Abstract
Objective
To measure the incidence of treatment failure and associated costs in patients with methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infections (SSTIs).
Methods
This was a prospective, observational study in 13 primary care clinics. Primary care providers collected clinical data, wound swabs, and 90-day follow-up information. Patients were considered to have “moderate or complicated” SSTIs if they had a lesion ≥ 5 cm in diameter or diabetes mellitus. Treatment failure was evaluated within 90 days of the initial visit. Cost estimates were obtained from federal sources.
Results
Overall, treatment failure occurred in 21% of patients (n=21/98) at a mean additional cost of $1,933.71 per patient. Treatment failure occurred in 27% of patients in the moderate or complicated group and 11% of patients in the mild or uncomplicated group (p = 0.08). In a subgroup analysis of patients who received I&D, patients with moderate or complicated SSTIs had higher rates of treatment failure than patients with mild or uncomplicated SSTIs (36% vs. 10%; p = 0.04).
Conclusions
One in five patients presenting to a primary care clinic for a MRSA SSTI will likely require additional interventions as a result of treatment failure at an associated cost of almost $2,000 per patient. Baseline risk stratification and new treatment approaches are needed to reduce treatment failures and costs in the primary care setting.
Introduction
Community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) skin and soft tissue infections (SSTIs) are commonplace in community health care settings.1–4 Furthermore, patients with CA-MRSA SSTIs have a high incidence of treatment failure and recurrence. Two recent studies involving urgent and primary care clinics found that 35% of patients with CA-MRSA SSTIs experienced treatment failure and 78% of patients reported a recurrent infection.5,6 Reasons for treatment failure may be multi-factorial including inappropriate antibiotics, antibiotic resistance, and disease severity; however, studies evaluating mechanisms of treatment failure are limited.
The Infectious Diseases Society of America (IDSA) has published two evidence-based practice guidelines related to the care of patients with MRSA SSTIs.7,8 The 2005 SSTI guidelines recommend incision and drainage (I&D) for purulent SSTIs.7 I&D is an important treatment modality for MRSA SSTIs, as approximately 80–90% will present as purulent infections.4,9 Systemic antibiotics are recommended for those patients with multiple lesions, impaired host defenses, extensive cellulitis surrounding the infection, cutaneous gangrene, and systemic manifestations.7
The 2011 MRSA guidelines also emphasize the use of I&D for MRSA SSTIs with drainable foci.8 These guidelines recommend adjunctive antibiotics for the same populations identified in the 2005 IDSA SSTI guidelines as well as patients at extremes of age, those with SSTIs in areas that are difficult to drain (e.g., face, hand, genitalia), and in those patients who do not respond to I&D alone. The 2011 IDSA MRSA guidelines define the “impaired host defenses” category as those patients with diabetes, neoplasms, and human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS). The guidelines reiterate that these patients should receive adjunctive antibiotics, regardless of other patient characteristics. These recommendations are supported by a recent systematic review which suggests oral generic anti-MRSA antibiotics (e.g., clindamycin, doxycycline, and trimethoprim-sulfamethoxazole) may provide additional benefit to I&D alone for purulent MRSA SSTIs.10 Taken together, this information indicates that I&D plus anti-MRSA antibiotics is the standard-of-care for most patients with purulent MRSA SSTIs.
There are several severity classification rules for SSTIs;7,8,11–13 however, the best classification rule is unknown.14 Without a well-recognized rule, patients with large abscesses or significant comorbidities, including diabetes, may receive less aggressive therapy than is warranted. This may be partially responsible for the high rate of treatment failure and recurrence in the community setting.
Several studies have quantified the prevalence of CA-MRSA SSTIs in the primary care setting and described the management of these infections.6,15,16 Levy et al. conducted a large prospective study assessing the management of SSTIs in primary care clinics within the Iowa Research Network. Among their many important findings, the investigators found that patients who received initial I&D had a shorter time to disease resolution (11.4 days versus 13.6 days) than patients who did not receive initial I&D.15 Members of our own research group have conducted two studies investigating CA-MRSA SSTIs involving the South Texas Ambulatory Research Network (STARNet), a practice-based research network (PBRN).6,16 The first study used a “card-system” to collect data on patients with CA-MRSA SSTIs seen between April 2007 and January 2008.6 The chief findings were identification of CA-MRSA risk factors and the high prevalence of CA-MRSA SSTIs (67%) in this region. The second study enrolled a new set of patients seen in STARNet clinics between 2009 and 2010.16 This largely descriptive study involved the collection of clinical data, microbiological specimens, and digital pictures of the infection. The major findings were the prevalence of CA-MRSA SSTIs (61%) and a description of the initial treatment patterns in the primary care setting.
