Abstract
Airway gene delivery is a promising strategy to treat patients with life-threatening lung diseases such as cystic fibrosis (CF). However, this strategy has to be evaluated in large animal preclinical studies in order to translate it to human applications. Because of anatomic and physiological similarities between the human and pig lungs, we utilized pig as a large animal model to examine the safety and efficiency of airway gene delivery with helper-dependent adenoviral vectors. Helper-dependent vectors carrying human CFTR or reporter gene LacZ were aerosolized intratracheally into pigs under bronchoscopic guidance. We found that the LacZ reporter and hCFTR transgene products were efficiently expressed in lung airway epithelial cells. The transgene vectors with this delivery can also reach to submucosal glands. Moreover, the hCFTR transgene protein localized to the apical membrane of both ciliated and nonciliated epithelial cells, mirroring the location of wild-type CF transmembrane conductance regulator (CFTR). Aerosol delivery procedure was well tolerated by pigs without showing systemic toxicity based on the limited number of pigs tested. These results provide important insights into developing clinical strategies for human CF lung gene therapy.
Keywords: cystic fibrosis, lung gene delivery, pig
Introduction
Gene therapy has been shown to offer clinical benefits to patients with diseases caused by genetic mutations such as Lebers congenital amaurosis, human X-linked retinitis pigmentosa, and adenosine deaminase-deficient severe combined immunodeficiency.1,2,3,4,5 However, efficient gene transfer to airway epithelial cells of cystic fibrosis (CF) patients has not been achieved. CF is the most common life-threatening autosomal recessive disease, caused by mutations in an epithelial chloride channel encoded by the CF transmembrane conductance regulator (CFTR) gene.6,7,8 CFTR is a membrane protein expressed on the surface of multiple epithelial cell types and submucosal glands of the conducting airways and it functions as a cAMP-activated chloride channel. The majority of CF patients carry the genetic mutation that lacks a phenylalanine at the amino acid position 508 of the protein. This mutation, ΔF508, decreases stability and shortens the functional half life of CFTR, and reduces CFTR trafficking to the apical membrane leading to impaired activation of chloride ion conductance.9,10 Although CFTR dysfunction involves multiple organs, lung disease is the main cause of morbidity and mortality in CF patients. Therefore, gene therapy strategies for CF abnormality are mainly focused on delivering the required amount of fully functional CFTR to airway epithelial cells and submucosal glands.
Although small animal models, such as CF mice, have been used for gene transfer vector testing and disease phenotype rescue, studies with mouse models alone are likely insufficient to predict the clinical efficacy of gene delivery methods in CF patients due to major differences in lung cell biology and anatomic scale between mice and humans. Development of large animal models for testing putative gene therapy strategies will facilitate in designing human clinical trials. Pigs are widely used as large animal models in medical research. They have been excellent for studying lung physiology, pathological mechanisms of lung diseases, and development of therapeutic strategies.11,12,13 Pigs share many features of lung biology with humans, including anatomy, histology, and immune responses. At the molecular level, pig and human CFTR are highly conserved in both the cDNA and the predicted polypeptide.14 In addition, the tissue-specific expression patterns of pig CFTR are very similar to human, with CFTR expression in bronchial epithelium and submucosal glands.15,16,17,18 The major disease manifestations of CF lungs in pigs are also similar to those observed in humans, including infection in the lung, airway inflammation, and accumulation of mucus in the airway.19 Another important advantage of the pig model is their longevity, which enables us to investigate the pathogenesis of lung diseases and also to assess long-term therapeutic outcomes.
In CF gene therapy, adenoviral (Ad) vectors have been widely used in animal and clinical studies. Of the 25 clinical trials for CF since 1993, 10 have used Ad vectors.20,21 Recombinant adenovirus with tropism for airway cells can efficiently transduce both dividing and nondividing cells. Because conventional Ad vectors inflict strong host immune reactions partially due to the leaky expression of viral genes, a new generation of Ad vector, helper-dependent Ad vector (HD-Ad), was created with all viral coding sequences deleted. Deletion of the viral coding sequences not only generates a larger gene-carrying capacity but also makes the vectors less immunogenic in systemic and local administration than the conventional Ad vectors.22,23,24 Because of the large gene carrying capacity, HD-Ad vectors can be used to deliver multiple genes with long promoters. With an epithelial cell-specific promoter, HD-Ad vectors have been shown to transduce both proximal and distal airway epithelial cells of mice, rabbits, and baboons.25,26,27,28,29,30 Furthermore, HD-Ad-mediated human CFTR gene expression was shown to be specifically localized in the appropriate target cell types in CFTR knockout mice and improved the resistance of these mice to acute lung infection.28
Our goal for this study was to develop a protocol to efficiently deliver human CFTR gene to pig lungs. Only when efficient therapeutic gene expression is achieved, other challenges such as maintaining long-term expression and vector readministration can be examined. The comparable size and volume of pig and human lungs make it advantageous to study lung gene delivery by using bronchoscopy to investigate the efficacy and safety of gene transfer procedures. In this study, we used wild-type, out-bred pigs as a large animal model for aerosolized airway gene transfer to investigate the technique of viral vector delivery, to explore the distribution and expression of foreign genes, and to analyze the host local and systemic toxicity to the HD-Ad vector. For the first time, we report that HD-Ad-delivered human CFTR protein was expressed at the apical membranes of pig airway epithelial cells and submucosal glands without systemic toxicity. Our data will be valuable for developing a safe and efficacious preclinical model of CFTR gene transfer with HD-Ad vectors that are suitable for use in future clinical trials.
Results
HD-Ad-vector formulation and aerosol delivery to pig lungs
To enhance delivery efficiency, HD-Ad vectors carrying the human CFTR or reporter gene LacZ were suspended in PBS with 0.01% lysophosphatidylcholine (LPC). We had previously shown that LPC at 0.1% dramatically enhanced HD-Ad vector delivery to rabbit lung airways, likely by opening intercellular tight junctions.27 To reduce the potential negative effects of LPC on the host, we used 0.01% LPC in this study. To confirm that the delivery process did not adversely affect viral vector activity, the suspended vector carrying the reporter gene LacZ was aerosolized through AeroProbe catheter rated at a mass median diameter of 15 µm and recovered. The recovered vector was used to transduce HeLa cells and the vector efficiency was tested by X-gal staining and activity assays. We found that more than 90% of the vector activity was sustained following aerosolization through the catheter (Supplementary Figure S1). Aerosol delivery was performed using a bronchoscope, catheter, and ventilation system as described in Methods. There were no apparent adverse effects on the pig's respiration during the delivery.
