Ther. 2000;2(2):188–198. [PubMed] [Google Scholar]
- 12.Robertson JS, Griffiths E. Assuring the quality, safety, and efficacy of DNA vaccines. Mol Biotechnol. 2001;17(2):143–149. doi: 10.1385/MB:17:2:143. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Dennis VA, Pillai SR, Singh SR. RSV fusion (F) protein DNA vaccine provides partial protection against viral infection. Virus Res. 2009;145(1):39–47. doi: 10.1016/j.virusres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Hou J, Li D, et al. Enhancement of HIV-1 DNA vaccine immunogenicity by BCG-PSN, a novel adjuvant. Vaccine. 2013;31(3):472–479. doi: 10.1016/j.vaccine.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Manoj S, Babiuk LA, van Drunen Littel-van den Hurk S. Approaches to enhance the efficacy of DNA vaccines. Crit Rev Clin Lab Sci. 2004;41(1):1–39. doi: 10.1080/10408360490269251. [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Li D, Hao Y, et al. Posttranscriptional regulatory elements enhance antigen expression and DNA vaccine efficacy. DNA Cell Biol. 2009;28(5):233–240. doi: 10.1089/dna.2009.0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerdts V, Mutwiri G, Richards J, van Drunen Littel-van den Hurk S, Potter AA. Carrier molecules for use in veterinary vaccines. Vaccine. 2013;31(4):596–602. doi: 10.1016/j.vaccine.2012.11.067. [DOI] [PubMed] [Google Scholar]
- 18.Ramos EA, Relucio JL, Torres-Villanueva CA. Gene expression in tilapia following oral delivery of chitosan-encapsulated plasmid DNA incorporated into fish feeds. Mar Biotechnol (NY) 2005;7(2):89–94. doi: 10.1007/s10126-004-3018-0. [DOI] [PubMed] [Google Scholar]
- 19.de Moura MR, Aouada FA, Mattoso LH. Preparation of chitosan nano-particles using methacrylic acid. J Colloid Interface Sci. 2008;321(2):477–483. doi: 10.1016/j.jcis.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Aspden TJ, Mason JD, Jones NS, Lowe J, Skaugrud O, Illum L. Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J Pharm Sci. 1997;86(4):509–513. doi: 10.1021/js960182o. [DOI] [PubMed] [Google Scholar]
- 21.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13(15):1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida A, Nagata T, Uchijima M, Higashi T, Koide Y. Advantage of gene gun-mediated over intramuscular inoculation of plasmid DNA vaccine in reproducible induction of specific immune responses. Vaccine. 2000;18(17):1725–1729. doi: 10.1016/s0264-410x(99)00432-6. [DOI] [PubMed] [Google Scholar]
- 23.Fan H, Lin Q, Morrissey GR, Khavari PA. Immunization via hair follicles by topical application of naked DNA to normal skin. Nat Biotechnol. 1999;17(9):870–872. doi: 10.1038/12856. [DOI] [PubMed] [Google Scholar]
- 24.Chen SC, Jones DH, Fynan EF, et al. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J Virol. 1998;72(7):5757–5761. doi: 10.1128/jvi.72.7.5757-5761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao K, Shi X, Zhao Y, et al. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine. 2011;29(47):8549–8556. doi: 10.1016/j.vaccine.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima Y, Handa T, Kasai A, Takenaka H, Lin SY, Ando Y. Novel method for the preparation of controlled-release theophylline granules coated with a polyelectrolyte complex of sodium polyphosphate-chitosan. J Pharm Sci. 1985;74(3):264–268. doi: 10.1002/jps.2600740308. [DOI] [PubMed] [Google Scholar]
- 27.Li XW, Lee DK, Chan AS, Alpar HO. Sustained expression in mammalian cells with DNA complexed with chitosan nanoparticles. Biochim Biophys Acta. 2003;1630(1):7–18. doi: 10.1016/j.bbaexp.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Yin Z, Liu N, et al. DNA-chitosan nanoparticles improve DNA vaccine-elicited immunity against Newcastle disease virus through shuttling chicken interleukin-2 gene. J Microencapsul. 2010;27(8):693–702. doi: 10.3109/02652048.2010.507881. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Dai W, Wang Z, Chen B, Li Z, Fan X. Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin Vaccine Immunol. 2011;18(1):75–81. doi: 10.1128/CVI.00263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25(11):2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koppolu B, Zaharoff DA. The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials. 2013;34(9):2359–2369. doi: 10.1016/j.biomaterials.2012.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng W, Wang Y, Shi X, et al. Optimization of codon usage of F gene enhanced efficacy of Newcastle disease virus DNA vaccine. Chinese Journal of Animal Infectious Diseases. 2009;17(2):8–16. [Google Scholar]
- 33.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd ed. Beijing: Gold Spring Harbor Laboratory; 2001. [Google Scholar]
