Abstract
In today's medical industry, the range of vaccines that exist for administration in humans represents an eclectic variety of forms and immunologic mechanisms. Namely, these are the live attenuated viruses, inactivated viruses, subunit proteins, and virus-like particles for treating virus-caused diseases, as well as the bacterial-based polysaccharide, protein, and conjugated vaccines. Currently, a new approach to vaccination is being investigated with the concept of DNA vaccines. As an alternative delivery route to enhance the vaccination efficacy, microneedles have been devised to target the rich network of immunologic antigen-presenting cells in the dermis and epidermis layers under the skin. Numerous studies have outlined the parameters of microneedle delivery of a wide range of vaccines, revealing comparable or higher immunogenicity to conventional intramuscular routes, overall level of stability, and dose-sparing advantages. Furthermore, recent mechanism studies have begun to successfully elucidate the biological mechanisms behind microneedle vaccination. This paper describes the current status of microneedle vaccine research.
Keywords: Microneedles, Transdermal delivery, Virus vaccines, Bacterial vaccines, DNA vaccines
Introduction
By definition, vaccines are pharmacological formulations that incorporate the disease-causing antigen which could innocuously induce an immune response when administered into a healthy human being, without causing the disease itself [1]. The vaccines licensed for human use in today's pharmaceutical industry is primarily divided into several subcategories. Firstly, there are the virus vaccines, which encompass the wide range of vaccines including the whole, live attenuated and killed inactivated virus vaccines, subunit vaccines, and the more recently devised technology of virus-like particles (VLPs). Furthermore, bacterial vaccines that take advantage of intrinsic structural components of bacteria, or more specifically the protein toxoids released by them, have been developed. These include the polysaccharide or protein subunit vaccines. Finally, one of the newest concepts in vaccine technology pertains to DNA plasmids that, upon in vivo administration, successfully express proteins that induce an immune response [2]. The spectrum of existing vaccine formulations is constantly expanding, as an ever growing number of studies are underway globally to figure out the optimal conditions for vaccine administration.
It has been noted in numerous studies that the measured efficacy of vaccines is highly affected by the route of administration. The potential benefits of transdermal delivery have been actively investigated by research communities, as the skin layer that lies beneath the stratum corneum is supported by a densely connected network of immune-response modulating antigen presenting cells (APCs), most significantly represented by the Langerhans cells and dermal dendritic cells in the epidermis and dermis of skin [3]. The prospect of transdermal delivery for vaccination has offered a vision of a promising alternative to the conventional intramuscular immunization, as skeletal muscle is loaded with a relatively sparse population of APCs and, correspondingly, a greater dosage of vaccine is required to induce a substantial immune response.
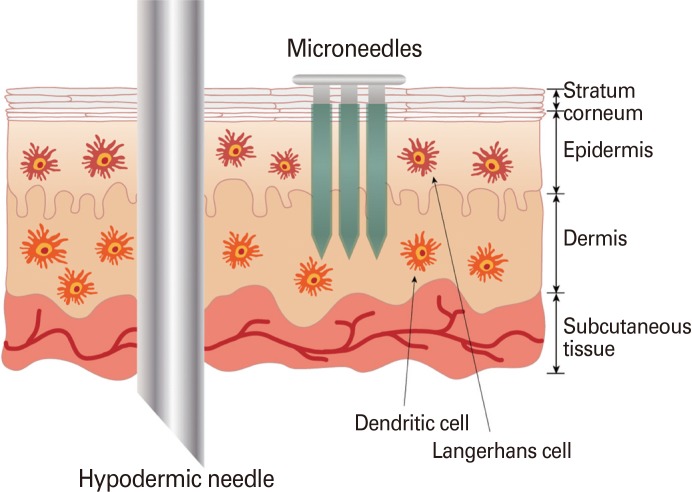
However, the present state of transdermal delivery techniques is not without setbacks. Firstly, the strategic targeting of the epidermis and dermis, without disrupting the underlying subcutaneous tissue, is a manually difficult technique that requires the hand of professionally trained healthcare personnel. As a potential solution to these drawbacks, microneedles have been proposed as an alternative delivery route that could replace hypodermic syringes (Fig. 1).
Fig. 1.
Comparison between conventional intramuscular injection by hypodermic needle and microneedles.
