Abstract
Alcohol use disorders (AUDs) are complex and developing effective treatments will require the combination of novel medications and cognitive behavioral therapy approaches. Epidemiological studies have shown there is a high correlation between alcohol consumption and tobacco use, and the prevalence of smoking in alcoholics is as high as 80% compared to about 30% for the general population. Both preclinical and clinical data provide evidence that nicotine administration increases alcohol intake and non-specific nicotinic receptor antagonists reduce alcohol-mediated behaviors. As nicotine interacts specifically with the neuronal nicotinic acetylcholine receptor (nAChR) system, this suggests that nAChRs play an important role in the behavioral effects of alcohol. In this review, we discuss the importance of nAChRs for the treatment of AUDs and argue that the use of FDA approved nAChR ligands, such as varenicline and mecamylamine, approved as smoking cessation aids may prove to be valuable treatments for AUDs. We also address the importance of combining effective medications with behavioral therapy for the treatment of alcohol dependent individuals.
Keywords: nAChRs, ethanol, nicotine, pharmacotherapy, smoking cessation aids, varenicline, mecamylamine
INTRODUCTION
Alcohol use disorders (AUDs) are progressive relapsing disorders that affect 4% of the adult population and is the third leading cause of preventable death worldwide (National Institute on Alcohol Abuse and Alcoholism, NIAAA). The estimated economic burden of addiction-related illnesses in the United States exceeds $366 billion a year. This estimate includes direct costs (medications, treatments, welfare services and disability benefits) and indirect costs (lost productivity and income due to reduced capability or incapacity). Left untreated, the individual’s health deteriorates and further increases susceptibility to other diseases and sometimes death. There has been a growing awareness that the treatment of AUDs will require a combination of medications and behavioral interventions. Although there are a few FDA approved medications for the treatment of AUDs, including disulfiram (Antabuse®), naltrexone (ReVia®, Depade® and Vivitrol®) and acamprosate (Campral®), there remains a need to improve and develop new pharmacotherapy and treatment strategy options.
Alcohol affects multiple brain neurotransmitter and neural systems including dopamine, serotonin, GABA, glutamate, opioid, and the nicotinic acetylcholine receptors (nAChRs) [1–22]. Although, the interaction of these various pathways with each other in the presence of alcohol is largely unknown (for review, see [23]), each of these neural systems can be potential targets for the treatment of alcohol use disorders.
It is clear that ~80% of alcohol dependent individuals are also smokers and that alcohol and nicotine are commonly co-abused [24–26]. Furthermore, in animal studies, nicotine increases ethanol intake and induces reinstatement of ethanol seeking, which is reduced by a non-specific nicotinic receptor antagonist, suggesting that the nAChR system plays a role in alcohol consumption and relapse-like behaviors [18, 27–31]. Targeting the nAChR system has shown promise in modifying alcohol consumption both in preclinical and some clinical studies but the lack of suitable ligands safe for the use in humans has impeded the progress so far. There are currently two FDA approved medications that target nAChRs mecamylamine (Inversine™) and varenicline (Chantix™ and Champix™). While mecamylamine has limited efficacy as a smoking cessation medication [32, 33], varenicline has been marketed as a smoking cessation aid with excellent therapeutic efficacy [34, 35]. In this review, we discuss the role of nAChRs in the effects of ethanol and argue for the use of nicotinic receptor ligands, as a possible therapeutic strategy for the treatment of AUDs.
THE SUBTYPES OF ALCOHOL USE DISORDERS
The development of effective pharmacotherapy for AUDs requires a thorough understanding of the disorders as well as the target population. AUDs are complex and heterogeneous disorders that has been shown to be modulated by family history, co-morbidity with other substances and/or psychiatric disorders, as well as environmental influences, thus making AUDs multi-faceted and difficult to treat [36–41]. Significant effort has been put into classifying individuals with alcohol dependence and has been an ongoing process over several years by largely employing either empirical or clinical/observational strategies primarily from treatment-seeking individuals [36, 37, 42–44]. The studies were mainly conducted with people in treatment programs for alcoholism and hence a large percentage of the population with AUDs were not included, as only ~25% of alcoholics seek treatment [45].
Recently, an empirical study was carried out by NIAAA where 1,484 subjects with AUDs were sub-typed from a non-institutionalized adult population and divided into five categories [40]. The largest group (31.5%) was young adults (~25 years) with no family history of alcoholism or co-occurrence with other mental disorders and exhibited a 32% probability of smoking. These individuals rarely seek any help for their drinking. The second group was young antisocial alcoholics (~26 years, 21.1% of sample) and had an early onset of drinking and alcohol problems. More than half of the subjects (~52.5%) showed a strong family history of alcoholism with major depression, anxiety and bipolar disorder (~37%). More than 75% also smoked cigarettes. The intermediate familial group (~38 years, ~19% of US alcoholics) were middle aged with multigenerational history (~57%), smoked cigarettes (~57%) and had been diagnosed with clinical depression and bipolar disorders (~35%). The functional group (~41years, ~19% of US alcoholics) consisted of older middle-aged individuals with stable jobs and was generally well educated. Nearly 31% of this group had families with multi-generational alcoholism and major depressive illness (~24%) at some point in their lives and nearly 45% of them were smokers. The fifth chronic severe group (~38 years, ~9% of US alcoholics) had a large multigenerational history (~77%), showed antisocial personality traits and criminal-like behavior. This group was reported to have the highest rate (~55%) of other psychiatric disorders such as depression, bipolar disorder, anxiety and high rates of smoking (~75%) and administering cocaine. Nearly two-thirds of this group sought help for their drinking making them the most prevalent type in alcohol treatment. It becomes clear from these studies that most alcoholics suffer from a host of other psychiatric disorders with a high incidence of other substance use disorders of which the most prevalent is smoking (50–80% of alcoholics smoked heavily).
Sub-typing alcohol dependent individuals into clinically relevant homogeneous groups with the consideration of the age of drinking onset, drinking history, family history, and the presence or absence of other substance abuse and psychiatric disorders has the advantage of providing guidance for the treatment selection, treatment outcomes and provides with suitable prognostic indicators. The Moss et al study [40] highlights the importance of considering the underlying cause for an alcoholic to drink uncontrollably or what induces them to relapse. For example, as nicotine is widely co-abused with alcohol, it may be essential for people who want to reduce drinking, to give up smoking as well. The occurrence of alcoholism is approximately 10 times more likely in smokers than non-smokers [46]. It has been shown that early onset of smoking is a significant predictor for later development of alcohol dependence [47] and there is a considerable genetic overlap between alcohol and nicotine addiction (for review see [48]). Hence, treatments that are effective in smoking cessation could be considered as beneficial therapeutics for alcoholism. In the following section, we discuss the biological basis behind the co-occurrence of alcohol and nicotine use.
THE BIOLOGICAL RATIONALE FOR THE CONCURRENT USE OF ALCOHOL AND NICOTINE
It may be argued that the legal availability of alcohol and cigarettes (nicotine) with no associated social stigma likely contributes to their high co-use. In addition, there is strong biological evidence that underlies the combined use of alcohol and nicotine. Human and animal studies have found that alcohol and nicotine exert both synergistic and antagonistic effects on behavior which may contribute to their concurrent use. For example, animal behavioral studies have shown that chronic nicotine treatment increases ethanol consumption and self-administration in rodents [18, 27–31]. The influence of nicotine on ethanol intake has been shown to be dependent on the dose and the treatment duration. For instance, acute treatment of nicotine has been shown to initially suppress alcohol operant self-administration, while prolonged treatment with nicotine reverses this suppression and increases alcohol administration thereafter [30]. The initial suppression and the later enhancement of alcohol acquisition has been postulated to be related to the development of tolerance to nicotine across exposure days [49, 50]. Nicotine treatment has also been shown to potentiate ethanol-appropriate responding in rats suggesting a change in the perception of ethanol reinforcement induced by nicotine [51].
Reinforcement: Dopamine
Neurochemical studies have shown that sub-chronic nicotine treatment increases both nicotine and ethanol-induced dopamine release in the limbic forebrain [28]. Researchers administering acute systemic ethanol or acute nicotine in the ventral tegmental area (VTA) have shown a dose dependent increase in the dopamine release measured in the nucleus accumbens of the mesolimbic dopaminergic pathway [52–54]. A simultaneous administration of low dose of both these drugs resulted in an additive effect on the release of dopamine measured in the nucleus accumbens of the mesolimbic pathway [53, 54]. The authors postulated that the reason why most people drink and smoke simultaneously is to increase their pleasure, as reflected by the increase in dopamine in rodents. Furthermore, low concentrations of nicotine when combined with ethanol has been shown to produce a synergistic effect of increased firing rate of dopamine-containing neurons in the VTA using slice recordings [55]. The mesolimbic dopamine pathway is thought to contribute to the rewarding and reinforcing properties of both these drugs (for review see [56–58]). Marszalec and colleagues, using cultured rat cortical neurons, showed that ethanol can potentiate nicotine-activated currents and thereby increase the positive reward and reinforcing effects of nicotine. Additionally, they demonstrated that ethanol can induce an opposite effect on the desensitization of receptors induced by continuous perfusion of nicotine, which they proposed as the explanation for the increase in tobacco use observed with excessive drinking [59]. It has been postulated that the co-administration of these two drugs can cause modification in the reward pathway via an interaction with nAChRs as reported with chronic infusion of nicotine or ethanol treatment [60, 61] and thereby cause altered responses. However it is not clear whether ethanol-nicotine interactions involve changes in the nicotinic receptors [62].
Reduction of Aversive Effects Such as Stimulant and Sedative Effects of Alcohol and Nicotine
While the co-administration of ethanol and nicotine has been shown to increase the reinforcing properties of the drugs, there is also evidence that they may help to reduce the aversive side effect of each drug. The stimulant effects of nicotine are thought to offset the acute sedative effects of alcohol, such as attenuation of ethanol-induced ataxia [63] and cognitive impairment [64–66]. Measurements using different genetic strategies suggest that the nicotinic cholinergic system modulates ethanol withdrawal symptoms [67]. These effects are also manifested in alcoholic individuals as it has been shown that increased nicotine administration can modify their cognitive deficits by decreasing the reaction time and increasing the correct responding to rapid visual information processing tasks [68]. Similarly, though not systematically investigated, the neuroprotective effects of nicotine may help mitigate some of the cytotoxic effects of alcohol measured using primary cultures of cerebellar granule cells [69, 70]. The development of cross-tolerance between alcohol and nicotine may be a possible motivation to consume higher amounts of the drug to get the same reward, as demonstrated by de Fiebre and Collins and coworkers in various rodent strains selected for differential sensitivity to ethanol [62, 71–75]. Individuals who drink and smoke regularly would need to smoke and drink more to get the same pleasurable feelings. Moreover, the combined use of alcohol and tobacco is considered more risky and harmful because of the development of cross-tolerance resulting in a higher intake of alcohol and nicotine than if the drugs were consumed individually [76].
Common Genetic Susceptibility
De Fiebre and Collins and co-workers were amongst the first to study the potential common genetic influences in the development of cross-tolerance between ethanol and nicotine using long-sleep and short-sleep mice [75]. They demonstrated that mice selectively bred for differences in duration of ethanol induced “sleep time” or anesthesia (long-sleep = sensitive line and short-sleep = insensitive line) differed in their initial behavioral sensitivities (tolerance) to ethanol and nicotine in several behavioral and physiological measures. Common genetic vulnerability has been shown to influence the development of alcohol and nicotine dependence in humans as well [77–80]. Several recent human genetic association studies have demonstrated that the gene cluster CHRNA3, CHRNA5, and CHRNB4, which encode the α3, α5 and β4 subunits of the nAChRs respectively, lead to an increase in susceptibility to develop nicotine and alcohol addiction [81–88]. Human and animal studies suggest a shared genetic predisposition to develop alcohol and nicotine dependence (for review see [89]). For instance, identical twins are twice as likely as fraternal twins to be alcohol and/or nicotine dependent if the other twin is dependent [79].