- 34.Boyoglu S, Vig K, Pillai S, et al. Enhanced delivery and expression of a nanoencapsulated DNA vaccine vector for respiratory syncytial virus. Nanomedicine. 2009;5(4):463–472. doi: 10.1016/j.nano.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Liu SW, Chen HY, Cao DJ, Lu JL. Effect on chicken lymphocytes by Newcastle disease virus. Chin J Animal Poult Infect Dis. 1998;20(3):140–142. [Google Scholar]
- 36.Aksungur P, Sungur A, Unal S, Iskit AB, Squier CA, Senel S. Chitosan delivery systems for the treatment of oral mucositis: in vitro and in vivo studies. J Control Release. 2004;98(2):269–279. doi: 10.1016/j.jconrel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhao K, Chen G, Shi XM, et al. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS One. 2012;7(12):e53314. doi: 10.1371/journal.pone.0053314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senel S, Kremer MJ, Kas¸ S, Wertz PW, Hincal AA, Squier CA. Enhancing effect of chitosan on peptide drug delivery across buccal mucosa. Biomaterials. 2000;21(20):2067–2071. doi: 10.1016/s0142-9612(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 39.Kang SW, Kim BS. PLGA microspheres in hyaluronic acid gel as a potential bulking agent for urologic and dermatologic injection therapies. J Microbiol Biotechn. 2005;15(3):510–518. [Google Scholar]
- 40.Kang ML, Kang SG, Jiang HL, et al. Chitosan microspheres containing Bordetella bronchiseptica antigens as novel vaccine against atrophic rhinitis in pigs. J Microbiol Biotechnol. 2008;18(6):1179–1185. [PubMed] [Google Scholar]
- 41.Gavini E, Hegge AB, Rassu G, et al. Nasal administration of carbamazepine using chitosan microspheres: in vitro/in vivo studies. Int J Pharm. 2006;307(1):9–15. doi: 10.1016/j.ijpharm.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Jin Z, Li W, Cao H, et al. Antimicrobial activity and cytotoxicity of N-2-HACC and characterization of nanoparticles with N-2-HACC and CMC as a vaccine carrier. Chem Eng J. 2013;221(1):331–341. [Google Scholar]
- 43.Liang HF, Chen CT, Chen SC, et al. Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials. 2006;27(9):2051–2059. doi: 10.1016/j.biomaterials.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 44.Boyoglu S, Vig K, Pillai S, et al. Enhanced delivery and expression of a nanoencapsulated DNA vaccine vector for respiratory syncytial virus. Nanomedicine. 2009;5(4):463–472. doi: 10.1016/j.nano.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51(1–3):81–96. doi: 10.1016/s0169-409x(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 46.Corsi K, Chellat F, Yahia L, Fernandes JC. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials. 2003;24(7):1255–1264. doi: 10.1016/s0142-9612(02)00507-0. [DOI] [PubMed] [Google Scholar]
- 47.Bozkir A, Saka OM. Chitosan-DNA nanoparticles: effect on DNA integrity, bacterial transformation and transfection efficiency. J Drug Target. 2004;12(5):281–288. doi: 10.1080/10611860410001714162. [DOI] [PubMed] [Google Scholar]
- 48.Mao HQ, Roy K, Troung-Le VL, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 49.Leong KW, Mao HQ, Truong-Le VL, Roy K, Walsh SM, August JT. DNA-polycation nanospheres as non-viral gene delivery vehicles. J Control Release. 1998;53(1–3):183–193. doi: 10.1016/s0168-3659(97)00252-6. [DOI] [PubMed] [Google Scholar]
- 50.Hejazi R, Amiji M. Chitosan-based gastrointestinal delivery systems. J Control Release. 2003;89(2):151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Jiang X, Ding Y, Ge H, Yuan Y, Yang C. Synthesis and characterization of chitosan-poly(acrylic acid) nanoparticles. Biomaterials. 2002;23(15):3193–3201. doi: 10.1016/s0142-9612(02)00071-6. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Dai W, Wang Z, Chen B, Li Z, Fan X. Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin Vaccine Immunol. 2011;18(1):75–81. doi: 10.1128/CVI.00263-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cripps AW, Kyd JM. Comparison of mucosal and parenteral immunisation in two animal models of pneumococcal infection: otitis media and acute pneumonia. Vaccine. 2007;25(13):2471–2477. doi: 10.1016/j.vaccine.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 54.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25(30):5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 55.McNeela EA, O’Connor D, Jabbal-Gill I, et al. A mucosal vaccine against diphtheria: formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19(9–10):1188–1198. doi: 10.1016/s0264-410x(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 56.Barnum SB, Subbarayan P, Vig K, et al. Nano-encapsulated DNA and/or protein boost immunizations increase efficiency of DNA vaccine protection against RSV. J Nanomedic Nanotechnol. 2012;3(2):1–11. [Google Scholar]