The study described in this paper is a continuation of the second STARNet CA-MRSA SSTI study. New support enabled us to expand the original cohort, collect 90-day treatment outcomes, and perform the cost-assessment. This study investigates possible causes for treatment failure, including failure to incise and drain purulent infections and a reluctance to administer adjunctive antibiotics to patients with more severe infections. Finally, it also provides estimates for the incidence of treatment failure and associated costs.
Methods
This was a multisite, prospective, community-based, observational cohort study pursued in collaboration with STARNet, composed of 108 urban, suburban, and rural primary care clinics distributed throughout the South Texas region. Health care providers at 13 STARNet clinics prospectively enrolled patients for this study. Similar to the other STARNet clinics, these 13 clinics serve a predominately Hispanic and non-Hispanic white population. Unlike the other STARNet clinics, these 13 clinics provide care to a largely underserved population, many without medical insurance. Patients were eligible for study enrollment if they provided informed consent, were 18 years of age or older, presented to one of the participating clinics with a SSTI, and if their managing clinician suspected MRSA. This analysis was further limited to patients confirmed to have MRSA via microbiology tests conducted in the principal investigator's research laboratory, those with baseline SSTI severity and treatment data, and those with 90-day follow-up data. No protocols or educational interventions were implemented as part of this observational study. Patients were excluded if they were pregnant, incarcerated, or had impaired decision-making capacity. The University of Texas Health Science Center Institutional Review Board granted approval for this study.
Study investigators provided the clinics with English and Spanish informed consent forms, a digital camera, a portable bio-hazard container, and individually labeled study kits, as previously described.16 STARNet clinicians were instructed to obtain informed consent from patients, record demographic and clinical information on the patient data card, obtain a wound culture, crush the ampoule to release Modified Stuart's Media onto the swab tip for preservation during transport, and capture digital pictures of the infection site. Clinic staff contacted the principal investigator when they enrolled a patient. The principal investigator retrieved the data cards, wound swabs, and pictures from the study sites and returned these materials to his research laboratory for processing. Clinics were compensated $50 for each patient enrolled in the study.
Wound swabs, submitted in transport media, were plated directly onto pre-filled Tryptic Soy Agar plates with 5% sheep blood (Remel, Lenexa, KS) and incubated for 18 to 24 hours at 35 to 37°C. If the isolate did not grow, the culture was re-plated up to two more times. If growth did not occur after three attempts, “no growth” was recorded. If growth occurred, isolates morphologically consistent with Staphylococcus aureus were sub-cultured once onto another blood agar plate under the same conditions to facilitate logarithmic growth. Next, the suspect isolates were plated onto MRSASelect™ chromogenic agar (Bio-Rad Laboratories, Hercules, CA) plates for identification and isolation of MRSA; incubation occurred for 18 to 28 hours at 35 to 37°C, protected from light. Pink colonies on the MRSASelect™ agar were indicative of MRSA. These methods have been used previously by this research group to identify MRSA SSTIs in the primary care setting.16
Severity Classification
The severity of the infection was categorized using a modified version of two Food and Drug Administration (FDA) classification schemes. This modified classification scheme has also been used in a prior study.16 The FDA's 1998 Guidance for Industry SSTI document defines “complicated” infections as those that penetrate the deeper soft tissues, those for whom significant surgical intervention is necessary, and those occurring in patients with comorbid conditions hindering treatment response (e.g., diabetes mellitus or HIV).12 A more recent FDA guidance document classifies abscesses affecting a body surface area ≥ 75 cm2 as “major abscesses” and those affecting an area of < 5 cm from the peripheral margin of the abscess as “minor abscesses”.13 The modified scheme classified patients as “moderate or complicated” if they had a lesion ≥ 5 cm in diameter, diabetes mellitus, or both. Patients not exhibiting these characteristics were considered to have “mild or uncomplicated” infections.