Reinstatement or Relapse
Nicotine can reinstate ethanol–seeking behavior in animals [15] and provoke relapse and craving in humans [90, 91]. In a rat model of drug relapse [92], Le and colleagues (2003) demonstrated that priming the animals with nicotine injections can reinstate ethanol seeking in rats by motivating them to press the lever previously associated with ethanol deliveries [15]. Furthermore, in human studies, nicotine containing cigarettes caused at least a subset of smokers to drink more alcohol than those that had the denicotinized cigarettes [90], and additionally, cigarette smoking increased the reinforcing value of alcohol [93]. While the involvement of nicotine in ethanol mediated behaviors is well established, the mechanisms underlying such effects have not been thoroughly investigated.
NEURONAL NICOTINIC ACETYLCHOLINE RECEPTORS: A COMMON BIOLOGICAL SUBSTRATE FOR ETHANOL AND NICOTINE ACTIONS
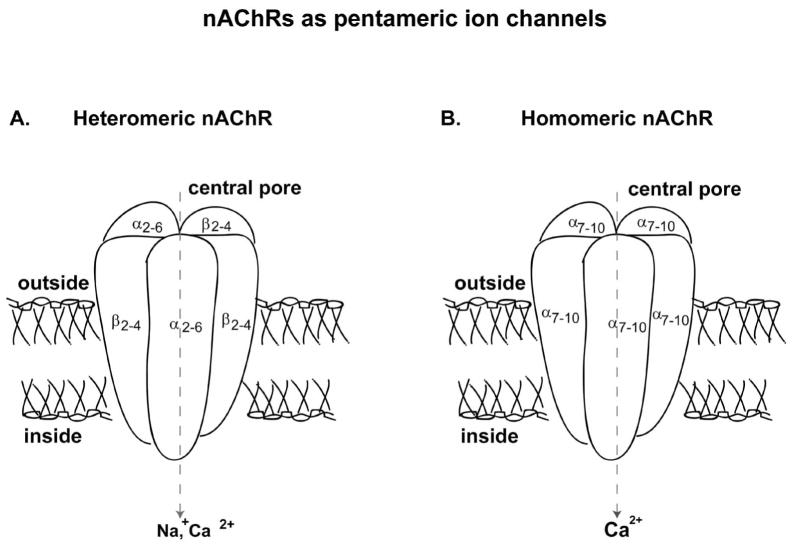
The neuronal nicotinic acetylcholine receptor (nAChR) system is a common target for the interaction of ethanol and nicotine in the brain. It was the first neurotransmitter receptor and ion channel to be purified and its molecular properties elucidated from the Torpedo electric organ [94, 95]. While Torpedos have a very rich and homogeneous source of the nAChR proteins, binding and gene expression studies have shown that nAChRs are also widely distributed in the peripheral and central nervous systems (CNS) of humans. The isolation of the nAChR followed several decades of research and discovery leading to the characterization of nAChRs as pentameric ligand-gated ion channels consisting of different combinations of α2 – α10 and β2 – β4 subunits (for review see [96–102]). The endogenous agonist acetylcholine (ACh) and the exogenous agonist nicotine are the most effective ligands at inducing the open conformation of the nAChR channel which allows cations to permeate this channel (Fig. 1).
Fig. 1.
The neuronal nicotinic acetylcholine receptor (nAChR) structure and the possible subtypes. The nAChRs are pentameric ligand-gated ion channels forming either heteromeric or homomeric receptors. The heteromeric receptors (A) are α-bungarotoxin insensitive receptors consisting of α (α2–α6) and β (β2–β4) subunits with the ligand-binding site at the interface of α and β subunits. The α-bungarotoxin insensitive receptors (B) are predominantly homomeric consisting of α7, α8 or α9 subunits and can seldom be heteromeric with α7–8 or α9–10 subunit compositions and contains five identical ligand binding site.
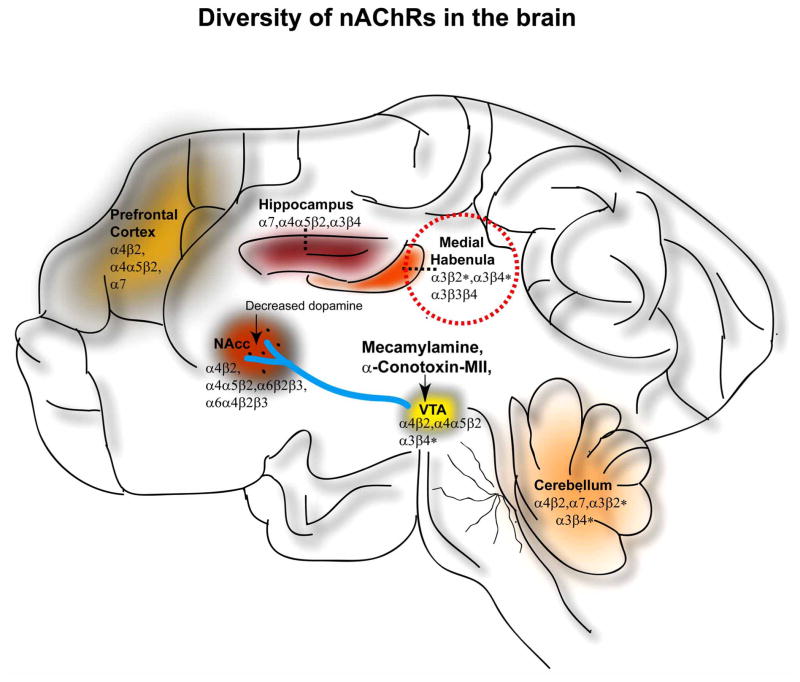
In the mammalian brain, most of the nAChRs contain both the α4* and β2* subunit that form heteromeric receptors and the homomeric α7 subunit-containing receptors [103–108]. The asterisks (i.e., α4*) here onward indicate that these subunits combine with other subunits to form functional receptors. In the peripheral nervous system, the predominant nAChR receptor subtype contains α7 and α3* subunits co-assembled with β2* or β4* subunits [109]. Although specific regions of the brain are often predominated with single classes of nAChRs, the formation of other subclasses can also occur (Fig. 2). The mesolimbic dopamine pathway consisting of the ventral tegmental area (VTA), and the nucleus accumbens (NAcc) have receptors mostly containing the α4* and β2* subunits with or without the co-assembly with α6* or α5* subunits [110, 111]. The medial habenula complex has predominantly receptors containing α3* and β4* subunits with few β2* subunits [112, 113] while α7 containing receptors are predominantly found in the hippocampus along with a few α4β2* and α3β4* containing receptors [114–116].
Fig. 2.
The diversity and localization of the possible nAChR subtypes in the brain. Several subunit compositions of nAChRs exist and are widely distributed as shown here in brain regions: ventral tegmental area (VTA), nucleus accumbens (NAcc), medial habenula, hippocampus, prefrontal cortex and cerebellum. The activation of nAChRs in the mid brain which consists of VTA and NAcc has been implicated to be important for ethanol mediated effects. Mecamylamine and α-conotoxin- MII are the only nAChR ligands that have been administered locally into the VTA resulting in a decrease of ethanol-induced dopamine overflow in the NAcc. Genetic-association studies have indicated nAChRs containing the α3*, α5* and β4* subunits to be important for alcohol dependence. Medial habenula (dashed line) indicates a brain region with the highest density of these subunits with afferent and efferent connections with the midbrain.
The importance of nAChRs in the human brain is now well established and appreciated as indicated by several excellent reviews on their molecular and functional structure [97, 117, 118], anatomical distribution [119, 120], physiology [121], as well as therapeutic indication for the treatment of several diseases such as Alzheimer’s disease, schizophrenia, epilepsy, pain, Parkinson’s disease, and nicotine and alcohol dependence [122–125].
Both ethanol and nicotine can activate the mesolimbic dopaminergic system via their actions at neuronal nicotinic acetylcholine receptors (nAChRs) [126–131]. The most widely studied role of nAChRs is in the modulation of neurotransmitter release such as dopamine, which in turn is thought to mediate the pleasurable feelings of alcohol and nicotine use. There is evidence that suggests that the mesolimbic dopaminergic system is a convergent site of action for these two drugs [52, 53]. However, it has also been suggested that nicotine and ethanol may elevate dopamine via different pathways and nAChR subtypes [132–134]. It is well established that the α4β2* nAChRs have an essential role in mediating the reinforcing properties of nicotine [135–137]. However, the subunit composition of the nAChR involved in the reinforcing effects of ethanol remains controversial. In the following section, we will discuss in detail the interaction of ethanol with the nAChR system and the various possible subunits that may be involved in its action.
INTERACTION OF ETHANOL WITH ION CHANNELS AND nAChRs
The ability of ethanol to interfere with ligand-gated ion channels [138, 139] was first demonstrated for the GABAA receptor at the behavioral [140, 141], cellular, and molecular level [142–144]. Then it was shown that ethanol can also act as an antagonist at NMDA receptors [145], a co-agonist at 5-HT3 receptors [146], as well as influence strychnine-sensitive glycine receptors [147]. The interaction of ethanol with nAChRs was first demonstrated using the Torpedo nAChR, where ethanol increased the binding affinity of acetylcholine (the endogenous ligand) to this receptor [148, 149]. It was then demonstrated that ethanol stabilized the open state of the receptor and short-chain n-alcohols increased the bursting frequency by increasing the rate of channel opening [150]. Ethanol is important for the open-close-inactivation kinetic properties of the nAChR, and several groups have examined the ethanol sensitivity at specific subunit compositions of nAChRs through both in vitro expressions in cell lines and in vivo.
Engel and colleagues (1992) were the first to establish that at least a part of the effect of ethanol is mediated by the activation of the central nAChRs, and this is a reason for the high concurrent consumption of ethanol and nicotine [151]. The investigators were able to show that the non-selective nAChR antagonist mecamylamine reduced an ethanol-induced dopamine overflow in the NAcc [28, 126, 127]. Furthermore, mecamylamine administered into the VTA but not the NAcc counteracted ethanol-induced dopamine overflow was shown using in vivo microdialysis in awake freely moving mice, suggesting the involvement of central nicotinic acetylcholine receptors for the ethanol-induced activation of the mesolimbic dopamine system (Fig. 2). Mecamylamine was first introduced as a therapeutic drug for hypertension in the 1950s [152]. It was later recognized as a potential smoking cessation aid [32, 33].
Acute ethanol treatment (75 mM) potentiates ACh-induced currents in Xenopus oocytes expressing α2β4, α4β4, α2β2 and α4β2 nAChRs [153] and low concentrations of ethanol (20 mM and 50 mM) inhibited nicotine activation of the α7 receptor [153, 154]. In contrast, the effects of ethanol on the activation of α3β4 nAChRs is more controversial, where both a potentiation of nicotine-inward current, as well as insensitivity to ethanol have been reported [153]. In rat pheochromocytoma (PC12) cells, low concentrations of ethanol (0.1–10 mM) in bath produced an increase in desensitization of nAChR responses [155]. It has been proposed that ethanol is a co-agonist that potentiates the effect of nicotine or acetylcholine but has no intrinsic agonist effect in the absence of acetylcholine, at least in cultured rat cortical neurons [59]. The ethanol concentration range (20 mM to 200 mM) used in cell culture studies and in in vitro brain slice recording [128, 156, 157] is considered behaviorally relevant [158–160]. Although it is important to note that in vitro conditions may be significantly different to behavior of a whole animal, these studies provide valuable directions in which to investigate the nAChR subunit compositions involved in ethanol-mediated behaviors.
