Abstract
Preoperative localization is necessary prior to video assisted thoracoscopic surgery for the detection of small or deeply located lung nodules. We compared the localization ability of a mixture of lipiodol and methylene blue (MLM) (0.6 mL, 1:5) to methylene blue (0.5 mL) in rabbit lungs. CT-guided percutaneous injections were performed in 21 subjects with MLM and methylene blue. We measured the extent of staining on freshly excised lung and evaluated the subjective localization ability with 4 point scales at 6 and 24 hr after injections. For MLM, radio-opacity was evaluated on the fluoroscopy. We considered score 2 (acceptable) or 3 (excellent) as appropriate for localization. The staining extent of MLM was significantly smaller than methylene blue (0.6 vs 1.0 cm, P<0.001). MLM showed superior staining ability over methylene blue (2.8 vs 2.2, P=0.010). Excellent staining was achieved in 17 subjects (81%) with MLM and 8 (38%) with methylene blue (P=0.011). An acceptable or excellent radio-opacity of MLM was found in 13 subjects (62%). An appropriate localization rate of MLM was 100% with the use of the directly visible ability and radio-opacity of MLM. MLM provides a superior pulmonary localization ability over methylene blue.
Keywords: Lung; Ethiodized Oil; Methylene Blue; Tomography, X-Ray Computed; Radiology, Interventional
INTRODUCTION
Preoperative localization is necessary for video-assisted thoracoscopic surgery (VATS) when pulmonary nodules are too small or distant from the visceral pleura to be detected (1-3). A failure to localize nodules disturbs the success of the thoracoscopic resection and leads to conversion to thoracotomy (4, 5). There are two kinds of localizing procedures: marking with thoracoscopically directly visible materials and marking with radio-opaque materials. Examples of directly visible materials are hook wire, methylene blue, and indocyanine green. Ethiodized oil (lipiodol), barium, and iodine contrast agents are used for radio-opaque markers. Each marking method has strong and weak points. Localization with a hook wire is easy to perform but carries a high risk of pneumothorax and a propensity to dislodge during transport and surgical preparation (6, 7). Methylene blue and indigo carmine have a tendency to diffuse over a large area by the time the operation is done and render localization features inadequate (8, 9). The use of a radio-opaque marker (such as barium or lipiodol) requires an intraoperative fluoroscopy to confirm an adequate excision as well as lead to increased radiation exposure (10-13).
The use of mixture has been reported to make up for the weakness of marking materials. For example, the problem of dye diffusion has led to attempts to use a mixture of dye with various materials such as cyanoacrylate adhesive or collagen or autologous blood (14-16). However, they have not been widely used for localization due to difficulties in making and manipulation. Lipiodol and methylene blue are commonly used materials for localization (17-20). We hypothesized that lipiodol reduces the spread of methylene blue and provides additional localization opportunities by its radio-opacity. The use of a mixture of lipidol and methylene blue (MLM) for a percutaneous injection material requires a high success rate for appropriate localization and a low complication rate. To our knowledge, there have been no reports that evaluate the availability of MLM as a percutaneous injection material in human lungs. This study compared MLM with methylene blue as a percutaneous injection material for pulmonary localization in rabbit lungs.
MATERIALS AND METHODS
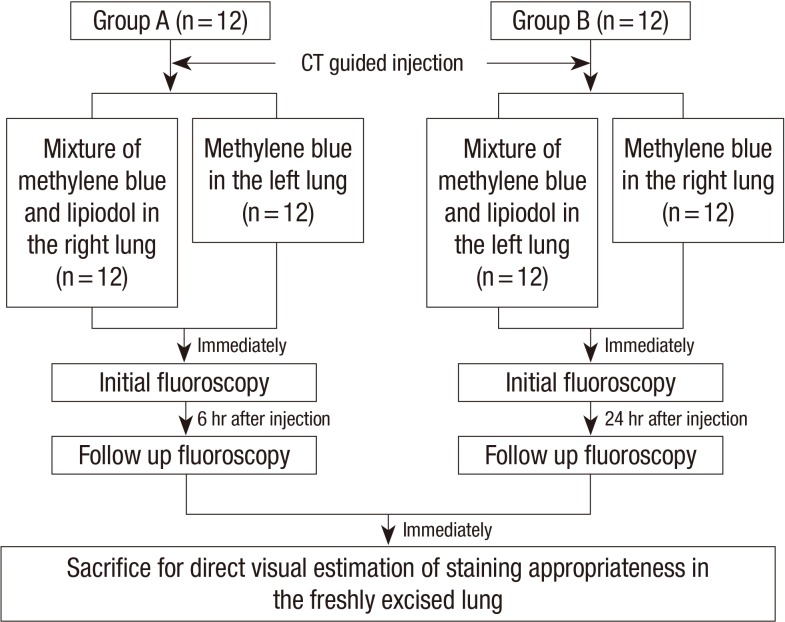
Animal preparation
This study was performed after approval by the Institutional Animal Care and Use Committee (IACUC) in Seoul National University Hospital biomedical research institute (IACUC approval No. 11-0356). Twenty-four adult New Zealand White rabbits were used. We recorded their weight before the procedures. The animals were randomly divided into two groups: Group A (n=12) and Group B (n=12), each sacrificed at about 6 hr and 24 hr after percutaneous injections, respectively (Fig. 1). Six hours after percutaneous injections were same day operations of the preoperative localization; and 24 hr after percutaneous injections were next day operations of the preoperative localization. The injection of each material was done in all 24 subjects because we injected methylene blue and MLM at two different lung sites for each subject.