Health Outcomes
Participating clinics were asked to collect additional follow-up information via medical chart review regarding events that transpired after the initial clinic visit. Clinic personnel used a standardized follow-up form that requested information regarding patient age, past medical history (e.g., peripheral vascular disease, chronic non-infectious skin disorder, HIV/AIDS, cancer, actively receiving chemotherapy, immunosuppressed at time of visit), type of infection (e.g., furuncle, abscess, cellulitis, other), and events occurring within 90 days of initial clinic visit. This form was collected by the principal investigator or returned electronically, void of any protected health information.
Ninety-day follow-up information was used to assess the incidence of treatment failure. Patients were considered to have experienced treatment failure if any of the following occurred within 90 days of their initial visit: (1) change in antibiotic therapy, (2) incision and drainage (after initial I&D or receipt of initial antibiotic(s) only) (3) SSTI at a new site, (4) SSTI at the same site, (5) emergency department visit, or (6) hospital admission.
Data and Statistical Analysis
JMP 9.0 statistical software (SAS Institute, Cary, NC) was used for all statistical analyses. Chi-square and Fisher's Exact test were used to compare nominal variables, Student's t-test was used to compare normally-distributed numeric variables, and the Wilcoxon Rank-Sum test was used to compare ordinal and non-normally distributed numeric variables.
The cost analysis was performed from the perspective of the health insurance payer. As such, indirect medical costs, such as loss of productivity, were not assessed in this study. Direct medical costs (e.g. hospital admission and I&D) were derived using estimates from the Agency for Healthcare Research and Quality (AHRQ).17–20 AHRQ National Average Drug Acquisition Costs, obtained from the Centers for Medicare and Medicaid Services, were used to estimate drug costs (Table 1). In patients with multiple interventions or repeated interventions of the same type during the 90-day follow-up period, the cost per intervention was multiplied by the total number of each type of intervention. All costs were then summed for patients with multiple types of failure (e.g. subsequent I&D and change in antibiotic therapy). All costs were incurred during a 32-month period (February 2009 to October 2011) and were therefore adjusted to 2011 U.S. dollars using national medical consumer price index (MCPI) inflation rates.21
Table 1.
Estimated Additional Costs of Treatment Failure
| Intervention | Cost (in 2011 U.S. Dollars)* | |
|---|---|---|
| Hospital admission 17 | $17,591.07 | |
| Outpatient incision & drainage 20 | $2,130.96 | |
| Emergency department visit 18 | $754.84 | |
| Antibiotics 34 | Bacitracin ointment | $5.23/tube |
| Mupirocin ointment | $0.31/25gm | |
| Cephalexin 500mg capsules | $0.11/capsule | |
| Ciprofloxacin 500mg tablets | $0.15/tablet | |
| Clindamycin 300mg capsules | $0.20/capsule | |
| Clindamycin 150mg capsules | $0.07/capsule | |
| Doxycycline 100mg capsules | $0.04/capsule | |
| Trimethoprim-sulfamethoxazole double-strength tablets | $0.04/tablet | |
| Clindamycin 600mg IV solution | $2.24/600mg | |
All costs were discounted to 2011 U.S. dollars using National Medical Consumer Price Index (MCPI) inflation rates20
The equation used to perform the cost discounting was as follows:
Results
Primary Analysis
A total of 265 cases were collected from 13 primary care clinics over a 32-month period. One hundred and thirty-seven cases from 12 clinics had MRSA-positive cultures (MRSA rate: 52%). Seven patients were missing initial treatment data and five were missing data necessary to classify SSTI severity. Of the 125 remaining cases, 98 patients from 10 clinics had 90-day follow-up information available for this analysis. The patients had a mean age of 42 years and most were Hispanic (74%). More than half of the patients (52%) had a body-mass index (BMI) ≥ 30 mg/m2, indicating obesity. Many patients had no medical insurance (43%).
Figure 1 depicts the distribution of patients into the two SSTI severity categories. Nearly two-thirds of patients had moderate or complicated MRSA SSTIs. The most common characteristic placing patients into the moderate or complicated severity category was lesion size ≥ 5 cm in diameter (57%); 18% of patients had diabetes alone and 25% had both characteristics. Patients with moderate or complicated MRSA SSTIs were more likely to be Hispanic (83% vs. 60%, p = 0.05) and were more likely to have BMI ≥ 30 kg/m2 (61% vs. 33%, p = 0.01) compared to those with mild or uncomplicated MRSA SSTIs. No other characteristics differed significantly between groups.