Efficient expression of reporter gene LacZ in pig airways
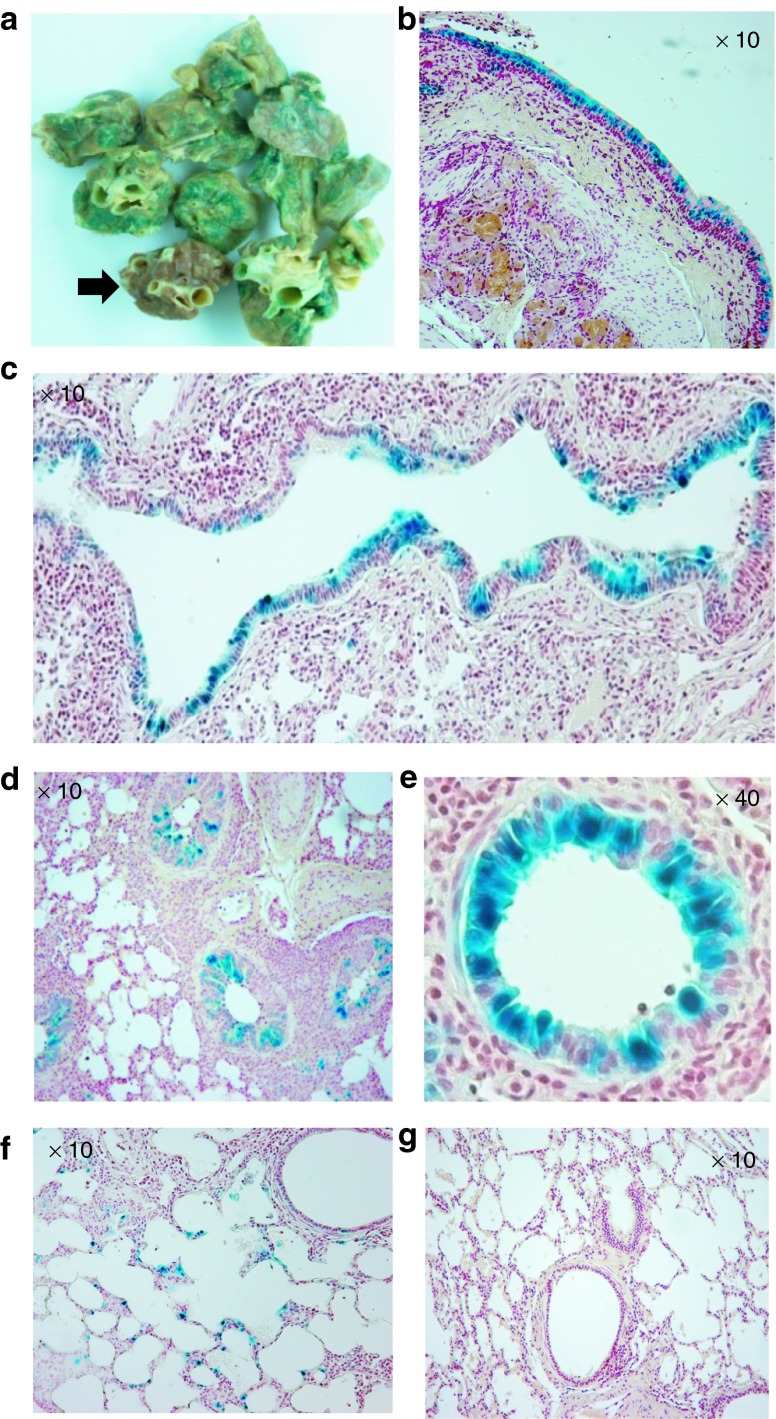
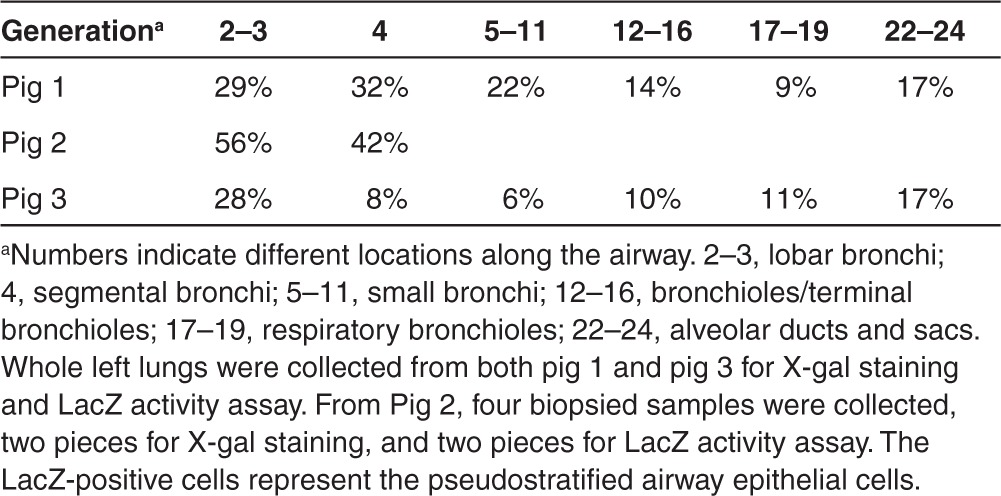
To examine whether pig lung epithelia can be efficiently transduced, 1 week following HD-Ad-lacZ vector transduction, three pigs received vector delivery were killed and the lungs were stained with X-gal to evaluate LacZ gene expression. We cut 2- to 3-cm thick sections from both the right and left lungs, making sure that sections were taken from the upper (proximal), middle, and lower (distal) regions of each lobe, including all four lobes of the right lung and two lobes of the left lung. X-gal staining of the bronchial tree revealed that all segments from the left lung had strong LacZ expression in the surface epithelium (Figure 1a). By contrast, segments from the right lung, which served as a negative control, did not show any positive staining (an arrow in Figure 1a). Histological analysis further confirmed that LacZ was expressed in the epithelial cells of the left lung with specific nuclear localization in the segmental bronchus (Figure 1b), bronchioles (Figure 1c,d,e), and respiratory bronchioles (Figure 1f). There were around 20% cells showing positive staining from the bronchial pseudostratified cells (Figure 1b and Table 1). This expression pattern conferred by the human cytokeratin 18 gene promoter in the LacZ HD-Ad vector transduced airway is similar to that observed in mice and rabbits.24,27 Alveolar tissue from the right lung (Figure 1g) is shown as a negative control for x-gal staining. LacZ gene expression levels were quantified in three pigs (Supplementary Table S1).
Figure 1.
Expression of the reporter gene LacZ in pig lungs. HD-Ad-K18-LacZ vector particles (5 × 1012) were delivered to the pig's left lung. One week following vector delivery, segments throughout the bronchial tree were collected for X-gal staining. The right lung acted as a negative control without vector delivery. (a) Gross examination of lung segments for X-gal staining. Sections stained blue are from the transduced left lung and a section shown with an arrow is a negative control from untransfected right lung. (b) Microscopic pictures of left main stem bronchus. (c–e) Left bronchiole, small bronchiole, small bronchiole with high magnification. (f) Left lung respiratory bronchiole, showing epithelial specific X-gal staining. (g) Right lung respiratory bronchiole and alveoli, negative for X-gal staining.
Table 1. LacZ positive cells in pig lung airways.
Reporter gene LacZ is expressed in submucosal glands in pig airways
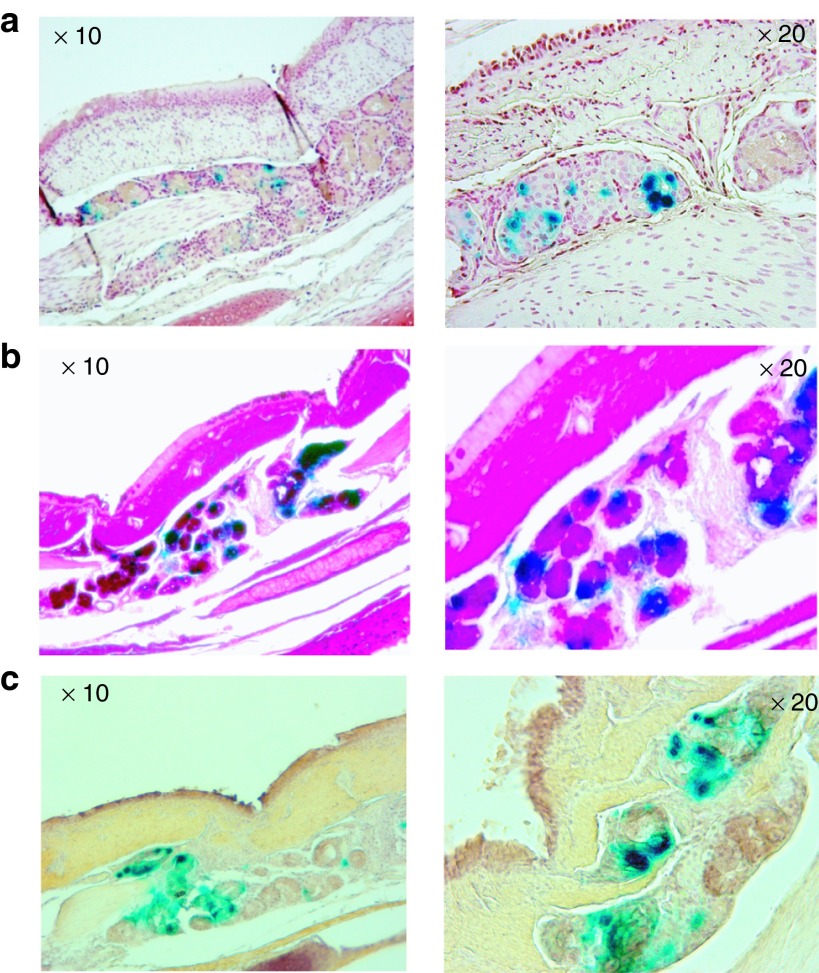
Because submucosal glands are considered an important target for CF lung gene therapy, we examined whether these glands could be transduced by vector delivery. Because of their anatomic location, delivery of gene therapy vectors to these glands would be expected to be difficult. Of note, we found that airway submucosal glands showed strong LacZ expression 1 week after vector transduction. Because the LacZ reporter contains a nuclear localization signal, the blue staining of the nuclei of the gland cells confirmed that these cells were indeed transduced by the vector (Figure 2a–c). Further, costaining with PAS and an anti-mucin 5 antibody confirmed that the positively stained cells were submucosal gland cells (Figure 2b,c).
Figure 2.