Microneedles are micron-sized needles that are constituted with appropriate drug formulations and directly penetrated into the stratum corneum in a direction that is perpendicular to the plane of the skin. The application of microneedles in vaccine delivery provides many clinical and logistic advantages. Most notably, the micron-scale dimensions of the microneedle shaft allow for simple and direct application into skin that does not require professional training. In addition, because of the small size of the needles, microneedle penetration is mostly pain-free. The production of self-administrable microneedle patches comprised of arrays of vaccine-coated microneedles will facilitate widespread dissemination of vaccines in times of rapid and uncontrolled onset of disease. The emergence of dry-coated microneedle vaccine formulations in the pharmaceutical industry will curtail the requirement of costly cold chain processes and promote the dissemination of vaccines to rural areas in developing countries. Furthermore, another important advantage of microneedles is its dose-sparing quality, in which the direct targeting of the rich network of immunogenic APCs produces higher immune responses for microneedles than the conventional intramuscular route. Many research efforts are being conducted globally to qualitatively compare the effective immune responses induced by microneedle vaccination as opposed to conventional delivery routes. Currently, there are four major types of microneedles in development: solid, coated, dissolving, and hollow microneedles [3]. This review paper discusses the current status of microneedle research in relation to vaccine development.
Virus Vaccines
The emergence of virus vaccines as a prophylactic initiative against disease has revolutionized the foundation of public healthcare. By educating the body's immune system against certain antigens that are unique to the disease-causing viruses, it has become possible to modulate a physiological response that suppresses the proliferation of viruses inside the human body in the initial stages of infection. Currently, there are four major categories of virus vaccines that are being investigated in the scientific community: live attenuated viruses, inactivated viruses, subunit vaccines, and VLPs.
Live attenuated viruses
Live attenuated virus vaccines are weakened forms of whole, normally pathogenic viruses in which their natural virulence has been compromised just enough to evade the occurrence of disease, while maintaining immunogenicity. Because these types of vaccines deal with live viruses, they are able to induce a potent immune response with even small dosages. However, the live viruses still have a potential to revert back to their original virulent form, so they pose a safety hazard in patient pools with compromised immune systems, including infants and the elderly. A study regarding the administration of a live attenuated Japanese encephalitis vaccine ChimeriVax in nonhuman primates provided evidence in support of the superior immunogenicity, in terms of higher neutralizing antibody titers and increased viremia duration, of cutaneous delivery by skin microabrasion and microneedle penetration than subcutaneous injection [4].
Inactivated viruses
Inactivated virus vaccines are formulated with whole, killed viruses that are less potent than their live attenuated counterparts, but still maintain sufficient immunogenicity to induce an immune response. Their subordinate immunogenicity may be compensated by the administration of multiple doses or immune response-boosting adjuvants, as is the case in commercially available influenza vaccines.
A case study involving intradermal injection of a clinically licensed rabies vaccine, delivered by a 1 to 3 mm BD Soluvia microneedle syringe, in a group of 66 healthy adult volunteers formally proved the safety and reliability of microneedle injection. In this study, as little as a 1/4 dosage of the rabies vaccine was sufficient to produce higher seroconversion rates than intramuscular injection, which displayed clear support of the dose-sparing advantage linked to the targeting of the immune-cell rich network of skin layers [4]. Another study examined the effects of the small hollow implantable dissolving-type microneedle, Bioneedle, that incorporated freeze-dried hepatitis B surface antigen formulated with aluminum hydroxide and lipopolysaccharide (LPS) adjuvants, which derived comparable immune responses as the liquid formulation of the vaccine after two immunizations [5].
The delivery of inactivated H1N1 A/PR/8/34 virus coated on metal microneedles in mice manifested significant serological antibody titers, protective immunity against virus infection, and a strong Th1 bias in comparison to intramuscular injection [6]. Moreover, studies of inactivated seasonal influenza virus vaccines coated on solid metal microneedles proved that microneedle delivery induced better recall and cellular immune responses, successful induction of virus-specific memory B cells, and improved lung viral clearance in mice than intramuscular delivery, providing empirical support that microneedles hold a promising potential as an alternative to conventional vaccine administration methods [6-8]. A stability quantification study relating to dry-coated vaccines was conducted using an inactivated influenza virus strain A/PR/8/34, which revealed that the addition of trehalose as a stabilizer was recommendable in order to circumvent the aggregation effects of antigen particles during coating and drying process [9]. Furthermore, dissolving-type microneedles encapsulating inactivated influenza antigens were fabricated and used for immunization in mice against H1N1 [10]. A dissolving microneedle patch consisting of the biocompatible polymer polyvinylpyrrolidone and encapsulating an inactivated virus vaccine against the influenza strain A/PR/8/34 induced robust antibody responses and enhanced cellular immune responses than IM [11].