Figure 1.
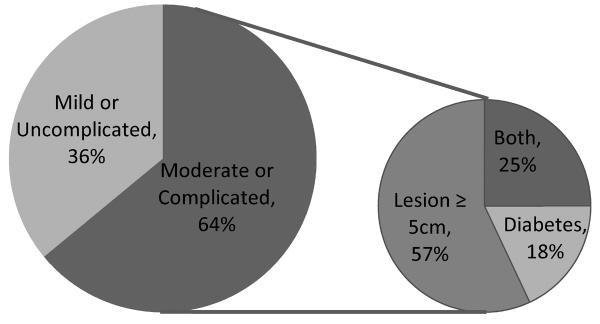
MRSA SSTI Severity Classification and Qualifying Characteristics, n=98
Twenty-one percent of all MRSA SSTI patients experienced treatment failure (n = 21 of 98 patients). Treatment failure occurred in 27% of patients in the moderate or complicated group and 11% of patients in the mild or uncomplicated group (p = 0.08). In the moderate-complicated group, the failure rate was 60% for those patients who received I&D alone and 32% in those patients who received I&D plus antibiotics (p = 0.33). This trend was not present in the mild-uncomplicated group. The mean additional cost of treatment failure was $1,933.70: $2,093.40 in the moderate or complicated group versus $1,255.02 in the mild or uncomplicated group.
Change in antibiotics was the most common indicator of treatment failure (81%). Forty-three percent of patients had a new SSTI, 24% required additional I&D, 24% were subsequently seen in the emergency department, 10% had a recurrent SSTI, and 5% were admitted to the hospital. There were no significant differences in the types of treatment failure between the two severity groups (p > 0.05 for all comparisons) (Figure 2). The mean time to treatment failure was 16.9 days. Time to treatment failure was 11.8 days in the moderate or complicated severity group and 38.8 days in the uncomplicated group (p = 0.06). More than half of all failures in the moderate or complicated severity group (59%) occurred within ten days of the initial clinic visit. In contrast, only 25% of mild or uncomplicated failures occurred within ten days of the initial clinic visit.
Figure 2.
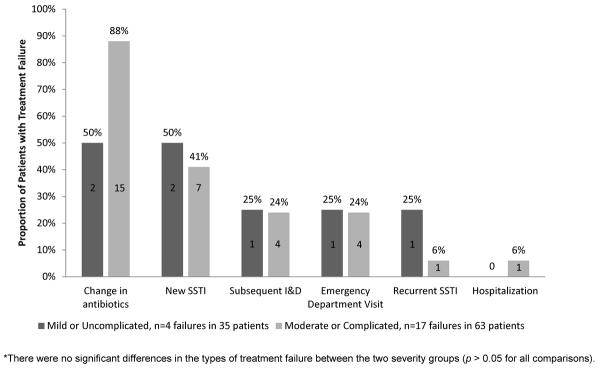
Type of Treatment Failure *
The trunk and lower extremity were the most common infection sites. SSTIs in difficult to drain areas (e.g., face, hand, genital area) accounted for 13% of all SSTIs. SSTI location (e.g., axilla, buttock, face, groin, hand, head/neck, lower/upper extremities) did not differ significantly between the two severity groups.
The most common initial treatments were: I&D plus antibiotics (57%), antibiotics alone (33%), and I&D alone (6%). Two patients did not receive I&D or antibiotics at the initial visit. Instead, they were instructed to return for follow-up at a later date. One patient was referred for surgery. There were no significant differences in initial treatment modality between the two severity groups (Table 2).
Table 2.
Initial Treatment Strategy, n=98
| Treatment Regimen | Total n=98 | Mild or Uncomplicated n=35 | Moderate or Complicated n=63 |
|---|---|---|---|
| I&D alone | 6 (6%) | 1 (3%) | 5 (8%) |
| I&D + Antibiotics | 56 (57%) | 19 (54%) | 37 (58%) |
| Antibiotics alone | 33 (34%) | 14 (40%) | 19 (30%) |
| Follow-up alone | 2 (2%) | 1 (3%) | 1 (2%) |
| Surgery | 1 (1%) | 0 | 1 (2%) |
There was no statistically significant difference in the initial treatment strategy between patients with Mild or Uncomplicated infections as compared to patients with Moderate or Complicated infections (Chi-square test for homogeneity, p = 0.66).