To determine the nAChR subtype associated with the behavioral effects of ethanol a variety of different nAChR antagonists have been investigated. Dihydro-β-erythroidine (DHβE), a potent antagonist at α4β2 nAChRs, does not have any effects on systemically administered ethanol in animals; specifically the locomotor stimulatory effect and ethanol-induced dopamine overflow in the NAcc [30, 131, 133, 161]. Similarly, methyllyca-conitine citrate (MLA), a potent and selective inhibitor of α7 nAChRs, had no effect on ethanol-induced locomotion or dopamine activating effects [133]. Additionally, DHβE does not reduce ethanol intake while the non-specific nAChR antagonist mecamylamine has been shown to decrease ethanol consumption in rats [30]. In marked contrast both DHβE and MLA have been shown to play a significant role in the behavioral effects of nicotine, including decreasing the stimulant, rewarding, and dopamine-enhancing effects of nicotine [162, 163]. Furthermore, it was shown that β2 nAChR knockout mice had significantly reduced nicotine self-administration [164, 165]. These studies lead to the suggestion that the α4β2* and α7* nAChRs are not directly involved in at least the neurochemical and behavioral effects of ethanol. The search for new compounds selective at different nAChRs resulted in the discovery of α-conotoxin MII, an antagonist at α3β2*, β3* and/or α6* nAChRs [102, 134]. Larsson and coworkers found α-conotoxin MII significantly reduced ethanol-induced accumbal dopamine overflow and locomotor stimulatory effects as well ethanol intake in rats and mice (Fig. 2). A second conotoxin was synthesized, α-conotoxin PIA, to discriminate nAChRs containing the α6* and α3* subunits [166]. It was shown that, α-conotoxin PIA with selectivity at α6* containing nAChRs, did not affect the stimulatory and/or accumbal dopamine-enhancing effect of ethanol [167]. This lead to the conclusion that α3β2* and/or β3* subunits rather than α6*, α4β2*, α7* nAChRs were important for ethanol induced locomotor stimulation and the dopamine-activating effects in the midbrain (VTA) and the NAcc [167].
A caveat in the drinking models that have commonly been used in ethanol research discussed so far is that rats rarely maintain high ethanol intake following the removal of the initiation procedures such as sucrose fading [168], food and water deprivation [169], limited access to ethanol [170] and pairing drinking with electrical brain stimulation [171]. The lack of suitable preclinical oral ethanol consumption paradigms that elicit high ethanol intake has been a major obstacle in the development of new medications for the treatment of AUDs. Other attempts to increase ethanol consumption have been to either increase the concentrations of ethanol [172, 173] or to use strains that are selectively bred for high preference to ethanol [174–176]. Although there were a group of studies done in the 1970’s that showed that intermittent-access to ethanol induce high voluntary ethanol consumption [177, 178], and more recently [179, 180], most research groups have largely used continuous-access drinking models or drinking models with initiation procedures that result in low ethanol consumption. Recently, our lab re-developed the intermittent-access 20% ethanol 2-bottle choice drinking paradigm [159] that can turn standard laboratory rats into high ethanol consuming rats. This paradigm allowed for robust and reproducible levels of high voluntary ethanol consumption in Wistar, Long-Evans, as well as P rats that were maintained over long period of time (up to 8 months). The intermittent-access 20% ethanol 2-bottle choice paradigms facilitated the study of effects of high ethanol consumption as well as examining the several stages of ethanol consumption including the escalation, maintenance and deprivation of ethanol consumption. Since the development of this paradigm, which is both elegant and can be replicated, it is being increasingly used by researchers as the preclinical tool for modeling several aspects of AUDs.
Despite the introduction of this new voluntary heavy drinking model, mecamylamine and α-conotoxin-MII were the only ligands that were pharmacologically effective in determining the site and the mechanism of action of ethanol at nAChRs (Fig. 2) until 2005. Then in 2006, the FDA approved varenicline (Chantix® and Champix®) as a smoking cessation aid, providing a valuable tool for probing nAChRs. Varenicline is a high affinity, partial agonist with efficacy at α4β2* nAChRs and three orders of magnitude lower affinity for α3β4*, α3β2*, α6β2* and, 10-orders of magnitude lower affinity for α7 nAChRs [181–183].
Our lab was the first to show that varenicline selectively reduces operant ethanol self-administration and voluntary ethanol consumption in heavy drinking rats using the intermittent-access 20% ethanol 2-bottle choice drinking paradigm following long-term ethanol exposure [19]. Furthermore, we showed that varenicline has a long-lasting effect on ethanol-mediated behaviors and multiple administrations of varenicline continue to reduce ethanol self-administration without inducing a rebound increase in the ethanol seeking after the cessation of the treatment. We showed that varenicline’s effect was selective for ethanol and showed no effect on sucrose seeking or water consumption. Later, varenicline was shown to improve ethanol-associated deficits in learning in mice [184] and modulate ethanol and nicotine induced dopamine overflow in the rat NAcc [52]. Hence, in recent years, the approval of varenicline as a smoking cessation aid has provided an excellent opportunity to better understand the role of nAChRs for ethanol modulation as well as evaluate the therapeutic potential of this novel compound for the treatment of AUDs.
Recently, human genetic association studies have implicated SNPs in the genes encoding the α5* (CHRNA5), α3* (CHRNA3), β4* (CHRNB4) nAChR subunits as playing a critical role in the development alcohol and nicotine dependence processes [81–87]. Therefore, there is a need for α3β4* nAChR ligands to be developed to pharmacologically assess the role of these receptors in addictive disorders. To the best of our knowledge, 18-methoxycoronaridine (18-MC) is the only other compound that has been reported to have activity at α3β4* nAChRs [185] and to reduce ethanol- [186] and nicotine- [187] seeking behavior in rats. However, 18-MC has rather weak affinity for α3β4* nAChRs (IC50 = 0.75 μM), as well as modest affinity at opioid and 5-HT3 receptors [188]. The α3β4* nAChRs are highly expressed in the habenular complex and have much lower densities in the mesolimbic pathway [110, 189–195] (Fig. 2). Furthermore, several studies have shown that the habenular complex can regulate midbrain dopamine neuronal activity [196–200], having both afferent and efferent connections with the ventral tegmental area (VTA), substantia nigra pars compacta, and the nucleus accumbens [97, 201–207]. In the future, a concerted effort of identifying new subtype-selective ligands at nAChRs that have a pharmacologic effect on alcohol reinforcing models, together with identifying the site of action in laboratory animals will be important in the development of efficacious therapeutics for the treatment of alcoholism.
THE RATIONALE TO DEVELOP NEURONAL NICOTINIC LIGANDS AS TREATMENTS FOR ALCOHOL USE DISORDERS
It is a long road for a target such as the nAChR or an experimental drug to be moved from the bench to one of standard care for patients, ranging anywhere from 10 to 15 or more years. Clinical trials take anywhere from months to years to determine if the treatments are safe and effective in humans. Of the several drugs tested in preclinical laboratories, only a small fraction gets tested in clinical trials and often only one of these is ultimately approved as medicine for patient use.
The FDA has approved only three drugs (disulfiram (Antabuse®), naltrexone (ReVia®, Depade® and Vivitrol®) and acamprosate (Campral®)) in 51 years to treat AUDs. The good news is that there are several drugs in the pipeline either in academic laboratories or in pharmaceutical companies that show therapeutic potential. The bad news is that many of these therapies still require expensive large scale Phase III clinical trials. Given the high probability of a heavy drinker to also be a heavy smoker, approved nicotinic ligands for smoking cessation present a unique opportunity to fast track development of novel medications for the treatment of AUDs. A promising candidate that can modify alcohol reactivity and drinking behavior can have immediate clinical relevance, with the added benefit of being FDA approved and can safely be administered to alcohol and nicotine dependent individuals seeking treatment.
Previously, mecamylamine was the only FDA approved drug, with activity at nAChRs, for humans showing limited efficacy as a smoking cessation agent. Recently, varenicline was approved as a smoking cessation aid with high efficacy. Given the effect of mecamylamine and varenicline for tackling the reinforcing properties of ethanol in animal studies and small scale human studies suggests that they also may have a predictive value in the medical treatment of AUDs. In the following section, we discuss the findings leading to the discovery of mecamylamine and varenicline and their new potential clinical use as a treatment for AUDs.
CLINICAL EVALUATION OF MECAMYLAMINE (INVERSINE®) AND VARENICLINE (CHANTIX™ AND CHAMPIX™) FOR ALCOHOL USE DISORDERS
Mecamylamine
Mecamylamine (Inversine®) was first made available as an oral antihypertensive drug in the early 1950’s originally distributed by Merck & Co Inc. Due to its ability as a useful peripheral ganglionic blocking agent, it was used clinically as a drug for hypertension. However, after a few decades in 1984, it fell out of favor because of its widespread ganglionic side effects at the high recommended doses (30–70 mg/kg). Recently, mecamylamine (2.5 mg tablet) was approved to be redistributed by Layton Biosciences, albeit at lower doses to prevent side effects, for severe and uncomplicated cases of malignant hypertension [152]. Unlike other ganglionic agents, mecamylamine has been shown to be completely absorbed and effectively cross the blood brain barrier where it acts as a nAChR antagonist [208]. It is this central activity at the nAChRs, demonstrated at low doses, that has made mecamylamine emerge as a potential therapeutic agent for other clinical indications such as substance abuse disorders. Mecamylamine became one of the first and most widely used pharmacological tools because of its potential to block nicotinic receptors in the brain at low doses (2.5–10 mg/day) without any significant effect on parasympathetic functions [209]. It has been investigated over several years both in the preclinical and clinical studies for the treatment of both smoking cessation [32, 33] and ethanol abuse [126, 210].
Mecamylamine as an Aid to Smoking Cessation
Preclinical studies have shown mecamylamine reduces the rewarding properties of nicotine effectively. In humans, Rose and colleagues demonstrated that mecamylamine co-administered with transdermal nicotine is useful as a smoking cessation aid [32, 33]. In a previous study by these investigators, eight subjects were asked to evaluate qualities of cigarette smoke after various dosages of mecamylamine with their subjective response [211]. Low doses of mecamylamine were shown to decrease the self-rated strength and harshness of cigarettes having high nicotine dose level. Precessation treatment with mecamylamine prolonged the duration of continued smoking abstinence and the combined intake of nicotine skin patches (21mg/24 hr) and mecamylamine (2.5–5.0 mg/day) has been shown to reduce smoking satisfaction and craving more than each drug alone [33]. Therefore, mecamylamine combined with nicotine patch therapy is more beneficial than nicotine patch treatment alone. The combined therapy of mecamylamine and transdermal nicotine in the above studies is thought to be favorable from the balance of agonist and the antagonist effect produced, causing a reduction in the nicotine withdrawal symptoms while providing some of the rewarding effects of nicotine delivery. However, no large scale studies were done confirming these earlier reports and the combined treatment of mecamylamine and transdermal nicotine have not yet become an established treatment for smoking cessation. Mecamylamine use has been limited by its dose limiting side effects such as drowsiness, dryness of the mouth and skin, dilation of the pupil, urinary retention, and constipation.
Mecamylamine as a Drug for Alcohol Use Disorders
Mecamylamine has been an important tool in determining the importance of the interaction of ethanol and nAChRs in animals [28, 30, 126, 133, 212]. A few human studies have evaluated the effect of mecamylamine on the subjective response of healthy individuals to moderate intake of alcohol. In a small scale study [213] of 10 men and woman, healthy volunteers with no history of other substance use disorders were given alcohol-containing drinks. Two hours prior to the drink (0.8 g/kg in men and 0.7 g/kg in women) the subject were given either mecamylamine (7.5–12.5 mg on the basis of body weight) or placebo. Primary measures included the Drug Effect Questionnaire, Alcohol Sensation Scale and the stimulant subscale of the Biphasic Alcohol Effects Scale. To monitor the pharmacokinetics of the interaction of mecamylamine and alcohol, breath alcohol levels were also measured. Mecamylamine reduced the breath alcohol levels in comparison to placebo during the ascending limb of the blood alcohol levels. Mecamylamine also reduced the drug effect questionnaire and alcohol sensation scale, thereby showing the potential to reduce both the pharmacokinetic and the rewarding profile of alcohol in human subjects. Another small scale study of non-smokers demonstrated mecamylamine pre-treatment (15 mg) followed by an alcohol beverage reduced the self-reported euphoric and stimulant effects of alcohol and the desire to consume additional alcohol beverages, an effect which was more pronounced in men [214].