Preclinical evaluation of whole inactivated influenza virus vaccines in mice revealed 100-fold dose sparing when the vaccine was delivered by intradermal route than by intramuscular route [12]. Furthermore, inactivated virus vaccine formulations against rotavirus were investigated with coated microneedles [13].
A recent mechanism study correlated the local increase of cytokines that play critical roles in the recruitment of macrophages, neutrophils, and dendritic cells after immunization with influenza vaccines in mice, serving as a cornerstone for elucidating the early immune events after microneedle immunization [14].
Subunit vaccines
Subunit vaccines contain only fragmented portions of disease-causing viruses that serve as the effective antigens. One notable study experimented with the use of a microfabrication material, poly[di(carboxylatophenoxy)phosphazene], both as the microneedle constituting core polymer and as a potent immunoadjuvant in delivering hepatitis B surface antigen in pigs [15]. The optimal vaccine formulation parameters have been researched for inducing maximal efficacy with microneedles coated with hemagglutinin (HA) subunits of influenza H1N1, H3N2, and B strains [16]. The inclusion of the sodium salt carboxymethylcellulose to enhance the viscosity of the coating solution has been shown to contribute to virus particle aggregation and the accompanying vaccine activity loss during the coating and drying process. Thus, incorporation of the sugar trehalose has been demonstrated to protect the antigen from destabilization when dry-coated onto microneedles. Another study investigated the effects of a trimeric soluble form of recombinant HA subunit of A/Aichi/2/68 influenza strain revealed the induction of higher immunogenicity in BALB/c mice, as measured by post-challenge lung titers, and more balanced IgG titers than delivery by traditional subcutaneous immunization [17]. Using a probability-based theoretical analysis for targeting skin APCs, a densely packed array of microneedle projections, Nanopatch, was devised to generate greater immune responses by directly contacting thousands of APCs. A study that investigated the effects of Nanopatch coated with a commercially approved inactivated split virion influenza vaccine, Fluvax, has demonstrated improved efficacy and a notable level of dose-sparing advantage that produced similar functional antibody levels with only a single vaccination and 1/100th of antigen delivered by intramuscular route [18]. A variation of this technology, utilizing a dissolving type Nanopatch, encapsulating Fluvax vaccine also produced higher systemic immune response in mice than intramuscular immunization [19]. With the apparent success of this technology, methods to site-selectively coat the micro-scale needle shafts have also been studied, which provided a solution for greater leverage of the dosage and uniformity of vaccine layering on top of microneedle shafts, by increasing the coating solution viscosity [20]. In addition, a study that investigated the effects of microneedle immunization with H1N1 A/Brisbane/59/2007 influenza subunit vaccine showed that both humoral and cellular arms of the immune response were activated, and conferred improved long-term protection in the mouse model compared to intramuscular vaccination [21].
A preclinical study of split inactivated trivalent influenza virus vaccines in mice revealed 5-fold dose-sparing when delivered by intradermal route compared to intramuscular route [12]. Randomized clinical studies involving healthy adult volunteers tracked the safety and immunogenicity profile of influenza vaccines delivered by a proprietary hollow intradermal microneedle system, BD Soluvia, which demonstrated significant dose-sparing advantages compared to intramuscular injection and no particular side reactions [22,23]. Other clinical studies involving influenza vaccination in the elderly population have noted superior seroprotection rates, seroconversion rates, and strain-specific HA inhibition mean titer increases, but also associated intradermal delivery with local injection site reaction, particularly erythema, but not pain [24,25].
Virus-like particles
VLPs are composed of self-assembling viral structural proteins, such as envelope and capsid. VLPs cannot replicate because they are devoid of viral genetic material. The portions of viral proteins expressed on the outer surface of VLPs serve as immunogenic epitopes that elicit strong B and T cell responses. A study involving the administration of Gardasil, a commercially approved prophylactic human cervical cancer vaccine composed of the L1 VLP capsid of human papillomavirus, was reported to induce higher virus neutralizing antibody titers in C57BL/6 mice when delivered via Nanopatch, a densely-packed microprojection array, than by intramuscular immunization [26].