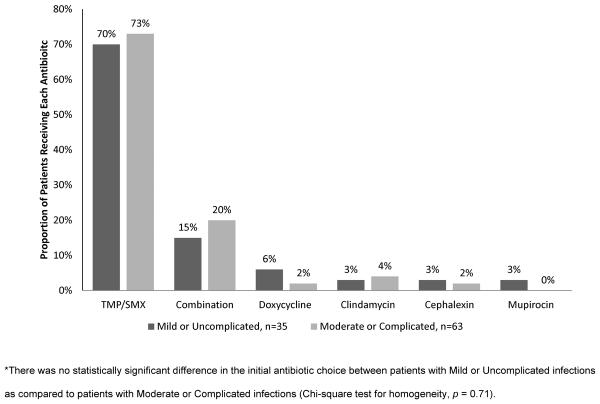
Overall, 89 of 98 patients (91%) received antibiotic therapy. The rate of antibiotic therapy was similar between the “mild or uncomplicated” and “moderate or complicated” severity groups (94% vs. 88%). Of those receiving antibiotic therapy, the most common antibiotic regimens were: TMP/SMX monotherapy (72%), combination therapy (18%), doxycycline monotherapy (3%), clindamycin monotherapy (3%), cephalexin monotherapy (2%), and mupirocin ointment monotherapy (1%). Seventy-five percent of the combination therapy regimens contained TMP/SMX. Figure 3 illustrates the initial antibiotic choice for the two severity groups. There were no significant differences in the use of antibiotics or the selection of initial antibiotic therapies between the two severity groups.
Figure 3.
Initial Antibiotic Choice, n=98
Subgroup Analysis: Patients Receiving I&D
When the analysis was limited to only those patients who received I&D, the most common initial treatment was I&D plus antibiotics (90%); I&D alone was performed on the remaining 10% of patients. Once again, there were no significant differences in initial treatments between the two severity groups.
Twenty-seven percent of MRSA SSTI patients who received I&D experienced treatment failure (n = 17 of 62 patients). Moderate or complicated patients were more than three times as likely to experience treatment failure as compared to mild or uncomplicated patients (36% vs. 10%; p = 0.04) (Figure 4). There were no significant differences in the type of treatment failure between the two severity groups (p > 0.05 for all comparisons). The mean time to treatment failure was 16.6 days. The mean time to treatment failure was 12.3 days in the moderate or complicated severity group and 49.0 days in the mild or uncomplicated group (p = 0.17). The mean additional cost associated with treatment failure was $2,174.05 overall and was similar in the two severity groups ($2,281.36 vs. $2,132.46).
Figure 4.
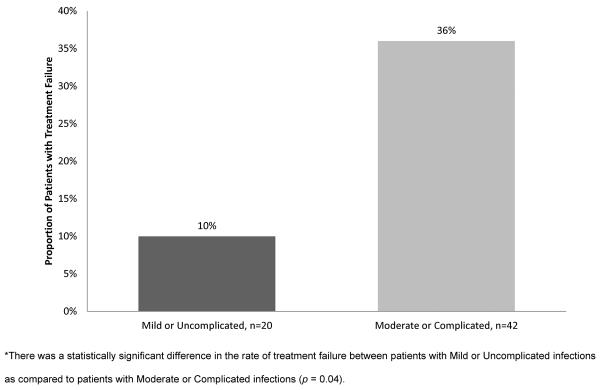
Proportion of Patients with Treatment Failure: Patients Receiving I&D, n=62
Discussion
This study measures the incidence of treatment failure and the additional costs associated with failure for outpatients receiving oral antibiotics for MRSA SSTIs. This study reveals a high rate of treatment failure (21%) and considerable associated costs (mean of $1,933.71 per patient). These results suggest that baseline risk stratification and new treatment approaches are needed to reduce treatment failures and costs in the primary care setting.
Incidence of Treatment Failure
A previous study of S. aureus SSTIs treated in the outpatient setting demonstrated a treatment failure rate of 18%, similar to that identified by our study.22 Other studies of MRSA SSTIs demonstrated higher rates of treatment failure.