Expression of the reporter gene LacZ in pig airway submucosal glands. Photomicrographs of submucosal glands stained with X-gal and (a) Neutral red as counter staining (red color), (b) PAS staining (magenta color), (c) Anti-human mucin 5 antibody (brown color).
Efficient expression of the hCFTR gene in pig airway epithelia following vector delivery
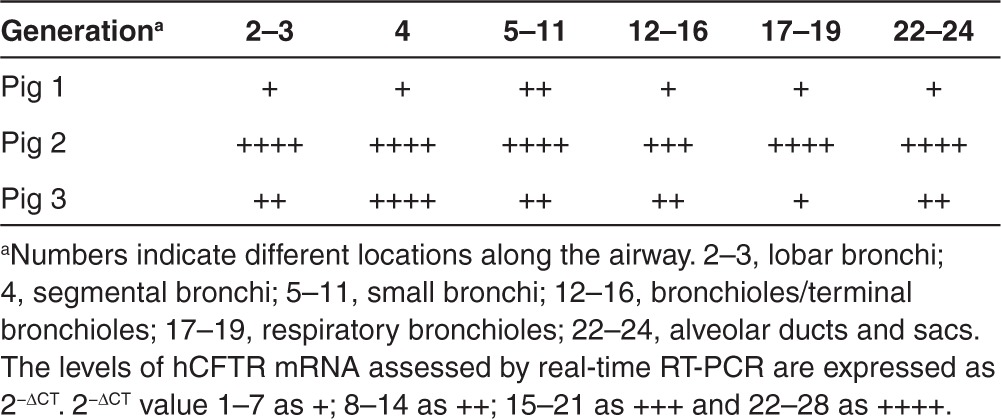
Demonstrating successful reporter gene delivery to pig airway epithelia and submucosal glands through an HD-Ad vector suggested that our vector system and delivery method were amenable to CFTR gene delivery. Using the same method, we delivered the HD-Ad vector carrying the human CFTR gene driven by the human cytokeratin 18 gene promoter to the left lung of pigs. The same numbers of vector particles were used as in the LacZ gene delivery experiment and CFTR mRNA and protein expression were analyzed 7 days after delivery in three pigs. From each pig, 15–18 pieces of lung tissue were collected from the left lung throughout the whole bronchial tree at intervals of around 5 mm per section and used to investigate tissue distribution of hCFTR mRNA and protein. Primers specific to the exogenous hCFTR were used to detect the mRNA by real-time RT-PCR. The hCFTR mRNA is expected to be expressed specifically at the airway epithelium because the expression of the hCFTR is under the control of the human cytokeratin-18 gene promoter. The hCFTR mRNA expression was normalized to that of the endogenous pig CFTR mRNA. Similar to LacZ expression, hCFTR mRNA was found in the entire left lung throughout all segments in three separate pigs (Table 2). All pieces of the pig's left lung tissues analyzed showed positive hCFTR mRNA expression while none of the biopsied lung tissues taken before vector delivery did (data not shown). We observed that one of the pigs had expressed lower levels of hCFTR mRNA, but subsequent investigation revealed that this pig suffered from pneumonia before delivery as evidenced by significant leukocyte infiltration present in the bronchoalveolar lavage fluid (BALF) collected before vector delivery. Endogenous pig CFTR expression levels were similar in all samples before and after vector administration indicating that our detection methods are valid.
Table 2. Expression levels of hCFTR mRNA in pig lung airways.
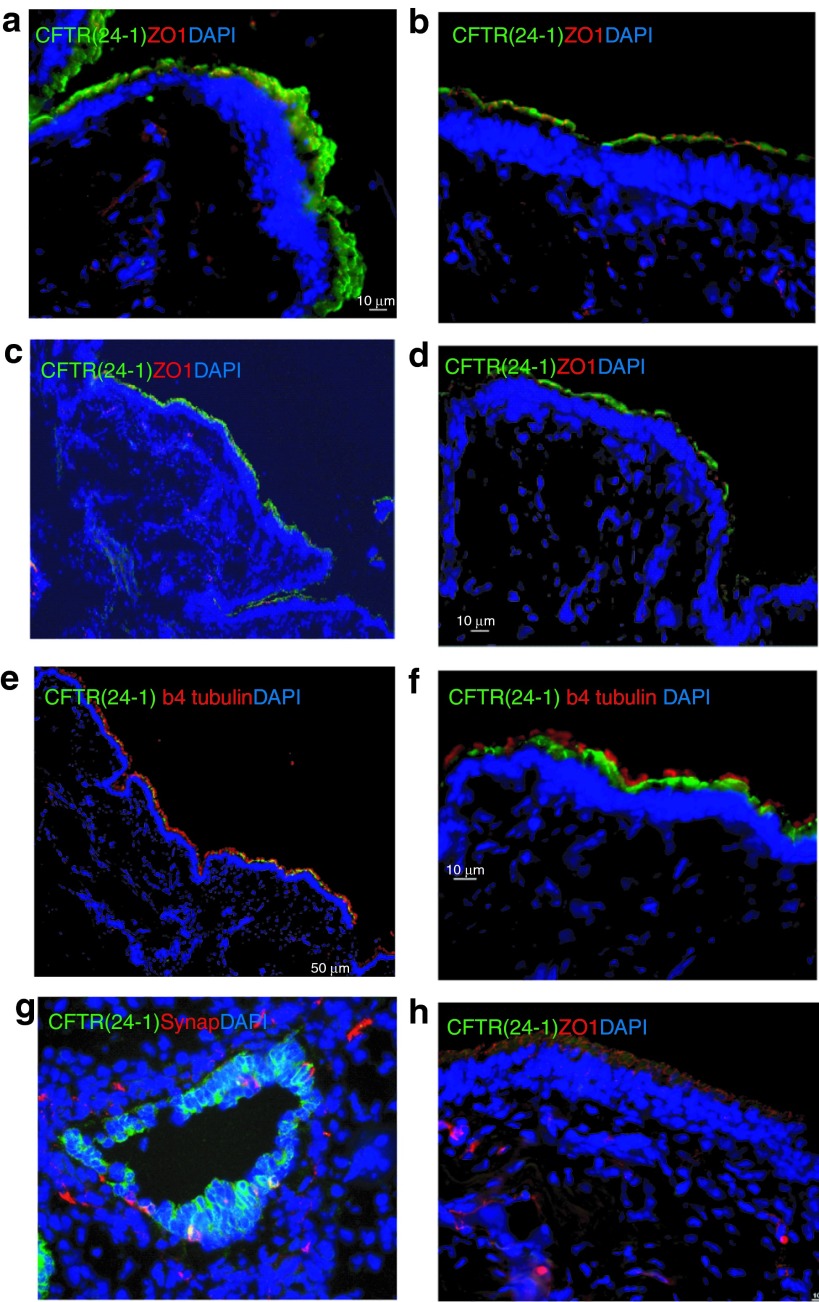
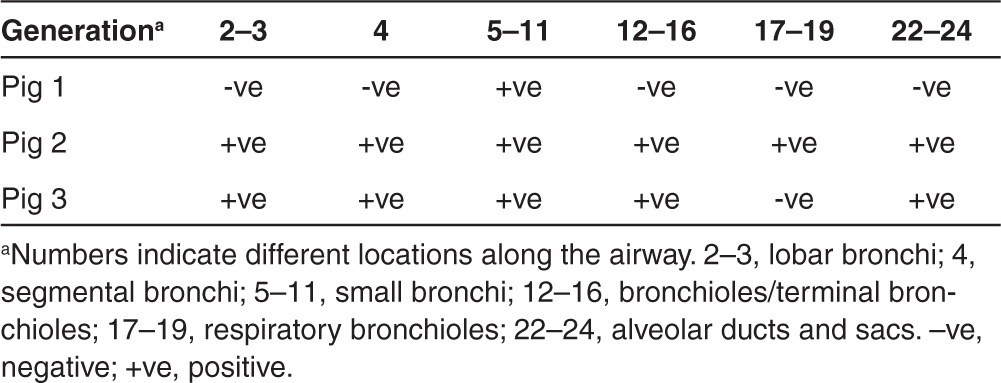
Biological function of CFTR depends on its level of apical expression. To assess the apical expression of the exogenous hCFTR protein in pig left lung tissues, immunostaining was carried out using an anti-hCFTR monoclonal antibody (clone 24-1). After testing a group of antibodies, we found that this monoclonal antibody bound only to human CFTR without crossreacting to pig CFTR protein. Human CFTR expression was seen throughout the lung in agreement with the mRNA expression (Figure 3 and Tables 3 and Table 4). As a positive control, a section of human fetal lung tissue was stained with this antibody showing strong immunofluorescence around the cell membranes in the epithelium of a small bronchiole (Figure 3g). The hCFTR immunofluorescence of the pig airway sections revealed a clear CFTR protein signal in the apical plasma membrane of surface epithelia throughout the whole lung segments including segmented bronchi, small bronchi, and bronchioles (Figure 3a–d). Most of the hCFTR stained cells were identified as ciliated cells by costaining with β-4-tubulin antibody, a marker for these cells (Figure 3e, f). No signal was observed in sections from lung tissues before vector delivery (Figure 3h). To further confirm that the signals were only from the exogenous hCFTR, but not from endogenous pig CFTR, polyclonal anti-CFTR antibodies (GTX101299, Gene Tex) were also used for immunofluorescence staining in these lung tissues. The monoclonal antibody (24-1) did not recognize the endogenous pig CFTR protein while polyclonal anti-CFTR antibodies did (Supplementary Figure S2). Furthermore, the expression level of CFTR protein is consistent with that of CFTR mRNA in all three pigs (Tables 2 and 3).