In a separate study, 12 healthy men and 12 healthy women volunteers were assessed for alcohol preference after being given a choice between money and alcohol following pretreatment with either mecamylamine or placebo. Mecamylamine (15 mg) treatment amongst these subjects was reported to reduce the stimulating ratings after alcohol intake, supporting the finding of the Blomqvist study [151]. When the subjects had the opportunity to choose alcohol versus money, mecamylamine did not necessarily reduce alcohol consumption in the group. However, the participants that experienced the most stimulant-like subjective effects from alcohol did show a tendency to reduce alcohol consumption with the treatment [210]. Interestingly, in this study, there was no difference in the effects of mecamylamine between the males and females.
It is encouraging that the effects of mecamylamine on the stimulant effects and the desire for alcohol in human studies draw parallel to the results in animals. It has been postulated that those individuals who experience a stimulating effect during the ascending limb and fewer sedative-like effects during the descending limb of the blood alcohol concentrations have a greater risk of having AUDs [215, 216] and mecamylamine effectively demonstrated the ability to reduce the stimulant effects of alcohol. Although the studies examining subjective alcohol reactivity are promising, a reduction in alcohol consumption with mecamylamine has yet to be demonstrated. However, the human studies, at that time, were largely limited by their small sample size when considering the variability in the behavioral and subjective response. In addition, only healthy volunteers participated in these studies and mecamylamine effect on subjects with AUDs, which are highly complex, were thought necessary. These studies were inconclusive of whether mecamylamine is more effective for men or for women as the difference could have been related to the characteristics of the participant sample such as weight, subjective stimulation from alcohol and alcohol dose. Most importantly, only the effects of acute administration of mecamylamine were demonstrated and, therefore, how well chronic administration of mecamylamine is tolerated in alcohol dependent individuals would have to be determined if mecamylamine is to be considered as a pharmacological agent for AUDs. Since these published results, new larger clinical trials are currently underway (started in 2004) initiated by Dr Petrakis and colleagues, to evaluate for the first time the treatment with mecamylamine in smoking and non-smoking alcohol dependent patients (Study NCT00342563-Yale University) (Table 1).
Table 1.
Preclinical, Small Scale Clinical Findings and Future Clinical Studies for Currently Approved Neuronal nAChRs Ligands
| Treatment | Pre-Clinical Findings | Small Scale Clinical Findings | Future Clinical Trials | |||
|---|---|---|---|---|---|---|
| Effect | Citation | Sample Size | Effect | Citation | ||
| Mecamylamine (Inversin®, marketed 1950) | Counter-acted ethanol induced dopamine overflow; Reduced ethanol intake; Reduced ethanol induced-stimulation of locomotor activity. | [30, 126, 133, 212] | 20–24 | Reduced self-reported euphoric and stimulant effects to alcohol, reduced breath alcohol levels | [210, 213, 214] | ClinicalTrials.gov Identifier: NCT00342563 Yale University Phase II: n=60; |
| Varenicline (Chantix® and Champix®, marketed 2006) | Reduced ethanol voluntary consumption and self-administration; Modulate dopamine mediated activity by ethanol and nicotine. | [19, 52] | 20 | Reduced self-reported alcohol reactivity; decreased alcohol self-administration | [222] | |
Varenicline
At the beginning of this decade, the only approved therapies available for tobacco dependence were nicotine replacement with multiple delivery methods (eg: patch, inhaler, nicotine gum and lozenge) and the antidepressant bupropion (Zyban®), which is thought to inhibit the reuptake of dopamine and norepinephrine [217, 218]. However these therapies had high long-term relapse rates [219]. The discovery of varenicline by Pfizer Inc as a smoking cessation aid (Chantix® and Champix® in 2006), was based on the need to find improved long-term efficacious treatment [220, 221]. Varenicline was developed with partial agonist activity (45% vs nicotine) and strong binding affinity (0.06 nM) at α4β2* nAChRs. Nicotine’s strong agonist activity at α4β2* nAChR leading to the dopamine activating effects in the mesolimbic pathway have been implicated in mediating several aspects of tobacco dependence. Varenicline was generated from the starting natural product cystine, with the key strategy of identifying partial agonist ligands with activity at α4β2* nAChRs for smoking cessation.
It was hypothesized that an effective agent would be one that exhibits intrinsic partial agonist activity at α4β2* nAChR eliciting a moderate sustained release of mesolimbic dopamine. It was anticipated that the intrinsic partial agonist activity would then counteract the low dopamine levels experienced in the absence of nicotine during smoking cessation attempts which mediate craving and eventually relapse to smoking behavior. The high-affinity partial agonist would have the advantage of blunting craving and withdrawal, as well as blocking nicotine-induced dopamine activation of α4β2* nAChRs by competitively binding to this receptor subtype. The blocking of the nicotine-induced elevation of dopamine would thereby prevent the reinforcing and rewarding properties of tobacco. Similar to the successful attempts of combining mecamylamine and transdermal nicotine, and thus providing a balance of agonist and antagonist, varenicline with its optimal partial agonist profile and physiochemical properties would be an optimal pharmcotherapeutic agent for patients trying to quit smoking.
Clinical Efficacy of Varenicline as a Smoking Cessation Agent
Large scale phase 3 clinical trials were conducted to determine the efficacy and safety of varenicline as a smoking cessation agent. Two different randomized double-blind parallel group placebo-active treatment-controlled trials were held between 2003 and 2005 comparing varenicline (1 mg twice daily), sustained-release bupropion (150 mg twice daily) or placebo on a 12 week treatment program and a follow-up of smoking status at week 52 [34, 35]. There were 1025 [35] and 1027 [34] healthy smokers in each study at 19 and 14 US research centers respectively. The outcome measures for the studies was exhaled carbon monoxide with measurement of continuous abstinence during the last 4 weeks of treatment (between 9 and 12 weeks) and through the non-drug follow up periods (week 9 through 24 and week 9 through 52). Varenicline was significantly more efficacious than placebo and bupropion for short-term continuous abstinence during the last 4 weeks of the treatment period as well as for long-term abstinence measured from the last 4 weeks of treatment to the non-drug period at the end of one year. Overall, varenicline was well tolerated with reduced craving and negative effects associated with smoking withdrawal in these individuals. Varenicline, marketed as CHANTIX®, became approved by regulatory agencies as an aid to smoking in USA in May 2006 based on its high binding affinity and partial agonist activity at α4β2* nAChR, good oral bioavailability, high absorbance, low plasma protein binding, and almost complete renal excretion as unchanged varenicline.
Varenicline as an Aid for Alcohol Dependence
Soon after varenicline was marketed as a smoking cessation aid, preclinical [19, 52, 184] and subsequently clinical studies investigating the pharmacotherapeutic potential of this novel drug for the treatment of AUDs were initiated. The first small scale human study of 20 heavy-drinking smokers was conducted by McKee and colleagues in a double-blind, placebo-controlled investigation to test the medication effects of varenicline on the reactivity to a priming drink and subsequent alcohol self-administration behavior [222]. After 7 days of pretreatment with varenicline (2mg/day) or placebo, a priming dose (0.3g/kg) of alcohol was administered for which the subjective and physiologic responses were assessed. This was followed with a 2-hour self-administration period during which the subjects could choose to consume up to 8 additional drinks (0.15 g/kg). In this study, varenicline significantly reduced the number of drinks compared to placebo and increased the likelihood of remaining abstinent during the 2-hour self-administrating period. Varenicline also attenuated alcohol craving and subjective reinforcing alcohol effects following the consumption of the priming alcohol drink. The authors suggested that since the effect of varenicline on drinks consumed and craving responses were similar to the effects of naltrexone using an identical laboratory paradigm [223], the observed effects, albeit in a small sample, had clinical relevance.
In this study, varenicline was well tolerated in heavy drinking smokers and the side effects experienced during the pretreatment were minimal and did not differ between the varenicline and placebo treatment groups. During the self-administration session, varenicline in combination with alcohol showed no visible effect on mood ratings, physiologic reactivity or adverse effects such as nausea, anxiety, or dizziness. Although, there are some reports that have raised concerns regarding varenicline’s association with neuropsychiatric side effects [224], a recent study showed varenicline to be effective and safe as a smoking cessation aid with or without mental illness (including alcohol dependence) [225].
Therefore, varenicline administered with low doses of alcohol appears safe and well tolerated in heavy-drinking smokers. Although this study was conducted in heavy drinking smokers, they were not deprived of cigarettes and hence varenicline’s effect was independent of nicotine withdrawal. Moreover, Bartlett and colleagues [19] have also demonstrated that the reductions in alcohol consumption and seeking with varenicline were in nicotine-naïve rats. Future studies with larger sample sizes of heavy drinkers who are smokers and non-smokers need to be conducted for the development of this compound for AUDs.
INCORPORATING OLD TREATMENT DESIGN AND STRATEGIES TO VARENICLINE FOR AN ALCOHOL DEPENDENT PERSON
So far, we have discussed the role of the nAChR system as a therapeutic target for AUDs and the emerging evidence that varenicline (Chantix® or Champix®) may represent a novel approach for the treatment of alcoholism. There is yet another strong component in the vulnerability of an alcohol dependent person which involves the individual’s behavioral components such as the drinking –abstinence- relapse history, as well as the influences of the environment. Social and cultural aspects can change the severity and the time course of this disorder. There is variability in AUDs amongst people as well as variability in the response to treatment. A single medication will not likely be effective for all those with these disorders. In addition genetic polymorphisms have been shown to lead to difference in the response to medication. Subjects with a specific allele variation have been shown to have a lower rate of relapse and longer time to heavy drinking with naltrexone than those without the genetic variation [226]. It is likely that a single approach with either medication or behavioral intervention, to treat AUDs won’t suffice as is the case with other chronic mental disorders. In a recent review, Mattson and colleagues from NIAAA highlight the important reasons and the various methods and strategies of combining therapies and/or behavioral interventions for effective alcoholism treatment [227].
One such program is Combining Medication and Behavioral Interventions (COMBINE) which is a multisite clinical trial launched by NIAAA in 1997 to determine if improvements of treatment outcomes could be achieved by combining pharmacotherapy and behavioral intervention. At the time of initiation of this program, the two most promising medications were naltrexone, which was FDA approved (1994) for alcohol dependence, and acamprosate, approved in Europe, was under consideration for review by the FDA in the United States (approved in 2006). Both medications had shown efficacy in the treatment of AUDs in placebo controlled clinical trials in the United States [228–232]. COMBINE aims to evaluate the efficacy of these medications either singly or together, with the combination of two types of behavioral treatments. The first type being Medical Management primarily delivered at primary care units by nurses to discuss medication information, to improve medication adherence and motivation and discuss any medication side effects. Secondly a more aggressive combined behavioral intervention method delivered by trained psychotherapists in specialized alcohol- treatment facilities
Since the introduction of the concept of COMBINE, various investigators have begun testing the strategies of combining medications with behavioral therapies. The first large-scale multisite randomized control trial was conducted between 2001 and 2004 amongst 1383 recently alcohol-abstinent volunteers [233] to evaluate the efficacy of the COMBINE program. Eight groups of subjects received medical management with naltrexone or acamprosate, both, and/or both placebos, with or without a combined behavioral intervention. The outcome of this clinical trial showed that subjects receiving medical management with naltrexone, combined behavioral intervention or both performed better on drinking outcomes than those that received medication management alone. Acamprosate treatment was not effective with or without behavioral support in this study. It remains controversial whether naltrexone adjunct with psychosocial intervention is superior to supportive medical advice [234–238]. Medical management of alcohol dependence with naltrexone appears to be feasible and, if implemented in primary, and other health care settings, has the potential to greatly extend patient access to effective treatment.