One study, by Menzin et al., identified a treatment failure rate of 37%; however, the study was conducted exclusively in hospitalized patients.23 Hospitalized patients are likely to have more severe infections than outpatients. Furthermore, hospitalized patients are followed more closely by health care providers who are better able to detect complications and new infections. Indeed, studies have shown that outpatients, even with verbal and printed instructions, may not be as likely as health care professionals to recognize new or recurrent skin infections.24,25 Also, the treatment failure definition used by Menzin et al. may have inflated the true rate of treatment failure. Menzin's definition classified patients who switched from intravenous to oral therapy as treatment failures. Transitioning from intravenous to oral antimicrobials is a common practice in hospitals and has been shown to have beneficial effects including decreased costs, reduced the incidence of catheter-related infections, and decreased hospital length of stay;26–29 therefore, the authors of this paper do not believe this type of change should be considered to be a treatment failure.
A second study by Frei et al. identified a treatment failure rate of 35%; however, nearly half of the patients in that study were hospitalized.5 As previously mentioned, this might have resulted in greater identification of treatment failure in comparison to an outpatient population. Also, the definition of treatment failure used by Frei et al. included subsequent positive MRSA cultures as one of the criteria for treatment failure. This criterion was not used in the present study. When this additional criterion was eliminated, the failure rate reported by Frei et al. becomes similar to that observed in the present study (25% vs. 21%).
Additional Cost of Treatment Failure
The patients in this study who failed therapy incurred additional health care costs of almost $2,000 per patient. This is the first study to quantify this cost in the primary care setting. The mean cost of treatment failure observed in this cohort is considerably less than those costs described in prior cohorts. Other studies have calculated the cost of failure for hospitalized patients23 and patients managed at skilled nursing facilities or outpatient infusion clinics.22 Hospital room and board and outpatient infusion services accounted for 50% and 25% of the total costs in prior studies, respectively. Oral therapy may be another reason for the lower costs observed in this cohort. Studies by Menzin et al.23 and Marton et al.22 both demonstrated decreases in total cost of 12% and 30%, respectively, when oral antibiotic therapy was chosen instead of IV antibiotic therapy.
This study also demonstrated higher treatment failure costs in patients with more severe MRSA SSTIs. This is due to the greater number of costly types of treatment failure (e.g., hospitalization, emergency department visits, subsequent I&D) in the moderate or complicated group as compared to the mild or uncomplicated group. This underscores the need for appropriate risk stratification at baseline.
Validation of the Disease Severity Rule
This study demonstrates that objective criteria, available at baseline, can be used to predict which patients are more likely to fail therapy with oral antibiotics. Simply, patients with diabetes or lesions ≥ 5 cm in diameter were categorized as having moderate or complicated infections. Patients with moderate or complicated infections had a failure rate more than twice as high as those patients with mild or uncomplicated infections. The difference was even more pronounced in a subgroup of patients who received I&D. Two prior studies have reported higher rates of treatment failure in patients with lesions ≥ 5 cm.30,31 Furthermore, patients with diabetes have been shown to have more SSTI-related hospital and emergency department visits, longer hospital stays, and more infection related deaths as compared to non-diabetics.32,33 This simple rule provides primary care clinicians with a quick and easy classification tool to estimate the risk of treatment failure in MRSA SSTI patients.
Limitations
This study has limitations. First, the cohort encompassed only patients in South Texas, which may limit the potential to generalize these results to other geographic regions. Second, there were more obese and Hispanic patients in the moderate or complicated severity group. This likely reflects a strong correlation between obesity, Hispanic ethnicity, and diabetes. Third, SSTIs can be caused by several pathogens, including staphylococci (MRSA and MSSA), streptococci, and gram-negative bacteria. This study focuses on the subset of patients with positive cultures for MRSA. These patients may be more severely ill than the general SSTI population; therefore, the failure rate reported in this study of patients with MRSA SSTIs cannot be extrapolated to all patients with SSTIs. Fourth, 90-day follow-up information was captured retrospectively by the clinic personnel, using the medical records available at their clinic. Patients who did not have additional visits (i.e., did not return to clinic or went to another clinic) were counted as treatment successes. In addition, clinicians may have changed their behavior to fit expected results of this observational study; a Hawthorne effect cannot be excluded. For these reasons, our study could have possibly underestimated the true rate of treatment failure. Finally, I&D techniques were not standardized among the clinics or treating clinicians. Differences in I&D techniques may have influenced SSTI outcomes.