Figure 3.
Expression of hCFTR protein in pig lung epithelia. HD-Ad-K18-hCFTR vectors were delivered to the pig's left lung and 1 week after delivery hCFTR protein in the airway was detected by immunofluorescence with anti-hCFTR monoclonal Ab (24-1). (a–d) The expression of vector-specific CFTR protein at pig segmental bronchi, small bronchi, and bronchiole epithelial cells. (e and f) Costaining with anti-hCFTR monoclonal Ab (24-1) and β-4-tubulin antibody. As antibody controls, (g) human fetal small bronchiole, (h) bronchi from nontranduced pig lung were stained with anti-hCFTR monoclonal Ab (24-1). Green: CFTR monoclonal Ab (24-1) (a–h), Red: ZO-1 (a, b, c, d, h), β4 tubulin (e and f), Synaptophysin (g); Blue: DAPI. Scale bar is 50 µm in low magnification images, 10 µm in high magnification images.
Table 3. Vector cystic fibrosis transmembrane conductance regulator (CFTR)-positive cells in pig airways.
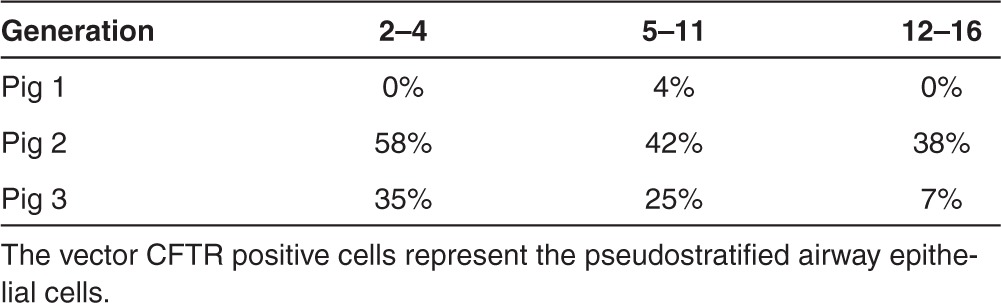
Table 4. Distribution of hCFTR protein in pig lung airways assessed by immunostaining.
Human CFTR protein expressed in pig airway submucosal glands
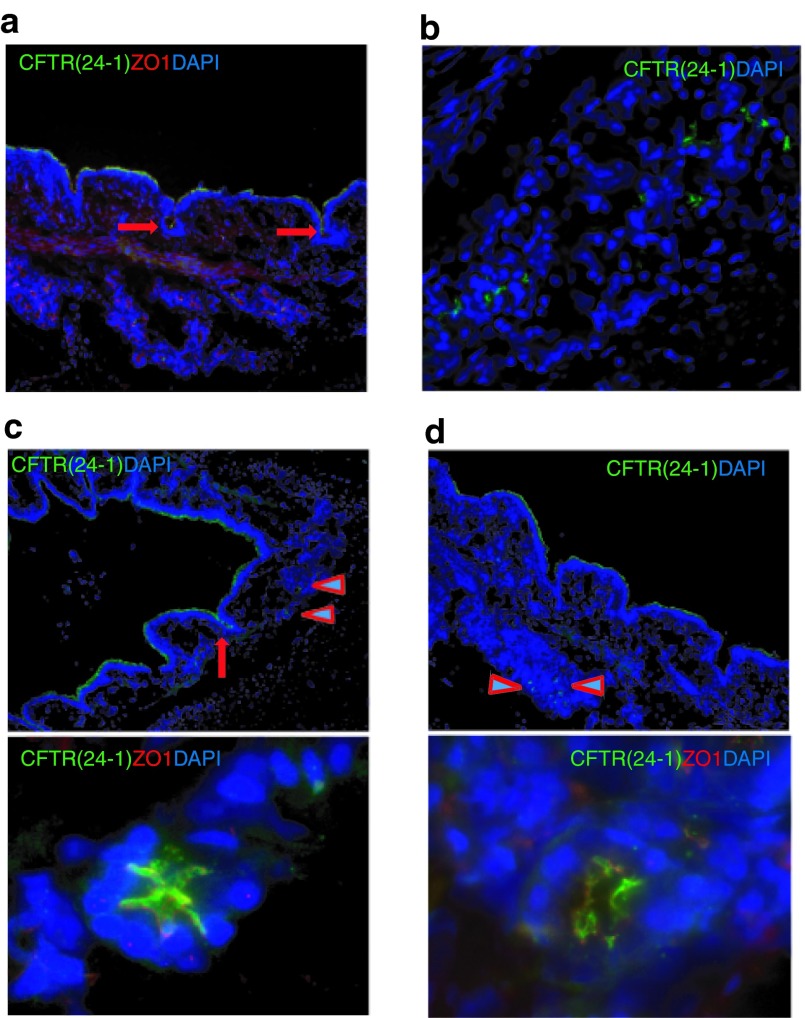
Human submucosal glands normally express high levels of CFTR and they secrete mucus and a variety of molecules for airway defense. They are considered important target tissues for CF gene therapy. With our vector delivery, we found that the hCFTR protein was expressed in submucosal glands as detected with the anti-hCFTR monoclonal antibody with around 1–2% submucosal glands showing positive signals. hCFTR protein appeared on the cell membranes of submucosal glands and ducts (Figure 4). This finding is consistent with the result of our reporter gene delivery (Figure 2). The successful delivery of HD-Ad vectors to submucosal glands may be due to the positive pressure generated during aerosolization.
Figure 4.
Expression of hCFTR protein in pig lung submucosal glands. hCFTR protein was detected by immunofluorescence with anti-hCFTR monoclonal Ab (24-1) in submucosal glands from (a–d) different areas of lung tissues. (a and c) Arrows indicate positively staining gland ducts. (c and d) Upper panels are low magnification and lower panels are high magnification. (c and d) Arrowheads in upper panels depict submucosal gland acini. Green: CFTR monoclonal Ab (24-1), Red: ZO1; Blue: DAPI.
No systemic toxicity was observed following aerosol delivery of HD-Ad vectors
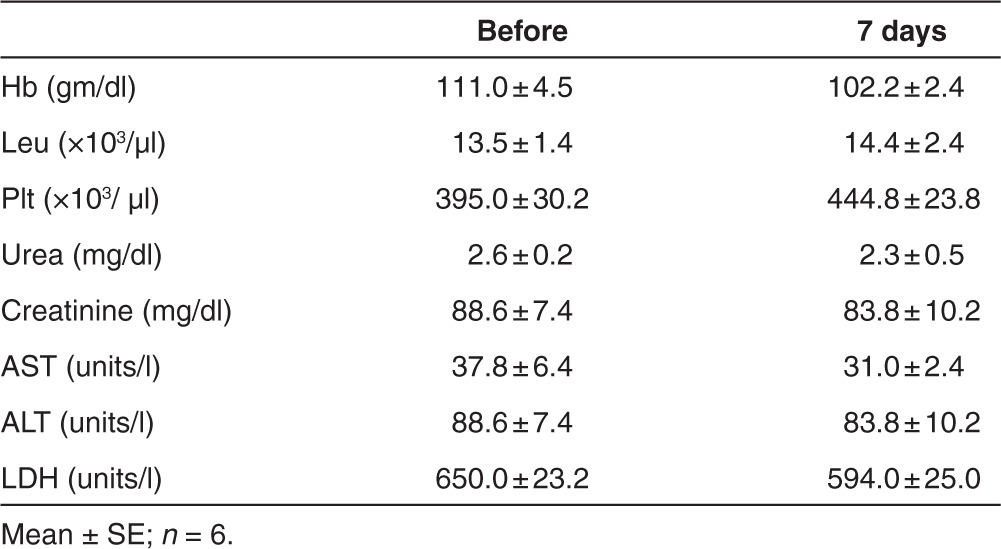
To assess the systemic toxicity following airway delivery of the HD-Ad vectors, the body weight, temperature, and clinical parameters of the pigs were monitored. The body weight increased with time similar to the untreated pigs. The body temperature did not change before and after vector delivery. No differences were observed between before and after 24 hours in hemoglobin, total white blood cells, neutrophils, lymphocytes, and platelets as well as liver and kidney function in three pigs (Table 5). Hemoglobin, leucocytes, and platelets 1 week after vector delivery were similar to that before delivery. Liver and kidney function did not change significantly before and after treatment as measured by aspartate transaminase, alanine transaminase, lactate dehydrogenase, and urea as well as creatinine at 7 days in six pigs (Table 6). These observations suggest that there was no major acute systemic toxicity after HD-Ad vector delivery.