However, both naltrexone and acamprosate are not effective medication treatments for all subjects. With the significant advances of several new potential pharmacotherapy interventions such as the neurokinin 1 (NK1) receptor antagonist LY686017 [239], 5-HT receptor antagonist ondansetron [240, 241] and now varenicline, studies need to be initiated on a systematic basis to test these new medications in combination with the existing options, as well as singly with effective behavioral therapies. Clinical trials should be initiated by both the pharmaceutical industry and academia alike to evaluate the efficacy of these treatments. With the high co-occurrence of smokers amongst heavy alcohol drinkers, varenicline with its convenient administration route can be made available at primary care settings. Varenicline with the support of medical therapy can potentially be suggested for those alcohol dependent individuals who are seeking help and show no adverse treatment effects.
In addition, medications that target different drinking behaviors could be administered in combination. For example, acamprosate which is known to attenuate alcohol craving, could be combined with varenicline that attenuates the rewarding properties of alcohol. Similar approaches with acamprosate and naltrexone have been studied previously. The naltrexone-acamprosate combination was shown to be superior to acamprosate alone but not to naltrexone alone [242, 243]. Similarly, varenicline and the NK1 receptor antagonist LY686017 with antiemetic properties, both targeting the rewarding properties of alcohol but not the same biological substrate (nAChRs vs neurokinin-1 receptor) could be combined to evaluate if the efficacy of the medication in combination is superior to either of them alone. Combinational drug trials use suboptimal dosages to get the desired effect and could produce reduced toxicity and side effects as an added benefit, especially since a large subset of alcohol dependent individuals can have other concurrent psychiatric or chronic disorders. These combinational therapies could be administered along with behavioral programs to engage subjects to adhering to the medications. Such protocols that pay attention to the subjects’ satisfaction with medication and the likelihood for them to adhere to the regimen have been established [244].
SIDE EFFECTS OF VARENICLINE USE
Although varenicline is the most effective smoking cessation aid available, anecdotal and case study reports of suicidal and other psychiatric adverse events have generated concerns for varenicline use [245–249]. The FDA has recently made a requirement for a boxed warning on the drug’s label to alert patients and physicians of the potential risks of behavioral changes, depression, aggression and suicidal thoughts [245]. FDA has urged the manufacturer of varenicline (Pfizer) to conduct randomized controlled trials that include individuals with pre-existing mental health conditions which forms a high proportionate of smokers. The belief is that the risks of taking varenicline needs to be weighed against the health benefits from quitting smoking [249–251].
To address the growing concerns of the risk of suicidal behavior associated with varenicline, a very large scale study (n=80,660) of men and women between the ages 18–95 was recently conducted [252]. Gunnell and colleagues (2009) measured outcomes such as fatal and nonfatal self-harm, suicidal thoughts, and depression following varenicline prescription and alternative smoking cessation treatments such as bupropion and nicotine replacement products (transdermal patch, inhaler, nasal spray, gum or lozenge). In this study, the authors found no clear evidence that varenicline treatment produced an increased risk of self-harm, depression or suicidal thoughts, compared with the alternative smoking cessation treatments. Additionally, others studies have shown that individuals, including some mentally-ill subjects, have benefited from taking varenicline with negligible side effects, with no worsening of neuropsychiatric symptoms or mood disturbances [225, 246, 253–257]. Therefore, it may be that varenicline is effective as a smoking cessation aid for some individuals and not for others, and patients prescribed this drug need to be monitored carefully for behavioral changes to prevent any serious psychiatric outcomes. It is worth noting that for the treatment of AUDs, the effect of varenicline appears to be effective, safe, and well tolerated in heavy-drinking smokers, albeit in a small scale study [222]; future large scale study is imperative.
DRUG ADDICTION, DECISION MAKING, AND nAChRs
Another aspect of prolonged drug exposure involves the modification of brain circuits that are crucial for the normal control of behavior, including the decision-making process, as demonstrated by a growing number of studies [258–260]. In this section, we will briefly discuss the effect of chronic use of alcohol on neural circuits regulating cognitive activities such as decision-making, behavioral inhibition and problem solving and the potential use of nAChR-targeted compounds to improve cognitive functions impaired by alcohol use in these brain regions.
The prefrontal cortex, which is the anterior part of the frontal lobes in the brain, is a highly evolved region involved in cognitive functions that are central to drug and alcohol dependence (see review see [261]). Apart from alcohol’s affect in modulating the rewarding sensation through the brain dopamine system, prolonged alcohol use has also been shown to alter frontal cortex structure and function impairing decision making and response inhibition operations [262–264]. For example, imaging studies in substance users reveal abnormalities in blood flow in the orbitofrontal cortex (OFC) which is part of the prefrontal cortex [265, 266]; over-activation of the OFC correlates with craving [267]. In addition, a reduction in spine density in the OFC of rat brain during abstinence from amphetamine self-administration has been shown [268]. An overall decline in frontal lobe function is seen in alcohol-dependent individuals with associated cognitive impairment [269–271]. During a decision making process, such as Iowa Gambling Task [272], alcohol dependent individuals were shown to have significant impairment in their performance compared with control subjects [273]. Studies have shown that active and abstinent alcoholics exhibit performance favoring larger immediate rewards while disregarding long-term consequences [266, 272, 274–276]. Compromised decision-making is also observed with other substance use such as cocaine, amphetamine, and heroin with functional abnormalities in the prefrontal neural circuitry [277–280]. The poor decision-making and behavioral inhibition to negative consequences of excessive drinking is thought to be a factor in compulsive drug-seeking behavior [281]. While cognitive deficits play a factor in developing alcohol dependence, a longitudinal study has shown that continuous substance use during adolescence can lead to larger neurocognitive deficits [282]. Investigators in this field hypothesize that the extent of decision-making impairment may reflect vulnerabilities to drug addiction and enhancement of these cognitive tasks may be important for treatment.
Understanding the role of nAChRs in cognitive functions has made progress over the years [283–285]. Cholinergic projections from the nucleus basalis magnocellularis to the cortex is thought to be particularly important in cognitive processes [286] (for review see [287]). The nAChRs target ligands have been of great interest for drug discovery research especially with regard to their capacity as cognitive enhancers [288–291] for diseases such as Alzheimer’s disease, Parkinson’s disease, attention deficit/hyperactivity disorder and schizophrenia [292–294]. Cognitive enhancers have been shown to facilitate the detection of signals by augmenting the transient increase of prefrontal cholinergic activity via the modulation of glutamatergic neurotransmission in an attention behavioral context [295]. nAChR target compounds can modulate the release of several neurotransmitters [127] and can improve cognitive function through the interaction of various neurotransmitter systems. This has lead to the development of several nicotinic compounds (ABT-418, SIB-1508Y, SIB-1553A, GTS-21, AZD3480, AZD1446) synthesized in various biotech and pharmaceutical companies, and the initiation of clinical trials for the above mentioned indications.
Given the large number of studies that have demonstrated cognitive impairment in alcohol-dependent individuals, novel nAChRs ligands that improve cognitive dysfunction should be developed. In order to find a treatment for AUD, serious initiatives with clinical evaluations of such ligands are imperative. A systematic screening of approved nicotinic ligands, such as those mentioned previously, with demonstrated safety in humans needs to be initiated in patients with alcohol dependence.
CONCLUSIONS
Lastly, even with the significant advances made both in preclinical and clinical settings, only about of 10% to 15% of those affected with alcohol use disorders get treated, mostly with the help of social and behavioral therapy. There is strong evidence from both biology and behavioral studies to show that alcohol addiction cannot be resolved with will power alone. Medication along with cognitive behavioral therapy is important and needs to be implemented by practitioners as part of the standard treatment options present to patients and their family as soon as possible in our society.
Acknowledgments
We thank Carolina Haass-Koffler, Jade Bito-Onon, Jeffrey Simms, and Joan Holgate for critiquing the manuscript. This work was supported by funding from the National Institutes of Health, RC2AA019429-01 (to S.E.B.), Department of Defense, #W81XWH-08-1-0016 (to S.E.B) and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (to S.E.B).