Despite these limitations, this study has important strengths. It is the first to estimate the additional cost of MRSA SSTI treatment failure in the primary care setting. One in five patients presenting to a primary care clinic for the treatment of a MRSA SSTI will likely require additional intervention at an associated cost of almost $2,000 per patient. Additionally, objective criteria, available at baseline, can be used to predict which patients are more likely to need additional interventions. Ultimately, baseline risk stratification and new treatment approaches are needed to reduce treatment failures and costs in the primary care setting.
Acknowledgements
The authors would like to thank their South Texas Ambulatory Research Network (STARNet) and Area Health Education Center (AHEC) colleagues who assisted with the administrative aspects of the study: Holly Hayes, Stephanie Reyes, Marisa Rodriquez, and Paula Winkler. The authors would also like to thank Kyllie Ryan-Hummel and Kyle Fischer (Research Assistants at the University of Texas Health Science Center, San Antonio, Texas) who formatted the final manuscript for submission to the journal.
Funding Statement:
This study was funded by an investigator-initiated research grant from Pfizer to Dr. Frei. Dr. Frei was also supported by the U.S. National Institutes of Health in the form of a KL2 Career Development Award (NIH/NCRR 5KL2 RR025766, PI-Robert Clark).
Footnotes
Conflicting and Competing Interests:
References
- 1.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin Infect Dis. 2006;42(3):389–91. doi: 10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 3.Styers D, Sheehan DJ, Hogan P, Sahm DF. Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: 2005 status in the United States. Ann Clin Microbiol Antimicrob. 2006;5:2. doi: 10.1186/1476-0711-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 5.Frei CR, Miller ML, Lewis JS, 2nd, Lawson KA, Hunter JM, Oramasionwu CU, et al. Trimethoprim-sulfamethoxazole or clindamycin for community-associated MRSA (CAMRSA) skin infections. J Am Board Fam Med. 2010;23(6):714–9. doi: 10.3122/jabfm.2010.06.090270. [DOI] [PubMed] [Google Scholar]
- 6.Parchman ML, Munoz A. Risk factors for methicillin-resistant Staphylococcal aureus skin and soft tissue infections presenting in primary care: a South Texas Ambulatory Research Network (STARNet) study. J Am Board Fam Med. 2009;22(4):375–9. doi: 10.3122/jabfm.2009.04.090003. [DOI] [PubMed] [Google Scholar]
- 7.Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373–406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 9.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–44. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 10.Forcade NA, Wiederhold NP, Ryan L, Talbert RL, Frei CR. Antibacterials as adjuncts to incision and drainage for adults with purulent methicillin-resistant Staphylococcus aureus (MRSA) skin infections. Drugs. 2012;72(3):339–51. doi: 10.2165/11599510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Managing skin and soft tissue infections: expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52(Suppl 1):i3–17. doi: 10.1093/jac/dkg466. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) [Accessed February 9, 2012];Guidance for industry: uncomplicated and complicated skin and skin structure infections: developing antimicrobial drugs for treatment. 1998 http://www.fda.gov/ohrms/dockets/ac/03/briefing/3997B1_02_GFI-Diabetic%20Foot.pdf.
- 13.US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) [Accessed February 9, 2012];Draft guidance for industry: acute bacterial skin and skin structure infections: developing antimicrobial drugs for treatment. 2010 http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guida nces/UCM071185.pdf.
- 14.Corey GR, Stryjewski ME. New rules for clinical trials of patients with acute bacterial skin and skin-structure infections: do not let the perfect be the enemy of the good. Clin Infect Dis. 2011;52(Suppl 7):S469–76. doi: 10.1093/cid/cir162. [DOI] [PubMed] [Google Scholar]
- 15.Levy BT, Daly J. Community-acquired skin infections in the age of methicillin-resistant organisms. Agency for Healthcare Research and Quality; Rockville, MD: Oct, 2010. AHRQ Publication No. 11-0007-1-EF. [Google Scholar]
- 16.Forcade NA, Parchman ML, Jorgensen JH, Du LC, Nyren NR, Trevino LB, et al. Prevalence, severity, and treatment of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) skin and soft tissue infections in 10 medical clinics in Texas: a South Texas Ambulatory Research Network (STARNet) study. J Am Board Fam Med. 2011;24(5):543–50. doi: 10.3122/jabfm.2011.05.110073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merrill CT. [Accessed March 5, 2010];HCUP Fact Book No. 6, Hospitalization in the United States. 2002 AHRQ Publication No. 05-0056, June 2005, www.ahrq.gov/data/hcup/factbk6/factbk6.pdf.