Table 5. No acute systemic toxicity observed following helper-dependent (HD) vector aerosol delivery.

Table 6. No systemic toxicity observed after 1 week of aerosol delivery of helper-dependent Ad vector.
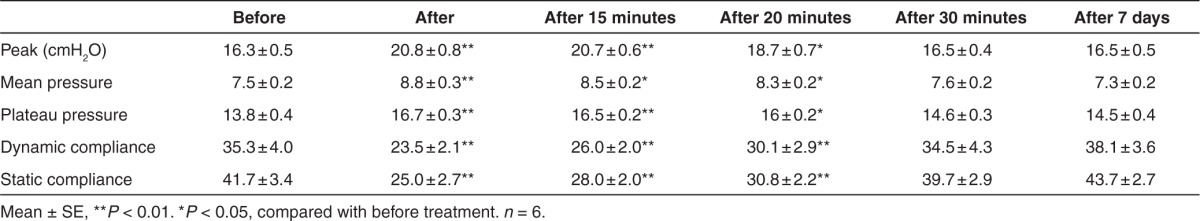
Minor changes in lung function were noticed, but recovered 30 minutes following vector delivery
Pulmonary function tests were performed on six animals immediately before and after delivery as well as before sacrifice. These tests included arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), peak-, mean-, and plateau-airway pressure, and dynamic and static compliance (Table 7). The mean, airway pressure the peak and plateau pressure increased transiently with delivery. The peak pressure is the pressure measured by the ventilator in major airways, and strongly reflects airway resistance. The plateau pressure is the pressure applied to the small airways and alveoli. The mean peak and plateau pressure increased marginally after vector delivery and returned to previous values within 30 minutes. Consequently, the static and dynamic compliance decreased marginally by 30 minutes (Table 7). These changes may have been caused by a small air leak by introduction of the bronchoscope through the trachea, transient partial airway occlusion by the bronchoscope, or by the vector itself. Results at 7 days postdelivery were similar to that of predelivery. These results suggest that the aerosol delivery of 5 × 1012 HD-Ad-vectors with LPC in 5-ml solution was well tolerated by pigs tested.
Table 7. Pulmonary mechanics before and after helper-dependent vector treatment with aerosol delivery.
Local inflammatory responses to aerosol delivery and vector transduction with cytokine production and neutrophil infiltration
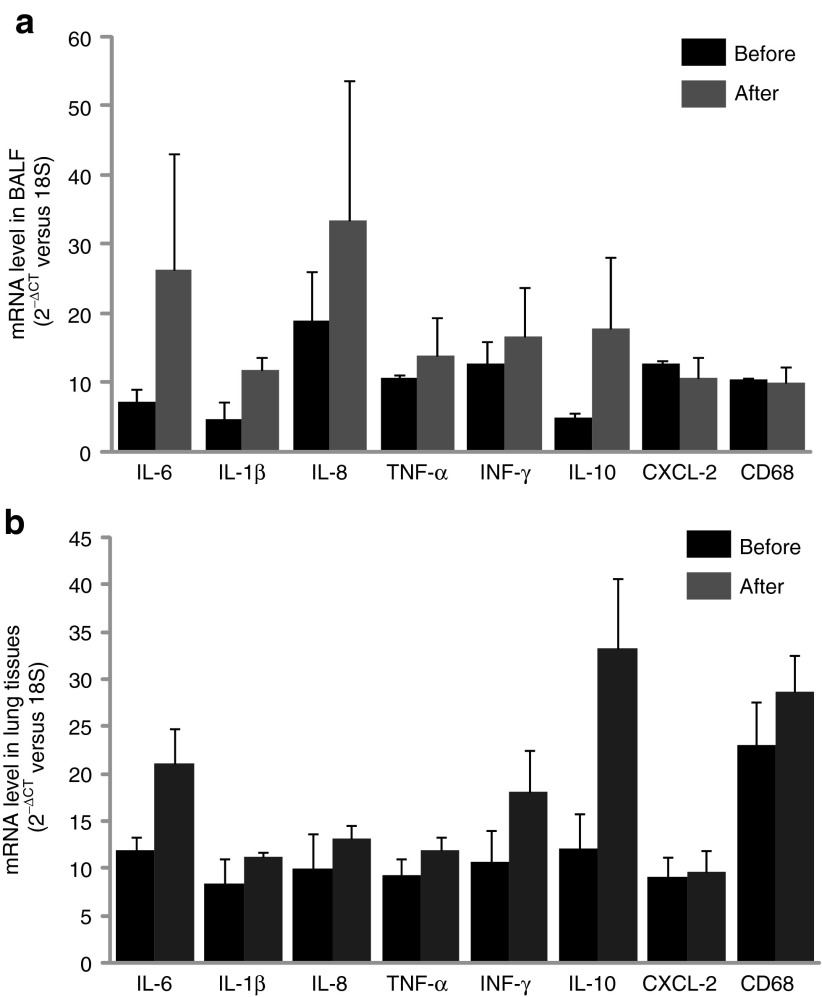
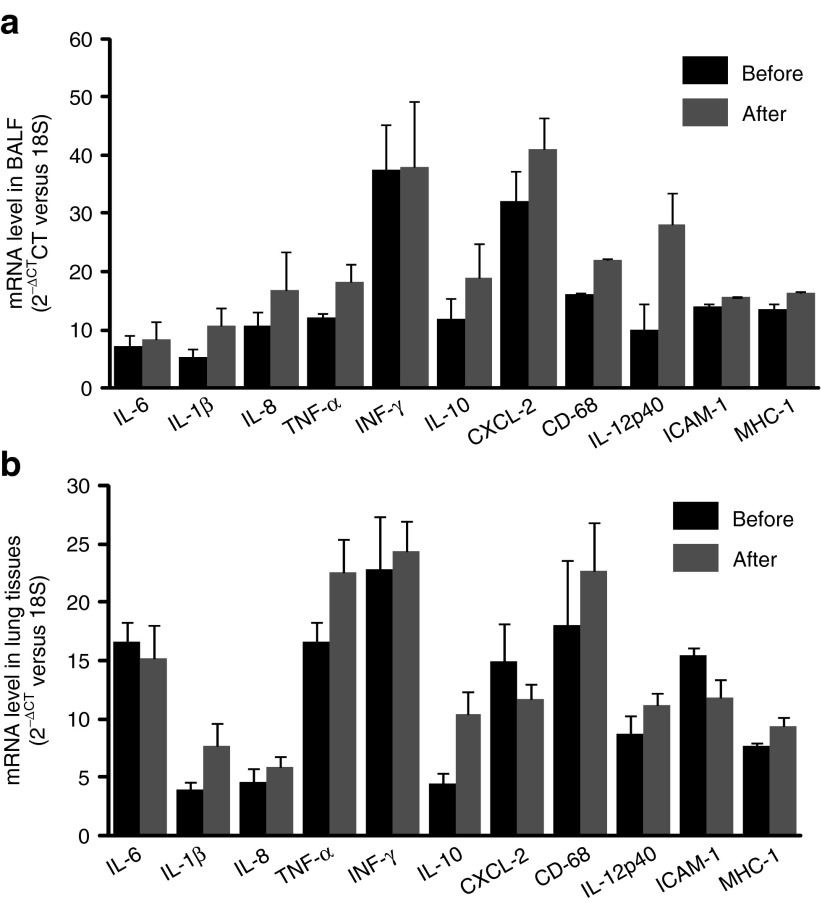
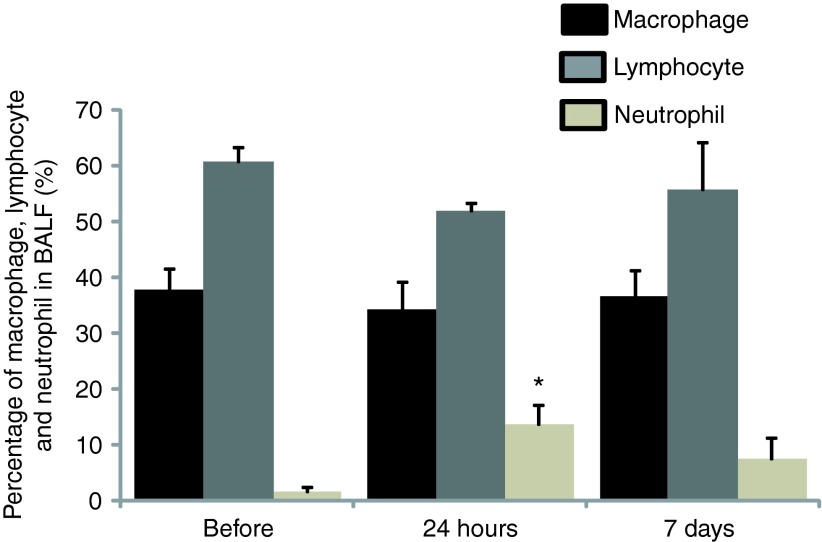
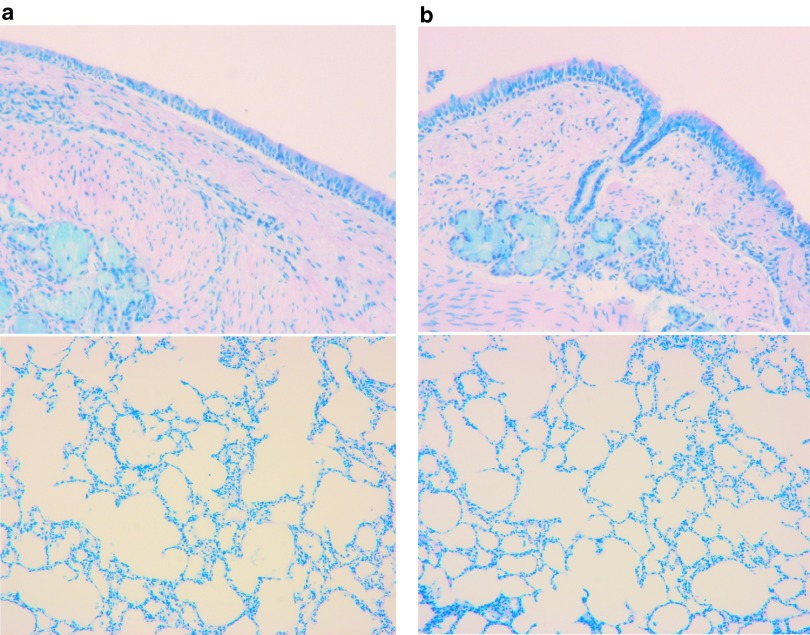
Levels of cytokine and chemokine gene expression were also determined in airway cells. BAL fluid and lung airway tissues were collected from all animals before treatment and at 24 hours (n = 3) as well as 7 days (n = 6) after gene transfer to measure the mRNA levels of cytokines and chemokines such as IL-6, IL-1β, IL-8, TNF-α, INF-γ, IL-10, CXCL-2, and CD68. The RNA expression was determined by quantitative RT-PCR with specific primers (Supplementary Table S2). ELISA for cytokines was not performed because antibodies against most pig cytokines are not available. We found that there was an increase in expression of some cytokine genes in 24 hours in BALF cells such as IL-6, IL-1β, IL-8, and IL-10 (Figure 5a). Similar to BALF cells, levels of IL-6, IL-1β, IL-8, INF-γ, and IL-10 in lung tissues 24 hours after vector delivery were also increased (Figure 5b). Although there was no significant difference of these products in both lung tissues and BAL cells with paired t-test analysis based on the three pigs, these changes may be significant given larger numbers of animals tested. We did not observe significant differences in cytokine/chemokine/adhesion molecule mRNA levels from BAL cells and lung tissues on 7th day between vector transduced and nontransduced (Figure 6a,b). Inflammatory cells in BALF were analyzed with Giemsa staining on cytospin preparations following vector administration at 24 hours and 7 days, and the percentage of neutrophils, macrophages, and lymphocytes were calculated. We found that there was an increase in neutrophils infiltrated in the airway at 24 hours after vector delivery compared with that before vector delivery (P < 0.05; Figure 7 and Supplementary Figure S3) with decrease in numbers by day 7. Inflammatory cells in lung tissue were analyzed by histology with Giemsa staining. There was no significant difference in inflammatory cell infiltration in bronchi and alveolar regions before and after 1 week vector delivery (Figure 8).
Figure 5.