Furthermore, the optimization parameters of VLP have been investigated with a vaccine formulation composed of the M1 matrix protein and the HA subunit of H1N1 A/PR/8/34 influenza virus strain [27]. A stability test comparing the antigenicity of influenza VLP vaccines including or devoid of trehalose was conducted, which showed that vaccine solutions without the stabilizer were not as effective as trehalose-inclusive formulations [28]. In vitro HA assay and in vivo challenge studies in mice provided clear evidence that the addition of trehalose as a stabilizer in vaccine coated microneedles reduced the extent of antigen destabilization. In addition, the administration of VLP vaccines via microneedles was shown to induce superior levels of recall immune responses compared to conventional intramuscular immunization [29]. Influenza VLPs expressing the HA subunit were coated on solid metal microneedles and manually applied onto the skin of mice, which induced comparable antibody responses to intramuscular administration, and full protection against viral challenge [30, 31]. Moreover, another study compared the immune responses elicited by low-dose microneedle and low-dose intramuscular routes, which reported that the low-dose microneedle induced higher immune responses that were similar to the serological antibody titers produced by high-dose intramuscular route [32].
In addition, a mechanism study examining the effect of microneedles in human skin presented a line of evidence indicating that H1 (A/PR/8/34) and H5 (A/Vietnam/1203/04) VLP vaccines delivered by microneedles stimulated Langerhans cells, which resulted in cell morphology change and a curtailed cell number in epidermal sheets [33].
Bacterial Vaccines
Although numerous types of virus vaccines have been compared so far, there are also a handful number of cases which have applied microneedles in the prevention of diseases caused by bacteria. Typically, these types of vaccines are constituted as a solution containing the intrinsic antigenic epitope portion of the bacterial polysaccharides or toxoids. The list of bacterial vaccines that have been formulated and administered by microneedles to provide immunity against bacterial disease is expansive, including anthrax [34-36], diphtheria [37-39], tetanus [40], tuberculosis [41], botulism, plague [42], and staphylococcal toxic shock [43].
In case of anthrax, there are numerous examples from literature that have successfully made use of the recombinant protective antigen (rPA) from Bacillus anthracis. One notable case introduced the serological protective effect seen in mice and rabbits after having been administered with rPA plus CpG by using a micro-enhancer array [34]. As an improvement over the previous case, a noteworthy dose-sparing effect and a higher immune response than intramuscular injection were reported in a rabbit model immunized by microcannula-shaped microneedles delivering rPA with aluminum adjuvant [36]. In yet another case, rats immunized by solid-state biodegradable microstructures consisting of polyvinyl alcohol, trehalose, maltitol, hydroxypropyl-β-cyclodextrin and rPA showed comparable antibody responses similar to traditional delivery methods [35].
Diphtheria, another disease caused by a bacterial exotoxin, were also tested in microneedle vaccine research. One study used the protein-based diphtheria toxin (DT) as a model antigen in order to compare the activity of several adjuvants, such as LPS, Quil A, CpG oligodeoxynucleotide and cholera toxin (CT), when jointly administered in mice after microneedle array pretreatment [37]. In addition, DT combined with CT applied on microneedle-pretreated mouse skin induced a similar immune response to subcutaneous injection that delivered DT with alum [38]. Furthermore, another study loaded DT into N-trimethyl chitosan nanoparticles and delivered the vaccine, along with CT, into mice by microneedle treatment in order to improve the immunogenicity [39].
In addition, tetanus toxoid was used in studies for evaluating the efficacy of Bioneedle, which are hollow implantable dissolving microneedles consisting of thermoplastic starch [40]. In another case study, it was shown that Bacillus Calmette-Guérin (BCG)-coated microneedle arrays could elicit strong cell-mediated immune responses in a guinea pig model, compared to vaccination by hypodermic syringe injected intradermally [41]. And lastly, F1-V fusion protein from Yersinia pestis injected into mice by a 34-gauge microneedle elicited similar IgG levels to traditional methods against plague [42].
An alternative approach for bacterial vaccine is the combinatorial vaccine, which is a pharmacological cocktail consisting of prophylactic vaccines against more than one bacteria. As a case in point, one research group immunized rhesus macaques by hollow stainless steel microneedles to deliver a vaccine solution containing 4 different proteins that would protect against anthrax, botulism, plague and staphylococcal toxic shock. To validate the predictions, the vaccinated monkeys were protected against lethal challenge of each source bacterium [43].