- 18.Machlin SR. [Accessed March 5, 2010];Medicare Expenditure Panel Survey (MEPS), Expenses for a hospital emergency room visit. 2003 AHRQ Statistical Brief #111, January 2006, www.meps.ahrq.gov/mepsweb/data_files/publications/st111/stat111.pdf.
- 19.Machlin SR. [Accessed March 5, 2010];Medicare Expenditure Panel Survey (MEPS), Expenses for office-based physician visits by specialty. 2004 AHRQ Statistical Brief #166, March 2007, www.meps.ahrq.gov/mepsweb/data_files/publications/st166/stat166.pdf.
- 20.Russo CA, et al. [Accessed March 5, 2010];HCUP Fact Book No. 9, Ambulatory surgery in U.S. hospitals. 2003 AHRQ Publication No. 07-0007, January 2007, www.ahrq.gov/data/hcup/factbk9/factbk9.pdf.
- 21.Bureau of Labor Statistics Archived Consumer Price Index detailed report information. [Accessed November 16, 2011];2003–2011 Consumer Price Index Detailed Reports. www.bls.gov/cpi/cpi_dr.htm.
- 22.Marton JP, Jackel JL, Carson RT, Rothermel CD, Friedman M, Menzin J. Costs of skin and skin structure infections due to Staphylococcus aureus: an analysis of managed-care claims. Curr Med Res Opin. 2008;24(10):2821–8. doi: 10.1185/03007990802365169. [DOI] [PubMed] [Google Scholar]
- 23.Menzin J, Marton JP, Meyers JL, Carson RT, Rothermel CD, Friedman M. Inpatient treatment patterns, outcomes, and costs of skin and skin structure infections because of Staphylococcus aureus. Am J Infect Control. 2009;38(1):44–9. doi: 10.1016/j.ajic.2009.04.287. [DOI] [PubMed] [Google Scholar]
- 24.Seaman M, Lammers R. Inability of patients to self-diagnose wound infections. J Emerg Med. 1991;9(4):215–9. doi: 10.1016/0736-4679(91)90416-d. [DOI] [PubMed] [Google Scholar]
- 25.Whitby M, McLaws ML, Collopy B, Looke DF, Doidge S, Henderson B, et al. Post-discharge surveillance: can patients reliably diagnose surgical wound infections? J Hosp Infect. 2002;52(3):155–60. doi: 10.1053/jhin.2002.1275. [DOI] [PubMed] [Google Scholar]
- 26.Davis SL, Delgado G, Jr, McKinnon PS. Pharmacoeconomic considerations associated with the use of intravenous-to-oral moxifloxacin for community-acquired pneumonia. Clin Infect Dis. 2005;41(Suppl 2):S136–43. doi: 10.1086/428054. [DOI] [PubMed] [Google Scholar]
- 27.Kuti JL, Le TN, Nightingale CH, Nicolau DP, Quintiliani R. Pharmacoeconomics of a pharmacist-managed program for automatically converting levofloxacin route from i.v. to oral. Am J Health Syst Pharm. 2002;59(22):2209–15. doi: 10.1093/ajhp/59.22.2209. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med. 2001;161(6):848–50. doi: 10.1001/archinte.161.6.848. [DOI] [PubMed] [Google Scholar]
- 29.Mertz D, Koller M, Haller P, Lampert ML, Plagge H, Hug B, et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother. 2009;64(1):188–99. doi: 10.1093/jac/dkp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, McCracken GH, Jr, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123–7. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 31.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45(3):311–20. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Bader MS. Diabetic foot infection. Am Fam Physician. 2008;78(1):71–9. [PubMed] [Google Scholar]
- 33.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–8. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34. [Accessed December 1, 2011];Alabama Medicaid Agency Average Acquisition Cost (AAC) rate listing for generic drugs: rates effective as of November 9, 2011. http://al.mslc.com/uploadedFiles/AL_AAC_Generic_Update_20111109.pdf.