Cytokine, chemokine, and adhesion molecule RNA expression in lung bronchoalveolar lavage fluid and lung tissues at 24 hours following vector delivery. RNA isolated from (a) pig's BAL cells and (b) lung tissues, before and after vector transduction. mRNA levels were detected with real-time RT-PCR and expressed as 2−ΔCT. Mean ± SEM, n = 3.
Figure 6.
Chemokine and adhesion molecule RNA expression in lung bronchoalveolar lavage fluid and lung tissues 1 week after vector delivery. RNA isolated from pig's (a) BAL cells and (b) lung tissues, before and after vector transduction. mRNA levels were detected with real-time RT-PCR and expressed as 2−ΔCT. Mean ± SEM, n = 6.
Figure 7.
Inflammatory cell infiltration in bronchoalveolar lavage fluid 24 hours and 7 days following vector administration. Cells were counted on Giemsa-stained cytospin preparations before, 24 hours and 7 days after vector delivery. Data were expressed as percentage of macrophages, lymphocytes, and neutrophils of the total cells counted. Mean ± SEM, n = 3, *P < 0.05 compared with before vector delivery. Note the relatively small change only in the neutrophil fraction which then diminishes by 7 days.
Figure 8.
Histological analysis of pig airway. Histological analysis of sections from pig bronchi and alveolar regions (a) before and (b) after 1 week vector delivery stained with Giemsa stain.
Discussion
In this study, we focused on examining the efficiency and localization of transgene expression in pig lungs at a single time point following bronchoscopically guided intratracheal aerosolized Hd-Ad vector administration. Large animal models for lung gene delivery, such as pigs, provide more relevant information for the design of clinical studies because their lungs are more similar to humans than small animals in anatomy, histology, and biology. We demonstrated that both the LacZ and vector-specific hCFTR gene products were strongly displayed at the airway epithelia throughout the pig lung following aerosol delivery. The transgene vector was evenly distributed, extending from the mainstream bronchus down to the respiratory bronchioles (Figure 1a). We used real-time RT-PCR with human CFTR-specific primers to detect the exogenous human CFTR mRNA expression. Even though we normalized the levels of the human CFTR mRNA to that of the pig CFTR mRNA, since different primers were used for the analysis, the levels of human CFTR mRNA cannot be directly compared with that of the endogenous CFTR mRNA in pig lung cells.
We found that vector-specific hCFTR protein was expressed in the apical plasma membrane of surface cells in the superficial epithelium, including ciliated cells and nonciliated cells. This mirrors the described localization of wild-type CFTR in the airway epithelium.31,32 Ciliated cells are involved in transepithelial ion transport and mucociliary clearance in the lung and some reports suggest that CFTR is expressed in ciliated cells within the surface epithelium, consistent with a role for CFTR in regulating airway surface liquid volume.32,33 In the non-CF adult human airway, CFTR exhibits a dual function as mediator of Cl− secretion and regulator of epithelial sodium channels mediated Na+ transport that maintains airway surface liquid volume homeostasis.9,34 Indeed, in CF patients with homozygous ΔF508 mutation, there is no significant level of mature CFTR protein or cAMP-dependent Cl− secretion present in airway epithelial cells.32,35 Defects in CFTR in these cells are believed to directly influence the ion transport in the airway surface liquid that cause obstructive lung disease and chronic bacterial infections leading to eventual respiratory failure. Therefore, therapeutic approaches might profit most by focusing on reestablishing CFTR expression and function in the superficial epithelium including ciliated cells. The efficient expression and, more importantly, the properly localized CFTR protein achieved in this study will be important in the design of future animal and clinical studies examining the effectiveness of CFTR gene therapy in reversing the CF phenotype.