ABBREVIATIONS
- AUDs
Alcohol use disorders
- ACh
Acetylcholine
- CNS
Central nervous system
- FDA
US Food and Drug Administration
- MHb
Medial habenula
- nAChRs
Nicotinic acetylcholine receptors
- NAcc
Nucleus accumbens
- OFC
Orbitofrontal cortex
- VTA
Ventral tegmental area
References
- 1.Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- 2.Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, Little HJ. Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology. 2001;41:989–999. doi: 10.1016/s0028-3908(01)00146-0. [DOI] [PubMed] [Google Scholar]
- 3.Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 4.Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn-Schmiedebergs Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- 5.Bisaga A, Sikora J, Kostowski W. The effect of drugs interacting with serotonergic 5HT3 and 5HT4 receptors on morphine place conditioning. Pol J Pharmacol. 1993;45:513–519. [PubMed] [Google Scholar]
- 6.Borg PJ, Taylor DA. Involvement of mu- and delta-opioid receptors in the effects of systemic and locally perfused morphine on extracellular levels of dopamine, DOPAC and HVA in the nucleus accumbens of the halothane-anaesthetized rat. Naunyn- Schmiedebergs Arch Pharmacol. 1997;355:582–588. doi: 10.1007/pl00004987. [DOI] [PubMed] [Google Scholar]
- 7.Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- 8.Charness ME, Simon RP, Greenberg DA. Ethanol and the nervous system. N Engl J Med. 1989;321:442–454. doi: 10.1056/NEJM198908173210706. [DOI] [PubMed] [Google Scholar]
- 9.Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- 10.Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- 12.Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCe. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman PL, Urwyler S, Tabakoff B. Alterations in opiate receptor function after chronic ethanol exposure. J Pharmacol Exp Ther. 1982;222:182–189. [PubMed] [Google Scholar]
- 14.Janak PH, Wolf FW, Heberlein U, Pandey SC, Logrip ML, Ron D. BIG news in alcohol addiction: new findings on growth factor pathways BDNF, insulin, and GDNF. Alcohol Clin Exp Res. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- 15.Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol. 1999;368:9–16. doi: 10.1016/s0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 17.Smith BR, Boyle AE, Amit Z. The effects of the GABAB agonist baclofen on the temporal and structural characteristics of ethanol intake. Alcohol. 1999;17:231–240. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- 18.Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berl) 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- 19.Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an a4b2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 24.Istvan J, Matarazzo JD. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychol Bull. 1984;95:301–326. [PubMed] [Google Scholar]
- 25.Sobell LC, Sobell MB, Kozlowski LT, Toneatto T. Alcohol or tobacco research versus alcohol and tobacco research. Br J Addict. 1990;85:263–269. doi: 10.1111/j.1360-0443.1990.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 26.Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the national epidemiologic survey on alcohol and related conditions. Alcohol Res Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- 27.Potthoff AD, Ellison G, Nelson L. Ethanol intake increases during continuous administration of amphetamine and nicotine, but not several other drugs. Pharmacol Biochem Behav. 1983;18:489–493. doi: 10.1016/0091-3057(83)90269-1. [DOI] [PubMed] [Google Scholar]
- 28.Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 29.Clark A, Lindgren S, Brooks SP, Watson WP, Little HJ. Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology. 2001;41:108–117. doi: 10.1016/s0028-3908(01)00037-5. [DOI] [PubMed] [Google Scholar]
- 30.Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- 31.Olausson P, Ericson M, Lof E, Engel JA, Soderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. Eur J Pharmacol. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- 32.Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- 33.Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy. Exp Clin Psychopharmacol. 1998;6:331–343. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- 34.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an a4b2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 35.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an a4b2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 36.Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- 37.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 38.Lesch OM, Dietzel M, Musalek M, Walter H, Zeiler K. The course of alcoholism. Long-term prognosis in different types. Forensic Sci Intl. 1988;36:121–138. doi: 10.1016/0379-0738(88)90225-3. [DOI] [PubMed] [Google Scholar]
- 39.Jacob T, Bucholz KK, Sartor CE, Howell DN, Wood PK. Drinking trajectories from adolescence to the mid-forties among alcohol dependent males. J Stud Alcohol. 2005;66:745–755. doi: 10.15288/jsa.2005.66.745. [DOI] [PubMed] [Google Scholar]
- 40.Moss HB, Chen CM, Yi HY. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Windle M, Scheidt DM. Alcoholic subtypes: are two sufficient? Addiction. 2004;99:1508–1519. doi: 10.1111/j.1360-0443.2004.00878.x. [DOI] [PubMed] [Google Scholar]
- 42.Bohman M, Wennberg P, Andersson T. Alcohol habits in a suburban male cohort. Scand J Public Health. 2000;28:275–282. [PubMed] [Google Scholar]
- 43.Chung T, Martin CS. Classification and course of alcohol problems among adolescents in addictions treatment programs. Alcohol Clin Exp Res. 2001;25:1734–1742. [PubMed] [Google Scholar]
- 44.Zucker RA, Davies WH, Kincaid SB, Fitzgerald HE, Reider EE. Conceptualizing and scaling the developmental structure of behavior disorder: the lifetime alcohol problems score as an example. Dev Psychopathol. 1997;9:453–471. doi: 10.1017/s0954579497002125. [DOI] [PubMed] [Google Scholar]
- 45.Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 46.Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, Jr, Morse RM, Palmen MA, Bruce BK. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res. 1994;18:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 47.Grant BF. Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subs Abuse. 1998;10:59–73. doi: 10.1016/s0899-3289(99)80141-2. [DOI] [PubMed] [Google Scholar]
- 48.Schlaepfer IR, Hoft NR, Ehringer MA. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev. 2008;1:124–134. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology (Berl) 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- 50.Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–342. doi: 10.1007/BF00429192. [DOI] [PubMed] [Google Scholar]
- 51.Signs SA, Schechter MD. Nicotine-induced potentiation of ethanol discrimination. Pharmacol Biochem Behav. 1986;24:769–771. doi: 10.1016/0091-3057(86)90589-7. [DOI] [PubMed] [Google Scholar]
- 52.Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther. 2009;329(1):225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- 53.Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- 54.Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- 55.Clark A, Little HJ. Interactions between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend. 2004;75:199–206. doi: 10.1016/j.drugalcdep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 57.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 58.Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–112. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- 59.Marszalec W, Aistrup GL, Narahashi T. Ethanol-nicotine interactions at a-bungarotoxin-insensitive nicotinic acetylcholine receptors in rat cortical neurons. Alcohol Clin Exp Res. 1999;23:439–445. [PubMed] [Google Scholar]
- 60.Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- 61.Yoshida K, Engel J, Liljequist S. The effect of chronic ethanol administration of high affinity 3H-nicotinic binding in rat brain. Naunyn-Schmied Arch Pharmacol. 1982;321:74–76. doi: 10.1007/BF00586353. [DOI] [PubMed] [Google Scholar]
- 62.Collins AC, Wilkins LH, Slobe BS, Cao JZ, Bullock AE. Long-term ethanol and nicotine treatment elicit tolerance to ethanol. Alcohol Clin Exp Res. 1996;20:990–999. doi: 10.1111/j.1530-0277.1996.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 63.Al-Rejaie S, Dar MS. Antagonism of ethanol ataxia by intracerebellar nicotine: possible modulation by mouse cerebellar nitric oxide and cGMP. Brain Res Bull. 2006;69:187–196. doi: 10.1016/j.brainresbull.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Gould TJ, Lommock JA. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav Neurosci. 2003;117:1276–1282. doi: 10.1037/0735-7044.117.6.1276. [DOI] [PubMed] [Google Scholar]
- 65.Gould TJ, Collins AC, Wehner JM. Nicotine enhances latent inhibition and ameliorates ethanol-induced deficits in latent inhibition. Nicotine Tob Res. 2001;3:17–24. doi: 10.1080/14622200020032060. [DOI] [PubMed] [Google Scholar]
- 66.Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- 67.Butt CM, King NM, Stitzel JA, Collins AC. Interaction of the nicotinic cholinergic system with ethanol withdrawal. J Pharmacol Exp Ther. 2004;308:591–599. doi: 10.1124/jpet.103.059758. [DOI] [PubMed] [Google Scholar]
- 68.Ceballos NA, Tivis R, Lawton-Craddock A, Nixond SJ. Nicotine and cognitive efficiency in alcoholics and illicit stimulant abusers: implications of smoking cessation for substance users in treatment. Subst Use Misuse. 2006;41:265–281. doi: 10.1080/10826080500409076. [DOI] [PubMed] [Google Scholar]
- 69.Tizabi Y, Manaye KF, Smoot DT, Taylor RE. Nicotine inhibits ethanol-induced toxicity in cultured cerebral cortical cells. Neurotox Res. 2004;6:311–316. doi: 10.1007/BF03033441. [DOI] [PubMed] [Google Scholar]
- 70.Tizabi Y, Al-Namaeh M, Manaye KF, Taylor RE. Protective effects of nicotine on ethanol-induced toxicity in cultured cerebellar granule cells. Neurotox Res. 2003;5:315–321. doi: 10.1007/BF03033151. [DOI] [PubMed] [Google Scholar]
- 71.Collins AC, Romm E, Selvaag S, Turner S, Marks MJ. A comparison of the effects of chronic nicotine infusion on tolerance to nicotine and cross-tolerance to ethanol in long- and short-sleep mice. J Pharmacol Exp Ther. 1993;266:1390–1397. [PubMed] [Google Scholar]
- 72.Collins AC, Burch JB, de Fiebre CM, Marks MJ. Tolerance to and cross tolerance between ethanol and nicotine. Pharmacol Biochem Behav. 1988;29:365–373. doi: 10.1016/0091-3057(88)90170-0. [DOI] [PubMed] [Google Scholar]
- 73.de Fiebre CM, Collins AC. A comparison of the development of tolerance to ethanol and cross-tolerance to nicotine after chronic ethanol treatment in long- and short-sleep mice. J Pharmacol Exp Ther. 1993;266:1398–1406. [PubMed] [Google Scholar]
- 74.Burch JB, de Fiebre CM, Marks MJ, Collins AC. Chronic ethanol or nicotine treatment results in partial cross-tolerance between these agents. Psychopharmacology (Berl) 1988;95:452–458. doi: 10.1007/BF00172954. [DOI] [PubMed] [Google Scholar]
- 75.de Fiebre CM, Marks MJ, Collins AC. Ethanol-nicotine interactions in long-sleep and short-sleep mice. Alcohol. 1990;7:249–257. doi: 10.1016/0741-8329(90)90014-4. [DOI] [PubMed] [Google Scholar]
- 76.Zacny JP. Behavioral aspects of alcohol-tobacco interactions. Recent Dev Alcohol. 1990;8:205–219. [PubMed] [Google Scholar]
- 77.Bierut LJ, Schuckit MA, Hesselbrock V, Reich T. Co-occurring risk factors for alcohol dependence and habitual smoking. Alcohol Res Health. 2000;24:233–241. [PMC free article] [PubMed] [Google Scholar]
- 78.Madden PA, Heath AC. Shared genetic vulnerability in alcohol and cigarette use and dependence. Alcohol Clin Exp Res. 2002;26:1919–1921. doi: 10.1097/01.ALC.0000040960.15151.30. [DOI] [PubMed] [Google Scholar]
- 79.True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 80.Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 81.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, Neale MC, Kendler KS. Variants in nicotinic acetylcholine receptors a5 and a3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(7):926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grucza RA, Wang JC, Stitzel JA, Hinrichs AL, Saccone SF, Saccone NL, Bucholz KK, Cloninger CR, Neuman RJ, Budde JP, Fox L, Bertelsen S, Kramer J, Hesselbrock V, Tischfield J, Nurnberger JI, Jr, Almasy L, Porjesz B, Kuperman S, Schuckit MA, Edenberg HJ, Rice JP, Goate AM, Bierut LJ. A risk allele for nicotine dependence in CHRNA5 is a protective allele for cocaine dependence. Biol Psychiatry. 2008;64:922–929. doi: 10.1016/j.biopsych.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 150B(4):453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci US A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, Thun MJ, Goate A, Calle EE. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–3525. doi: 10.1158/1055-9965.EPI-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamens HM, McKinnon CS, Li N, Helms ML, Belknap JK, Phillips TJ. The a3 subunit of the nicotinic acetylcholine receptor is a candidate gene for ethanol stimulation. Genes Brain Behav. 2009 doi: 10.1111/j.1601-183X.2008.00444.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- 90.Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 91.Marks JL, Hill EM, Pomerleau CS, Mudd SA, Blow FC. Nicotine dependence and withdrawal in alcoholic and nonalcoholic ever-smokers. J Subst Abuse Treatment. 1997;14:521–527. doi: 10.1016/s0740-5472(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 92.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 93.Perkins KA, Fonte C, Grobe JE. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behav Pharmacol. 2000;11:63–70. doi: 10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt J, Raftery MA. Purification of acetylcholine receptors from Torpedo californica electroplax by affinity chromatography. Biochemistry. 1973;12:852–856. doi: 10.1021/bi00729a011. [DOI] [PubMed] [Google Scholar]
- 95.McNamee MG, Weill CL, Karlin A. Purification of acetylcholine receptor from Torpedo californica and its incorporation into phospholipid vesicles. Ann NY Acad Sci. 1975;264:175–182. doi: 10.1111/j.1749-6632.1975.tb31482.x. [DOI] [PubMed] [Google Scholar]