DNA Vaccines
Currently, many studies are underway to elaborate on the concept of DNA vaccines for prophylactic applications. DNA vaccines are formulations created by DNA plasmid vectors that express specific antigenic proteins of interest for which an immune response is modulated. The idea of DNA vaccines for medical administration accompanies several attractive advantages over traditional vaccination methods, including a better safety profile because it does not contain whole viruses or bacteria, enhanced vaccine stability, and ease of rapid and large-scale production.
Previously, a proof-of-concept study utilized a Nanopatch dry-coated with a DNA coating solution expressing West Nile virus particles to induce immune responses in mice [44]. Furthermore, another study investigating the immunological efficacy of a dry-coated DNA vaccine expressing hepatitis C viral particles revealed the stimulation of virus-specific cytotoxic T lymphocytes [45]. In addition, a new type of microneedle composed of micron-scale silicon projections, termed microenhancer arrays, was invented and tested for its efficacy in vivo by dry-coating with DNA vaccines expressing hepatitis B surface antigen [46]. Furthermore, in order to forgo the requirement for animal models for in vivo studies of cutaneous DNA vaccination, a human skin organ culture ex vivo system has been developed for analyzing expression levels and immunological activation over a prolonged period of 72 hours [8]. Another study examined the effects of DNA vaccination of plasmids that encode a low dose of the vaginal herpes simplex virus (HSV) protein gD2, and proved that the immune response was comparable to that of conventional intramuscular DNA vaccination at a high dose, and provided protection against lethal challenge with vaginal HSV-2 in a mouse model [47]. In another study, positively charged poly (lactic-co-glycolic) acid nanoparticles were coated with DNA plasmid solution expressing anthrax protective antigen, and the vaccine solution was used to immunize mice by dripping the solution onto skin that were pretreated with microneedle rollers [48]. As a result, transcutaneous DNA vaccination induced comparable responses to intramuscular administration, but a specific mucosal immunity and more balanced type 1 and 2 helper T cell responses. In another study, microneedles composed of dissolvable polyelectrolyte multilayers encapsulating DNA vaccines for a model human immunodeficiency virus antigen has been developed, which promoted local transfection and prolonged persistence of antigens in the skin [49].
Conclusion
Since microneedles were first invented in the late 1990's, there has occurred a conspicuous paradigm shift in terms of modulating the delivery route of vaccines to induce the greatest response with the most economic doses. The past decade has seen a burgeoning of research efforts worldwide to investigate the parameters of microneedle-mediated vaccine delivery in terms of eliciting antigen-specific B and T cell responses, effective doses, patient compliance, and stability issues in comparison to conventional intramuscular or subcutaneous delivery methods. The gamut of microneedle applications in therapeutic vaccines is manifold, ranging from the diverse types of virus and bacterial vaccines commonly available to the novel concept of DNA vaccines yet to be utilized for human use. On a similar note, a resultant diversification in the types of microneedle constitution have emerged, from the earlier forms of vaccine coated solid microneedles to injectable hollow and dissolving implantable types. The variation in the microneedle types would prove useful in controlling the kinetics of vaccine release. Finally, while the original intent of the creation of microneedles was a facilitated administration to a rich network of APCs underneath the skin, recent studies in basic immunology have begun to elucidate the biological mechanisms that are responsible for the positive results that have been seen in many microneedle studies to date. In addition, clinical studies in the elderly pertaining to influenza vaccination using microneedles have indicated higher serological protection over conventional subcutaneous injection and verified the painlessness and safety of microneedles, as there were no notable side reactions save for a mild local erythemia in some patients. Thus, it is plausible that microneedles, by virtue of its dose-sparing advantage, safety, improved serological conversion rate, and better patient compliance, will be sure to establish a firm stand as one of the most effective and easily practiced drug delivery routes, if not replace some of the existing methods, in the near future.
Footnotes
No potential conflict of interest relevant to this article was reported.
This study was supported in part by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Project number: HI13C0826).