Submucosal glands are a major site of CFTR expression in the human airway and play an important role in the pathogenesis of CF lung disease, thus they are an important target for CF lung gene therapy. In non-CF individuals, CFTR is primarily localized in the cells of submucosal glands in bronchial tissues. Submucosal glands generate most of the airway fluid, and defects in CFTR expression leads to abnormal production of mucus31 and failure to clear mucus in human CF lungs. Previously, it was thought that strategies for gene targeting to submucosal glands will likely be dependent on in utero gene transfer to submucosal gland progenitors because of the inaccessibility of these regions from the fully developed airway.36 We report here for the first time that human CFTR can be targeted to the bronchial submucosal glands through aerosol delivery in pig airway with the HD-Ad vector and that the resulting CFTR protein is strongly expressed in the submucosal gland mainly at the cell surface membrane. The HD-Ad vector contains the human cytokeratin-18 gene regulatory elements that specifically target epithelial cells and tracheal submucosal glands in transgenic mice.37 Our previous study has demonstrated that HD-Ad-hCFTR vector can be efficiently delivered to the epithelium of sweat glands in primary culture of human skin tissue.38 How HD-Ad vectors transduce the pig submucosal glands with aerosol delivery is not clear. Griesenbach has reported that the submucosal gland of sheep airway can be transduced with Sendai viral vector using the Trudell AeroProbe.21 It is possible that the aerosol stream generated by 50 or 80 psi can push the viral vector into the submucosal glands through the ducts. If this is the case, viral vectors with therapeutic genes could be delivered to submucosal glands through aerosol generation as a minimally invasive method for clinical CF lung gene therapy.
Safety is always a primary issue for gene transfer in clinical studies. Small animal models are limited for gene delivery safety testing because of the significant differences in physiology and scale between small animals and humans. By contrast, pigs share many features of lung biology with humans, including anatomy, histology, and immune responses. Pig and human bronchi show similar patterns of branching and histology. The devices and techniques used for pig lung gene delivery can be easily adapted to clinical studies for CF lung gene therapy. For example, the size 8 endotracheal tube used in this study for the 25–35 kg (12–15 weeks) pigs matches the tube size suitable for the 12-year-old human lung. The volume of gene vectors is also scaled to humans because of the comparable size of human and pig lungs. In our study, the aerosol delivery of HD-Ad vectors had a temporary impact on some measures of pig lung mechanics, but recovered completely within 30 minutes. Because of this short timeframe, these changes are likely caused by the aerosol delivery procedure itself rather than the vector. All bronchoscopic examinations by virtue of introduction of the bronchoscope will cause some partial airway occlusion and pulmonary atelectasis and be reflected by transiently increased airway pressures and decreases in compliance. There was no evidence of systemic toxicity based on the analyses for blood clinical parameters, including liver and kidney function as well as body temperature 24 hours and 7 days after vector transduction. This indicates that pigs tolerate this strategy of vector administration for therapeutic gene delivery well. Because all equipment utilized is of clinical grade, we anticipate that translation to human use would be straightforward. In this study, HD-Ad vectors were formulated with 0.01% LPC to enhance transduction by disruption of epithelial cells tight junctions in pig lung.27 LPC is a natural component of lung surfactant and is converted in the lung into phosphatidylcholine.39 The final concentration of LPC in this study (0.01%) is similar to that in normal human serum.40 Although there is an increasing chance of airway infection while vector administrated with LPC since LPC may transiently open the airway epithelial cells tight junction, we did not observe signs of infection in our experiments. We expect that this formula of vector treatment could be adapted in future clinical trials.
It is well known that first-generation Ad vectors cause acute production of cytokines and chemokines in a dose-dependent manner after local or systemic administration. One of our recent studies showed that HD-Ad vectors delivered to mouse airways can induce production of inflammatory cytokines and chemokines 6 hours after vector administration.29 In this study, we observed an increase in expression of inflammatory cytokine genes and infiltration of neutrophils in pig airway cells 24 hours after vector delivery. Although the increased levels are well tolerated by pigs, future studies can be designed to further minimize the inflammatory responses by reducing particle numbers of trasgene vector or transiently modulating host immune system.
We recognize that gene delivery in CF airways would be more challenging because of preexisting mucus plugging and inflammation barriers. An important next step will be to utilize CF pig models for testing our gene therapy method. However, currently, the cost associated with these pigs makes it difficult for us to examine our gene transfer technology at this stage of development. We will consider using the CF pig models for testing our gene delivery protocol after we demonstrate long-term transgenic CFTR expression in wild-type pigs.
In this study, we demonstrated that the HD-Ad vector can efficiently transduce pig airway epithelium and submucosal glands through aerosol delivery without significant side effects despite only a small number of pigs tested. The vector-specific human CFTR protein was properly expressed in pig lung epithelial cells at their apical membranes and the surface membranes of submucosal gland cells, similar to normal human CFTR protein expression pattern. This result provides important insights toward the design of clinical strategies for CF lung gene therapy in patients and for studies with the CF pig model.
Materials and methods
Animals. Male Yorkshire pigs weighing 25–35 kg were used for this study. This size range of pigs was compatible with clinically utilized endotracheal tubes and bronchoscopes. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care of Laboratory Animals” published by the National Institutes of Health. The Animal Care Committee of the Toronto General Research Institute approved all studies.
Anesthesia. Pigs were sedated with ketamine (40 mg/kg intramuscularly), anesthetized with inhaled isoflurane (5%), and maintained with propofol (5–8 mg/kg/hour intravenously) and fentanyl citrate (2–20 mg/kg/hour intravenously) for the duration of the procedure. The airway was secured by intubation with a size 8 French endotracheal tube.
HD-Ad vectors and aerosol delivery to pig lung. Design and production of the HD-Ad vectors expressing LacZ or hCFTR used in this study were described previously.24,28 Before vector delivery, 10 ml of BALF was collected using a bronchoscope. For vector aerosolization, an AeroProbe catheter27 was inserted into the working port of the bronchoscope and directly positioned into the center of the left mainstem bronchus. Five milliliter of vector formulation containing 5 × 1012 viral particles and 0.01% of LPC in PBS were aerosolized to the left lung with 80 psi of air pressure through the catheter under bronchoscopic guidance. Pulses of aerosol were manually activated and delivered to the segmental bronchi coming off the left mainstem bronchus by direct visualization. The particle size distribution of the aerosol was in the order of 13 µm.27 The whole procedure took about 20 minutes.
X-gal staining and β-galactosidase activity assay. Pig lungs were serially cut into 2 × 2 cm blocks along the bronchial tree and fixed with a fixative mixture for 4 hours at 4 °C with continuous shaking. Staining was performed with X-gal solution at 37 °C overnight and then washed with 70% ethanol and kept in 10% formaldehyde for photography.24,41 The stained lung tissues were sectioned to reveal LacZ gene expression at the cellular level with neutral red as the counter stain. For β-galactosidase activity assays, pig lung tissues were homogenized in ice-cold buffer containing of 100 mmol/l K-phosphate, 0.2% Triton X-100, 0.5 mmol/l dithiothreitol and proteinase inhibitor cocktail. The homogenate was cleared by centrifugation and supernatants were incubated at 48 °C for 50 minutes to inactivate endogenous enzymes. Samples were assayed for β-galactosidase activity using a chemiluminescence kit (Galacto-Light; Applied Biosystems, Foster City, CA). Values, expressed as relative light units, were normalized for total protein content.
RNA isolation and quantitative RT-PCR. Total RNA from pig lungs was isolated using an RNAeasy kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA), followed by DNase digestion. For quantification, 1 µg of total RNA was reverse transcribed using random hexamers and SuperScript II reverse transcriptase (Invitrogen) following the manufacturer's protocol. Ten nanograms of cDNA were then used as templates for real-time PCR using SYBR Green.29 The primers for detecting hCFTR were designed as one from vector sequence and the other from hCFTR cDNA that cross vector intron (K18CFTR-F CCTGAGTCCTGTCCTTTCTC and R-CGCTGTCTGTATCCTTTCCTC). The following primers, F-GAGAATGGGACAGAGAACTGG and R-CAGTAAGAGAGGCTGGACTG, were used for detecting pig CFTR mRNA.
CFTR immunofluorescence. Double immunofluorescent labeling was performed in pig lung tissues taken before and after vector transduction. To obtain cryosections, pig lungs were embedded in OCT and 5-µm sections were cut. For each pig, a total of 18 blocks were prepared from left cranial and caudal lobes. Cryosections were fixed in methanol for 5 minutes at −20 °C, soaked in 0.2% TritonX-100 in PBS for 5 minutes, blocked with 4% of BSA in PBS for 30 minutes, and then incubated for 2 hours with primary antibodies including CFTR monoclonal antibody (24-1)(R & D, Minneapolis, MN), CFTR polyclonal (Gene Tex, Irvine, CA), and β-4-tubilin (Sigma, Oakville, Ontario), ZO-1 (Zymed, Burlington, Ontario), E-cadherin (Zymed) as well as synaptophysin (Cedarlane, Burlington, Ontario). This procedure was followed by Alexa 488 or Alexa 594-conjugated secondary antibodies (Invitrogen, Burlingto, Ontario).