- 96.Sargent PB. The diversity of neuronal nicotinic acetylcholine receptors. Annu Rev Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [DOI] [PubMed] [Google Scholar]
- 97.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 98.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luetje CW, Patrick J, Seguela P. Nicotine receptors in the mammalian brain. FASEB J. 1990;4:2753–2760. doi: 10.1096/fasebj.4.10.2197155. [DOI] [PubMed] [Google Scholar]
- 100.Patrick J, Sequela P, Vernino S, Amador M, Luetje C, Dani JA. Functional diversity of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1993;98:113–120. doi: 10.1016/s0079-6123(08)62387-0. [DOI] [PubMed] [Google Scholar]
- 101.Colquhoun LM, Patrick JW. Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv Pharmacol. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- 102.Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of a4 and b2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 104.Zoli M, Lena C, Picciotto MR, Changeux JP. Identification of four classes of brain nicotinic receptors using b2 mutant mice. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Le Novere N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- 106.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 107.Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pabreza LA, Dhawan S, Kellar KJ. [3H]cytisine binding to nicotinic cholinergic receptors in brain. Mol Pharmacol. 1991;39:9–12. [PubMed] [Google Scholar]
- 109.Gotti C, Fornasari D, Clementi F. Human neuronal nicotinic receptors. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 110.Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RA. a3b4 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- 113.Fonck C, Nashmi R, Salas R, Zhou C, Huang Q, De Biasi M, Lester RA, Lester HA. Demonstration of functional a4-containing nicotinic receptors in the medial habenula. Neuropharmacology. 2009;56:247–253. doi: 10.1016/j.neuropharm.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grybko M, Sharma G, Vijayaraghavan S. Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J Mol Neurosci. 2009 doi: 10.1007/s12031-009-9266-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adams CE, Stitzel JA, Collins AC, Freedman R. a7-nicotinic receptor expression and the anatomical organization of hippocampal interneurons. Brain Res. 2001;922:180–190. doi: 10.1016/s0006-8993(01)03115-8. [DOI] [PubMed] [Google Scholar]
- 116.Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F. Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci. 1994;6:1596–1604. doi: 10.1111/j.1460-9568.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 117.Lindstrom J, Schoepfer R, Conroy WG, Whiting P. Structural and functional heterogeneity of nicotinic receptors. Ciba Found Symp. 1990;152:23–42. doi: 10.1002/9780470513965.ch3. [DOI] [PubMed] [Google Scholar]
- 118.Deneris ES, Connolly J, Rogers SW, Duvoisin R. Pharmacological and functional diversity of neuronal nicotinic acetylcholine receptors. Trends Pharmacol Sci. 1991;12:34–40. doi: 10.1016/0165-6147(91)90486-c. [DOI] [PubMed] [Google Scholar]
- 119.Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. doi: 10.1002/neu.10153. [DOI] [PubMed] [Google Scholar]
- 120.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 121.McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- 122.D’Hoedt D, Bertrand D. Nicotinic acetylcholine receptors: an overview on drug discovery. Expert Opin Ther Targets. 2009;13:395–411. doi: 10.1517/14728220902841045. [DOI] [PubMed] [Google Scholar]
- 123.Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 124.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 125.Vazquez-Palacios G, Bonilla-Jaime H. Nicotine acetylcholine receptors and neuropsychiatric disorders. Rev Neurol. 2004;39:1146–1160. [PubMed] [Google Scholar]
- 126.Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 127.Blomqvist O, Ericson M, Engel JA, Soderpalm B. Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- 128.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- 129.Clarke PB, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988;246:701–708. [PubMed] [Google Scholar]
- 130.Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- 131.Larsson A, Edstrom L, Svensson L, Soderpalm B, Engel JA. Voluntary ethanol intake increases extracellular acetylcholine levels in the ventral tegmental area in the rat. Alcohol Alcohol. 2005;40:349–358. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- 132.Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- 133.Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- 134.Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA. Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol. 2004;34:239–250. doi: 10.1016/j.alcohol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 135.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 136.Picciotto MR, Zoli M, Zachariou V, Changeux JP. Contribution of nicotinic acetylcholine receptors containing the b2-subunit to the behavioural effects of nicotine. Biochem Soc Trans. 1997;25:824–829. doi: 10.1042/bst0250824. [DOI] [PubMed] [Google Scholar]
- 137.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of a4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- 138.Grant KA. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav Pharmacol. 1994;5:383–404. doi: 10.1097/00008877-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 139.Lovinger DM. Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:267–282. doi: 10.1007/pl00005051. [DOI] [PubMed] [Google Scholar]
- 140.Cott J, Carlsson A, Engel J, Lindqvist M. Suppression of ethanol-induced locomotor stimulation by GABA-like drugs. Naunyn-Schmiedebergs Arch Pharmacol. 1976;295:203–209. doi: 10.1007/BF00505087. [DOI] [PubMed] [Google Scholar]
- 141.Liljequist S, Engel J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology (Berl) 1982;78:71–75. doi: 10.1007/BF00470592. [DOI] [PubMed] [Google Scholar]
- 142.Allan AM, Harris RA. Gamma-aminobutyric acid and alcohol actions: neurochemical studies of long sleep and short sleep mice. Life Sci. 1986;39:2005–2015. doi: 10.1016/0024-3205(86)90324-3. [DOI] [PubMed] [Google Scholar]
- 143.Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci USA. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ticku MK, Lowrimore P, Lehoullier P. Ethanol enhances GABA-induced 36Cl-influx in primary spinal cord cultured neurons. Brain Res Bull. 1986;17:123–126. doi: 10.1016/0361-9230(86)90168-1. [DOI] [PubMed] [Google Scholar]
- 145.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 146.Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- 147.Engblom AC, Akerman KE. Effect of ethanol on gamma-aminobutyric acid and glycine receptor-coupled Cl− fluxes in rat brain synaptoneurosomes. J Neurochem. 1991;57:384–390. doi: 10.1111/j.1471-4159.1991.tb03764.x. [DOI] [PubMed] [Google Scholar]
- 148.Forman SA, Righi DL, Miller KW. Ethanol increases agonist affinity for nicotinic receptors from Torpedo. Biochim Biophys Acta. 1989;987:95–103. doi: 10.1016/0005-2736(89)90459-8. [DOI] [PubMed] [Google Scholar]
- 149.Ei-Fakahany EF, Miller ER, Abbassy MA, Eldefrawi AT, Eldefrawi ME. Alcohol modulation of drug binding to the channel sites of the nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 1983;224:289–296. [PubMed] [Google Scholar]
- 150.Wu G, Tonner PH, Miller KW. Ethanol stabilizes the open channel state of the Torpedo nicotinic acetylcholine receptor. Mol Pharmacol. 1994;45:102–108. [PubMed] [Google Scholar]
- 151.Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- 152.Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR. Mecamylamine (Inversine): an old antihypertensive with new research directions. J Hum Hypertens. 2002;16:453–457. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- 153.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- 154.Yu D, Zhang L, Eisele JL, Bertrand D, Changeux JP, Weight FF. Ethanol inhibition of nicotinic acetylcholine type a7 receptors involves the amino-terminal domain of the receptor. Mol Pharmacol. 1996;50:1010–1016. [PubMed] [Google Scholar]
- 155.Nagata K, Aistrup GL, Huang CS, Marszalec W, Song JH, Yeh JZ, Narahashi T. Potent modulation of neuronal nicotinic acetylcholine receptor-channel by ethanol. Neurosci Lett. 1996;217:189–193. [PubMed] [Google Scholar]
- 156.Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 157.Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- 158.Majchrowicz E, Hunt WA. Temporal relationship of the induction of tolerance and physical dependence after continuous intoxication with maximum tolerable doses of ethanol in rats. Psychopharmacology (Berl) 1976;50:107–112. doi: 10.1007/BF00430477. [DOI] [PubMed] [Google Scholar]
- 159.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- 161.Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology (Berl) 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- 162.Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-b-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- 163.Curzon P, Brioni JD, Decker MW. Effect of intraventricular injections of dihydro-beta-erythroidine (DHbE) on spatial memory in the rat. Brain Res. 1996;714:185–191. doi: 10.1016/0006-8993(95)01536-1. [DOI] [PubMed] [Google Scholar]
- 164.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the b2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 165.Whiteaker P, Marks MJ, Grady SR, Lu Y, Picciotto MR, Changeux JP, Collins AC. Pharmacological and null mutation approaches reveal nicotinic receptor diversity. Eur J Pharmacol. 2000;393:123–135. doi: 10.1016/s0014-2999(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 166.Dowell C, Olivera BM, Garrett JE, Staheli ST, Watkins M, Kuryatov A, Yoshikami D, Lindstrom JM, McIntosh JM. Alpha-conotoxin PIA is selective for a6 subunit-containing nicotinic acetylcholine receptors. J Neurosci. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol. 2006;41:486–493. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- 168.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 169.Meisch RA, Thompson T. Ethanol intake during schedule-induced polydipsia. Physiol Behav. 1972;8:471–475. doi: 10.1016/0031-9384(72)90331-9. [DOI] [PubMed] [Google Scholar]
- 170.Sinclair JD, Hyytia P, Nurmi M. The limited access paradigm: description of one method. Alcohol. 1992;9:441–444. doi: 10.1016/0741-8329(92)90045-c. [DOI] [PubMed] [Google Scholar]
- 171.Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiol Behav. 1972;9:737–740. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- 172.Cicero TJ, Myers RD. Selection of a single ethanol test solution in free-choice studies with animals. Q J Stud Alcohol. 1968;29:446–448. [PubMed] [Google Scholar]
- 173.Kiefer SW, Bice PJ, Badia-Elder N. Alterations in taste reactivity to alcohol in rats given continuous alcohol access followed by abstinence. Alcohol Clin Exp Res. 1994;18:555–559. doi: 10.1111/j.1530-0277.1994.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 174.Eriksson K. Genetic selection for voluntary alcohol consumption in the albino rat. Science. 1968;159:739–741. doi: 10.1126/science.159.3816.739. [DOI] [PubMed] [Google Scholar]
- 175.Li TK, Lumeng L, McBride WJ, Murphy JM. Rodent lines selected for factors affecting alcohol consumption. Alcohol Alcohol Suppl. 1987;1:91–96. [PubMed] [Google Scholar]
- 176.Mardones J, Segovia-Riquelme N. Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol. 1983;5:171–178. [PubMed] [Google Scholar]
- 177.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 178.Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav. 1976;16:693–698. doi: 10.1016/0031-9384(76)90238-9. [DOI] [PubMed] [Google Scholar]
- 179.Tomie A, Miller WC, Dranoff E, Pohorecky LA. Intermittent presentations of ethanol sipper tube induce ethanol drinking in rats. Alcohol Alcohol. 2006;41:225–230. doi: 10.1093/alcalc/agl002. [DOI] [PubMed] [Google Scholar]
- 180.Tomie A, di Poce J, Derenzo CC, Pohorecky LA. Autoshaping of ethanol drinking: an animal model of binge drinking. Alcohol Alcohol. 2002;37:138–146. doi: 10.1093/alcalc/37.2.138. [DOI] [PubMed] [Google Scholar]
- 181.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an a4b2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 182.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the a4b2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 183.Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE. Preclinical pharmacology of the a4b2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 184.Gulick D, Gould TJ. Varenicline ameliorates ethanol-induced deficits in learning in C57BL/6 mice. Neurobiol Learn Mem. 2008;90:230–236. doi: 10.1016/j.nlm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Glick SD, Maisonneuve IM, Kitchen BA, Fleck MW. Antagonism of a3b4 nicotinic receptors as a strategy to reduce opioid and stimulant self-administration. Eur J Pharmacol. 2002;438:99–105. doi: 10.1016/s0014-2999(02)01284-0. [DOI] [PubMed] [Google Scholar]
- 186.Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- 187.Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking a3b4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- 188.Glick SD, Maisonneuve IM. Development of novel medications for drug addiction: the legacy of an African shrub. Ann NY Acad Sci. 2000;909:88–103. doi: 10.1111/j.1749-6632.2000.tb06677.x. [DOI] [PubMed] [Google Scholar]
- 189.Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of a4b2, a3b2 and a3b4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- 190.Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, Collins AC, Marks MJ. Involvement of the a3 subunit in central nicotinic binding populations. J Neurosci. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of a2, a3, a4, and b2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 192.Duvoisin RM, Deneris ES, Patrick J, Heinemann S. The functional diversity of the neuronal nicotinic acetylcholine receptors is increased by a novel subunit: b4. Neuron. 1989;3:487–496. doi: 10.1016/0896-6273(89)90207-9. [DOI] [PubMed] [Google Scholar]