References
- 1.Schwartz M. The life and works of Louis Pasteur. J Appl Microbiol. 2001;91:597–601. doi: 10.1046/j.1365-2672.2001.01495.x. [DOI] [PubMed] [Google Scholar]
- 2.Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV) Vaccine. 2006;24:4062–4081. doi: 10.1016/j.vaccine.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 3.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dean CH, Alarcon JB, Waterston AM, et al. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin. 2005;1:106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 5.Hirschberg HJ, van de Wijdeven GG, Kraan H, Amorij JP, Kersten GF. Bioneedles as alternative delivery system for hepatitis B vaccine. J Control Release. 2010;147:211–217. doi: 10.1016/j.jconrel.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204:582–591. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O'Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012;101:1021–1027. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007;14:375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31:3396–3402. doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.del Pilar Martin M, Weldon WC, Zarnitsyn VG, et al. Local response to microneedle-based influenza immunization in the skin. MBio. 2012;3:e00012-12. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrianov AK, DeCollibus DP, Gillis HA, et al. Poly[di(carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci U S A. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weldon WC, Martin MP, Zarnitsyn V, et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol. 2011;18:647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernando GJ, Chen X, Prow TW, et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One. 2010;5:e10266. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010;6:1785–1793. doi: 10.1002/smll.201000326. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Corbett HJ, Yukiko SR, et al. Site-selectively coated, densely-packed microprojection array patches for targeted delivery of vaccines to skin. Adv Funct Mater. 2011;21:464–473. [Google Scholar]
- 21.Koutsonanos DG, Vassilieva EV, Stavropoulou A, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leroux-Roels I, Vets E, Freese R, et al. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–6619. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 23.Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 25.Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 26.Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010;5:e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11:1193–1201. doi: 10.1208/s12249-010-9471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YC, Quan FS, Song JM, et al. Influenza immunization with trehalose-stabilized virus-like particle vaccine using microneedles. Procedia Vaccinol. 2010;2:15–19. doi: 10.1016/j.provac.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan FS, Kim YC, Vunnava A, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84:7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song JM, Kim YC, Lipatov AS, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010;17:1381–1389. doi: 10.1128/CVI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song JM, Kim YC, Barlow PG, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88:244–247. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147:326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearton M, Kang SM, Song JM, et al. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine. 2010;28:6104–6113. doi: 10.1016/j.vaccine.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikszta JA, Sullivan VJ, Dean C, et al. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191:278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 35.Wendorf JR, Ghartey-Tagoe EB, Williams SC, Enioutina E, Singh P, Cleary GW. Transdermal delivery of macromolecules using solid-state biodegradable microstructures. Pharm Res. 2011;28:22–30. doi: 10.1007/s11095-010-0174-y. [DOI] [PubMed] [Google Scholar]
- 36.Mikszta JA, Dekker JP, 3rd, Harvey NG, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006;74:6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding Z, Van Riet E, Romeijn S, Kersten GF, Jiskoot W, Bouwstra JA. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res. 2009;26:1635–1643. doi: 10.1007/s11095-009-9874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Z, Verbaan FJ, Bivas-Benita M, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009;136:71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Bal SM, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations. Pharm Res. 2010;27:1837–1847. doi: 10.1007/s11095-010-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirschberg HJ, van de Wijdeven GG, Kelder AB, van den Dobbelsteen GP, Kersten GF. Bioneedles as vaccine carriers. Vaccine. 2008;26:2389–2397. doi: 10.1016/j.vaccine.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 41.Hiraishi Y, Nandakumar S, Choi SO, et al. Bacillus Calmette-Guerin vaccination using a microneedle patch. Vaccine. 2011;29:2626–2636. doi: 10.1016/j.vaccine.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, D'Souza AJ, Alarcon JB, et al. Protective immunity in mice achieved with dry powder formulation and alternative delivery of plague F1-V vaccine. Clin Vaccine Immunol. 2009;16:719–725. doi: 10.1128/CVI.00447-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morefield GL, Tammariello RF, Purcell BK, et al. An alternative approach to combination vaccines: intradermal administration of isolated components for control of anthrax, botulism, plague and staphylococcal toxic shock. J Immune Based Ther Vaccines. 2008;6:5. doi: 10.1186/1476-8518-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prow TW, Chen X, Prow NA, et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010;6:1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 45.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 47.Kask AS, Chen X, Marshak JO, et al. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine. 2010;28:7483–7491. doi: 10.1016/j.vaccine.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, Wonganan P, Sandoval MA, Li X, Zhu S, Cui Z. Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles. J Control Release. 2012;163:230–239. doi: 10.1016/j.jconrel.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeMuth PC, Min Y, Huang B, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013;12:367–376. doi: 10.1038/nmat3550. [DOI] [PMC free article] [PubMed] [Google Scholar]