Airway inflammatory cells analysis. To analyze inflammatory cells in the airway, BALF was collected after injecting 10-ml saline into the left or right lung separately. Cell counts in BALF were performed using microscopy on cytospin preparations after Giemsa staining. More than 2,500 cells were counted for each time point. Neutrophils were clearly identified by their multilobar nuclei.
Cytokine/chemokine/adhesion molecule RNA analysis in lungs. RNA was isolated from pig lung tissues and BALF cells. Inflammatory cytokine gene products were analyzed by real-time RT-PCR. 18S was used to normalize as an internal reference. The primers for cytokine gene detection are listed in Supplementary Table S2.
Statistic analysis. Paired t-test analysis was used to compare data pairs. Data were presented as mean ± SEM. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Activity of HD-Ad-LacZ vector after aerosolization. Figure S2. hCFTR protein detected with anti-hCFTR mono- and poly-antibodies in pig airways. Figure S3. Macrophages, lymphocytes, and neutrophils in BALF. Table S1. Activity of β-galactosidase in pig lung tissues. Table S2. The list of cytokine genes primers for real-time RT-PCR.
Acknowledgments
We thank Simon Lam and Manjunatha Ankathatti Munegowda for reading the manuscript and providing suggestions, and Paul Chartrand for assistance in animal protocol applications. We also thank the Staff in Toronto General Hospital animal facility for taking care of the animals. This work was partially supported by Canadian Cystic Fibrosis Foundation grants to JH and ALC, and JCY was supported by the Surgeon-Scientist Program at the University of Toronto.
Supplementary material
References
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV. Leber congenital amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran WA, Cideciyan AV, Lewin AS, Iwabe S, Khanna H, Sumaroka A, et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc Natl Acad Sci USA. 2012;109:2132–2137. doi: 10.1073/pnas.1118847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Davis PB, Drumm M, Konstan MW. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Engelhardt JF. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur J Hum Genet. 1998;6:12–31. doi: 10.1038/sj.ejhg.5200158. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Chang XB, Bear C, Kartner N, Mohamed A, Riordan JR, et al. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–21598. [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS. From the farm to the lab: the pig as a new model of cystic fibrosis lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L238–L239. doi: 10.1152/ajplung.90311.2008. [DOI] [PubMed] [Google Scholar]
- Ibrahim Z, Busch J, Awwad M, Wagner R, Wells K, Cooper DK. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation. 2006;13:488–499. doi: 10.1111/j.1399-3089.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- Tebbutt SJ, Wardle CJ, Hill DF, Harris A. Molecular analysis of the ovine cystic fibrosis transmembrane conductance regulator gene. Proc Natl Acad Sci USA. 1995;92:2293–2297. doi: 10.1073/pnas.92.6.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, Jr, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Rich DP, DeBerg LG, Welsh MJ. Association of domains within the cystic fibrosis transmembrane conductance regulator. Biochemistry. 1997;36:1287–1294. doi: 10.1021/bi962174s. [DOI] [PubMed] [Google Scholar]
- Plog S, Mundhenk L, Bothe MK, Klymiuk N, Gruber AD. Tissue and cellular expression patterns of porcine CFTR: similarities to and differences from human CFTR. J Histochem Cytochem. 2010;58:785–797. doi: 10.1369/jhc.2010.955377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ST, Trout L, Bebök Z, Sorscher EJ, Crews A. CFTR involvement in chloride, bicarbonate, and liquid secretion by airway submucosal glands. Am J Physiol. 1999;277 4 Pt 1:L694–L699. doi: 10.1152/ajplung.1999.277.4.L694. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakland M, Sinn PL, McCray PB., Jr Advances in cell and gene-based therapies for cystic fibrosis lung disease. Mol Ther. 2012;20:1108–1115. doi: 10.1038/mt.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesenbach U, McLachlan G, Owaki T, Somerton L, Shu T, Baker A, et al. Validation of recombinant Sendai virus in a non-natural host model. Gene Ther. 2011;18:182–188. doi: 10.1038/gt.2010.131. [DOI] [PubMed] [Google Scholar]
- Kim IH, Józkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal WK, Zhou H, Morral N, Langston C, Parks RJ, Graham FL, et al. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol Med. 2000;6:179–195. [PMC free article] [PubMed] [Google Scholar]
- Toietta G, Koehler DR, Finegold MJ, Lee B, Hu J, Beaudet AL. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol Ther. 2003;7 5 Pt 1:649–658. doi: 10.1016/s1525-0016(03)00059-5. [DOI] [PubMed] [Google Scholar]
- Koehler DR, Hitt MM, Hu J. Challenges and strategies for cystic fibrosis lung gene therapy. Mol Ther. 2001;4:84–91. doi: 10.1006/mthe.2001.0435. [DOI] [PubMed] [Google Scholar]
- Koehler DR, Hannam V, Belcastro R, Steer B, Wen Y, Post M, et al. Targeting transgene expression for cystic fibrosis gene therapy. Mol Ther. 2001;4:58–65. doi: 10.1006/mthe.2001.0412. [DOI] [PubMed] [Google Scholar]
- Koehler DR, Frndova H, Leung K, Louca E, Palmer D, Ng P, et al. Aerosol delivery of an enhanced helper-dependent adenovirus formulation to rabbit lung using an intratracheal catheter. J Gene Med. 2005;7:1409–1420. doi: 10.1002/jgm.797. [DOI] [PubMed] [Google Scholar]
- Koehler DR, Sajjan U, Chow YH, Martin B, Kent G, Tanswell AK, et al. Protection of Cftr knockout mice from acute lung infection by a helper-dependent adenoviral vector expressing Cftr in airway epithelia. Proc Natl Acad Sci USA. 2003;100:15364–15369. doi: 10.1073/pnas.2436478100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Yang T, Li XF, Wu J, Duan C, Coates AL, et al. Readministration of helper-dependent adenoviral vectors to mouse airway mediated via transient immunosuppression. Gene Ther. 2011;18:173–181. doi: 10.1038/gt.2010.125. [DOI] [PubMed] [Google Scholar]
- Hiatt P, Brunetti-Pierri N, Koehler D, McConnell R, Katkin J, Palmer D, et al. Aerosol delivery of helper-dependent adenoviral vector into nonhuman primate lungs results in high efficiency pulmonary transduction with minimal toxicity. Mol Ther. 2005;11:317. [Google Scholar]
- Engelhardt JF, Wilson JM. Gene therapy of cystic fibrosis lung disease. J Pharm Pharmacol. 1992;44 suppl. 1:165–167. [PubMed] [Google Scholar]
- Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, et al. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E, Gaillard D, Ploton D, Hinnrasky J, Fuchey C, Boutterin MC, et al. Differential localization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis airway epithelium. Am J Respir Cell Mol Biol. 1992;7:485–491. doi: 10.1165/ajrcmb/7.5.485. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol, Cell Physiol. 2003;284:C2–15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Chow YH, Plumb J, Wen Y, Steer BM, Lu Z, Buchwald M, et al. Targeting transgene expression to airway epithelia and submucosal glands, prominent sites of human CFTR expression. Mol Ther. 2000;2:359–367. doi: 10.1006/mthe.2000.0135. [DOI] [PubMed] [Google Scholar]
- Lee H, Koehler DR, Pang CY, Levine RH, Ng P, Palmer DJ, et al. Gene delivery to human sweat glands: a model for cystic fibrosis gene therapy. Gene Ther. 2005;12:1752–1760. doi: 10.1038/sj.gt.3302587. [DOI] [PubMed] [Google Scholar]
- Seidner SR, Jobe AH, Ikegami M, Pettenazzo A, Priestley A, Ruffini L. Lysophosphatidylcholine uptake and metabolism in the adult rabbit lung. Biochim Biophys Acta. 1988;961:328–336. doi: 10.1016/0005-2760(88)90080-x. [DOI] [PubMed] [Google Scholar]
- Portman OW, Alexander M. Lysophosphatidylcholine concentrations and metabolism in aortic intima plus inner media: effect of nutritionally induced atherosclerosis. J Lipid Res. 1969;10:158–165. [PubMed] [Google Scholar]
- Chow YH, O'Brodovich H, Plumb J, Wen Y, Sohn KJ, Lu Z, et al. Development of an epithelium-specific expression cassette with human DNA regulatory elements for transgene expression in lung airways. Proc Natl Acad Sci USA. 1997;94:14695–14700. doi: 10.1073/pnas.94.26.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.