- 193.Mulle C, Changeux JP. A novel type of nicotinic receptor in the rat central nervous system characterized by patch-clamp techniques. J Neurosci. 1990;10:169–175. doi: 10.1523/JNEUROSCI.10-01-00169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wong ET, Holstad SG, Mennerick SJ, Hong SE, Zorumski CF, Isenberg KE. Pharmacological and physiological properties of a putative ganglionic nicotinic receptor, a3b4, expressed in transfected eucaryotic cells. Mol Brain Res. 1995;28:101–109. doi: 10.1016/0169-328x(94)00189-l. [DOI] [PubMed] [Google Scholar]
- 195.Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- 196.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 197.Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003;23:4308–4314. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lisoprawski A, Herve D, Blanc G, Glowinski J, Tassin JP. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183:229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- 199.Sutherland RJ, Nakajima S. Self-stimulation of the habenular complex in the rat. J Comp Physiol Psychol. 1981;95:781–791. doi: 10.1037/h0077833. [DOI] [PubMed] [Google Scholar]
- 200.Sasaki K, Suda H, Watanabe H, Yagi H. Involvement of the entopeduncular nucleus and the habenula in methamphetamine-induced inhibition of dopamine neurons in the substantia nigra of rats. Brain Res Bull. 1990;25:121–127. doi: 10.1016/0361-9230(90)90262-x. [DOI] [PubMed] [Google Scholar]
- 201.Araki M, McGeer PL, McGeer EG. Retrograde HRP tracing combined with a pharmacohistochemical method for GABA transaminase for the identification of presumptive GABAergic projections to the habenula. Brain Res. 1984;304:271–277. doi: 10.1016/0006-8993(84)90330-5. [DOI] [PubMed] [Google Scholar]
- 202.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 203.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 204.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 205.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 206.Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 207.Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- 208.Martin BR, Onaivi ES, Martin TJ. What is the nature of mecamylamine’s antagonism of the central effects of nicotine? Biochem Pharmacol. 1989;38:3391–3397. doi: 10.1016/0006-2952(89)90106-8. [DOI] [PubMed] [Google Scholar]
- 209.Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- 210.Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- 211.Rose JE, Sampson A, Levin ED, Henningfield JE. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacol Biochem Behav. 1989;32:933–938. doi: 10.1016/0091-3057(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 212.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 213.Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- 214.Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- 215.Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- 216.Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- 217.Holm KJ, Spencer CM. Bupropion: a review of its use in the management of smoking cessation. Drugs. 2000;59:1007–1024. doi: 10.2165/00003495-200059040-00019. [DOI] [PubMed] [Google Scholar]
- 218.Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 219.Cryan JF, Gasparini F, van Heeke G, Markou A. Non-nicotinic neuropharmacological strategies for nicotine dependence: beyond bupropion. Drug Discov Today. 2003;8:1025–1034. doi: 10.1016/s1359-6446(03)02890-3. [DOI] [PubMed] [Google Scholar]
- 220.Coe JW, Vetelino MG, Bashore CG, Wirtz MC, Brooks PR, Arnold EP, Lebel LA, Fox CB, Sands SB, Davis TI, Schulz DW, Rollema H, Tingley FD, 3rd, O’Neill BT. In pursuit of a4b2 nicotinic receptor partial agonists for smoking cessation: carbon analogs of (−)-cytisine. Bioorg Med Chem Lett. 2005;15:2974–2979. doi: 10.1016/j.bmcl.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 221.Coe JW, Brooks PR, Wirtz MC, Bashore CG, Bianco KE, Vetelino MG, Arnold EP, Lebel LA, Fox CB, Tingley FD, 3rd, Schulz DW, Davis TI, Sands SB, Mansbach RS, Rollema H, O’Neill BT. 3,5-Bicyclic aryl piperidines: a novel class of a4b2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett. 2005;15:4889–4897. doi: 10.1016/j.bmcl.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 222.McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- 224.Kuehn BM. Studies linking smoking-cessation drug with suicide risk spark concerns. JAMA. 2009;301:1007–1008. doi: 10.1001/jama.2009.293. [DOI] [PubMed] [Google Scholar]
- 225.Stapleton JA, Watson L, Spirling LI, Smith R, Milbrandt A, Ratcliffe M, Sutherland G. Varenicline in the routine treatment of tobacco dependence: a pre-post comparison with nicotine replacement therapy and an evaluation in those with mental illness. Addiction. 2008;103:146–154. doi: 10.1111/j.1360-0443.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 226.Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the m-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 227.Mattson ME, Litten RZ. Combining treatments for alcoholism: why and how? J Stud Alcohol Suppl. 2005:8–16. doi: 10.15288/jsas.2005.s15.8. [DOI] [PubMed] [Google Scholar]
- 228.Kranzler HR, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1335–1341. [PubMed] [Google Scholar]
- 229.Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- 230.Mason BJ. Acamprosate in the treatment of alcohol dependence. Expert Opin Pharmacother. 2005;6:2103–2115. doi: 10.1517/14656566.6.12.2103. [DOI] [PubMed] [Google Scholar]
- 231.Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 232.Mason BJ, Ownby RL. Acamprosate for the treatment of alcohol dependence: a review of double-blind, placebo-controlled trials. CNS Spectr. 2000;5:58–69. doi: 10.1017/s1092852900012827. [DOI] [PubMed] [Google Scholar]
- 233.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study - a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 234.Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 235.O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatr. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 236.Balldin J, Berglund M, Borg S, Mansson M, Bendtsen P, Franck J, Gustafsson L, Halldin J, Nilsson LH, Stolt G, Willander A. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003;27:1142–1149. doi: 10.1097/01.ALC.0000075548.83053.A9. [DOI] [PubMed] [Google Scholar]
- 237.O’Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, O’Connor PG. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med. 2003;163:1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- 238.Donovan DM, Anton RF, Miller WR, Longabaugh R, Hosking JD, Youngblood M. Combined pharmacotherapies and behavioral interventions for alcohol dependence (The COMBINE Study): examination of posttreatment drinking outcomes. J Stud Alcohol Drugs. 2008;69:5–13. doi: 10.15288/jsad.2008.69.5. [DOI] [PubMed] [Google Scholar]
- 239.George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- 240.Johnson BA, Ait-Daoud N, Prihoda TJ. Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypotheses to preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:737–742. [PubMed] [Google Scholar]
- 241.Swift RM, Davidson D, Whelihan W, Kuznetsov O. Ondansetron alters human alcohol intoxication. Biol Psychiatr. 1996;40:514–521. doi: 10.1016/0006-3223(95)00432-7. [DOI] [PubMed] [Google Scholar]
- 242.Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatr. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- 243.Johnson BA, O’Malley SS, Ciraulo DA, Roache JD, Chambers RA, Sarid-Segal O, Couper D. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. J Clin Psychopharmacol. 2003;23:281–293. doi: 10.1097/01.jcp.0000084029.22282.bb. [DOI] [PubMed] [Google Scholar]
- 244.Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud Alcohol Suppl. 2005;150:170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- 245.Kuehn BM. Varenicline gets stronger warnings about psychiatric problems, vehicle crashes. JAMA. 2009;302:834. doi: 10.1001/jama.2009.1153. [DOI] [PubMed] [Google Scholar]
- 246.McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, Deprey M, Richards J, Javitz H. Mood, side-effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. J Gen Int Med. 2009;24:563–569. doi: 10.1007/s11606-009-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kintz P, Evans J, Villain M, Cirimele V. Smoking cessation with varenicline: a suicidal fatality. J Anal Toxicol. 2009;33:118–120. doi: 10.1093/jat/33.2.118. [DOI] [PubMed] [Google Scholar]
- 248.Purvis TL, Mambourg SE, Balvanz TM, Magallon HE, Pham RH. Safety and effectiveness of varenicline in a veteran population with a high prevalence of mental illness. Ann Pharmacother. 2009;43:862–867. doi: 10.1345/aph.1L661. [DOI] [PubMed] [Google Scholar]
- 249.Kasliwal R, Wilton LV, Shakir SA. Safety and drug utilization profile of varenicline as used in general practice in England: interim results from a prescription-event monitoring study. Drug Saf. 2009;32:499–507. doi: 10.2165/00002018-200932060-00006. [DOI] [PubMed] [Google Scholar]
- 250.Hays JT. The risk-benefit balance of varenicline for smoking cessation. J Thorac Oncol. 2008;3:949–950. doi: 10.1097/JTO.0b013e31818593fc. [DOI] [PubMed] [Google Scholar]
- 251.Stapleton J. Do the 10 UK suicides among those taking the smoking cessation drug varenicline suggest a causal link? Addiction. 2009;104:864–865. doi: 10.1111/j.1360-0443.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 252.Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the general practice research database. BMJ. 2009;339:b3805. doi: 10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ramon JM, Bruguera E. Real world study to evaluate the effectiveness of varenicline and cognitive-behavioural interventions for smoking cessation. Intl J Environ Res Public Health. 2009;6:1530–1538. doi: 10.3390/ijerph6041530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Grosshans M, Mutschler J, Hermann D, Mann K, Diehl A. Reduced affective symptoms during tobacco dependence treatment with varenicline. Addiction. 2009;104:859–861. doi: 10.1111/j.1360-0443.2009.02537.x. [DOI] [PubMed] [Google Scholar]
- 255.Philip NS, Carpenter LL, Tyrka AR, Whiteley LB, Price LH. Varenicline augmentation in depressed smokers: an 8-week, open-label study. J Clin Psychiatr. 2009;70:1026–1031. doi: 10.4088/jcp.08m04441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ochoa EL. Varenicline reduced smoking behaviour in a mentally ill person. J Psychopharmacol. 2009;23:340–341. doi: 10.1177/0269881108089601. [DOI] [PubMed] [Google Scholar]
- 257.Smith RC, Lindenmayer JP, Davis JM, Cornwell J, Noth K, Gupta S, Sershen H, Lajtha A. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009;110:149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 258.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 259.Clark L, Robbins T. Decision-making deficits in drug addiction. Trends Cogn Sci. 2002;6:361. doi: 10.1016/s1364-6613(02)01960-5. [DOI] [PubMed] [Google Scholar]
- 260.Stalnaker TA, Roesch MR, Franz TM, Burke KA, Schoenbaum G. Abnormal associative encoding in orbitofrontal neurons in cocaine-experienced rats during decision-making. Eur J Neurosci. 2006;24:2643–2653. doi: 10.1111/j.1460-9568.2006.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 262.Glass JM, Buu A, Adams KM, Nigg JT, Puttler LI, Jester JM, Zucker RA. Effects of alcoholism severity and smoking on executive neurocognitive function. Addiction. 2009;104:38–48. doi: 10.1111/j.1360-0443.2008.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A. Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol Alcohol. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- 264.Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 2009;44:372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- 265.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 266.Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- 267.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 268.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 269.Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- 270.Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 271.Dom G, De Wilde B, Hulstijn W, van den Brink W, Sabbe B. Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol Clin Exp Res. 2006;30:1670–1677. doi: 10.1111/j.1530-0277.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 272.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 273.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 274.Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 275.Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. NeuroImage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 279.Vassileva J, Petkova P, Georgiev S, Martin EM, Tersiyski R, Raycheva M, Velinov V, Marinov P. Impaired decision-making in psychopathic heroin addicts. Drug Alcohol Depend. 2007;86:287–289. doi: 10.1016/j.drugalcdep.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 280.Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 281.Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 282.Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- 283.Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–305. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 284.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 285.Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 286.Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- 287.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 288.Ochoa EL, Lasalde-Dominicci J. Cognitive deficits in schizophrenia: focus on neuronal nicotinic acetylcholine receptors and smoking. Cell Mol Neurobiol. 2007;27:609–639. doi: 10.1007/s10571-007-9149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- 290.Zanardi A, Leo G, Biagini G, Zoli M. Nicotine and neurodegeneration in ageing. Toxicol Lett. 2002;127:207–215. doi: 10.1016/s0378-4274(01)00502-1. [DOI] [PubMed] [Google Scholar]
- 291.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 292.Allen SJ, MacGowan SH, Tyler S, Wilcock GK, Robertson AG, Holden PH, Smith SK, Dawbarn D. Reduced cholinergic function in normal and Alzheimer’s disease brain is associated with apolipoprotein E4 genotype. Neurosci Lett. 1997;239:33–36. doi: 10.1016/s0304-3940(97)00872-0. [DOI] [PubMed] [Google Scholar]
- 293.Tiraboschi P, Hansen LA, Alford M, Masliah E, Thal LJ, Corey-Bloom J. The decline in synapses and cholinergic activity is asynchronous in Alzheimer’s disease. Neurology. 2000;55:1278–1283. doi: 10.1212/wnl.55.9.1278. [DOI] [PubMed] [Google Scholar]
- 294.Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]