Fig. 1.
Overview of the experimental design. Animals were randomly divided into two groups: Group A (n = 12) was sacrificed 6 hr after percutaneous injection and Group B (n = 12) was sacrificed 24 hr after a CT guided percutaneous injection of MLM and methylene blue.
Percutaneous injection materials: mixture of lipiodol and methylene blue versus methylene blue
A pilot study was performed to decide the optimal amount of materials for percutaneous injections. Methylene blue (1% 100 mg/mL, TERA Pharmaceuticals, Buena Park, CA, USA) of 0.3 to 0.9 mL was used for human lung localization in previous studies by Wicky et al. (18) and Vandoni et al. (19). In the pilot study with rabbit lungs, we injected 0.1 mL and 0.05 mL of methylene blue and MLM in four subjects. We found that staining was extensive (more than half height of one lobe) with 0.1 mL and localized (about 1 cm of staining diameter) with 0.05 mL for both methylene blue and MLM. Extensive dispersion made it difficult to find exact injecting sites; subsequently, 0.05 mL of methylene blue was administered.
We made variable mix ratios of lipiodol and methylene blue in vitro; 1:1, 1:2, 1:3, 1:4, and 1:5 in order to find an appropriate mixing ratio of lipiodol (480 mg Iodine/mL, Andre Guerbet, Aulnay-sous-Bois, France) and methylene blue. The separation of two materials occurred instantly after mechanical blending to the fat-soluble character of lipiodol and the water-soluble character of methylene blue. A higher concentration of lipiodol in MLM resulted in increased uneven blending and rapid separation. A mixture with a 1:6 (or lower) mixing ratio contained a minimal amount of lipiodol and it might make it difficult to be detected on the fluoroscopy; subsequently, we decided that 1:5 was an appropriate mixing ratio for injection. A total of 0.06 mL of MLM (0.01 mL of lipiodol plus 0.05 mL of methylene blue) was administrated in each subject to avoid the effect of different volumes of methylene blue to the diffusion extent of the materials.
CT guided percutaneous injections
Percutaneous injection was performed with computed tomography (CT) guidance (Discovery CT750 HD; GE Healthcare, Waukesha, WI, USA). We performed pre-procedural CT scans in order to determine an appropriate skin entry site for the successful placement of a needle in the desired location. The desired location was the basal portion of both caudal lobes around the mid-scapula line. We tried to situate the needle tip at 5 mm depth from the visceral pleura and avoid passing through the pulmonary vessels. We placed the needle of 20 gauze and 3.5 cm length in the lung parenchyma after marking the appropriate skin entry site. The parameters of CT used in our study were: tube voltage of 120 kV, tube current of 25 mA, slice thickness of 2 mm thickness, and gantry rotation speed of 350 milliseconds. We connected 1 mL syringe to the needle hub and retracted the syringe piston to confirm that no blood was aspirated after the needle tip was accurately located within the desired location. We then injected the materials and immediately removed the needle. On the procedural CT scan, we measured the distance from the skin-entry to the needle tip and the depth from visceral pleura to the needle tip. A post-procedural CT scan identified procedure-related complications that included the leakage of injecting materials and pneumothorax; in addition, we recorded the extent, shape, and density of radio-opacity of MLM after injection. The extent of MLM was defined as a maximum diameter of the radio-opacities. The shape of radio-opacity was categorized into 3 groups (small faint nodular, scattered nodular, and discrete compact nodular). We recorded the injection time to measure the time interval between injection and sacrifice.
Fluoroscopic examinations
A successful localization of lipiodol was determined by fluoroscopic examination; subsequently, we evaluated the radio-opacity of MLM using the fluoroscopy X-ray unit (BV Pulsera; Philips Medical Systems, Best, The Netherlands) at the immediate post-procedure session and the follow up session at 6 hr in Group A and 24 hr in Group B. The parameters of fluoroscopy were: tube voltage of 59 kV and tube current of 946 mA. We obtained anteroposterior fluoroscopic images of the thorax of the rabbit with a 17 cm of field of view. A radio-opaque ruler of 5 cm was located near the rabbit in order to estimate the exact size of lipiodol opacity. We recorded the time of the fluoroscopic examinations and the radiographic findings of MLM (size and shape of the radio-opacity).
Evaluation of the staining and radio-opacity
We assessed the directly visible staining on the freshly excised lung surface and radio-opacity of MLM on the fluoroscopic examinations using 4-point scoring in order to compare the localization ability of MLM and methylene blue as a percutaneous injection material. A blind reviewer who was unaware of the injection materials assessed the staining ability. In order to evaluate the staining ability, the blind reader reviewed the photographic images of the freshly excised lung specimens obtained before formalin fixations and rated the staining by 4-point scores: 0=non-visualization of staining, 1=inappropriate; extensive dispersion made it difficult to find accurate injecting locations, 2=acceptable; available to estimate injecting locations in spite of the dispersion and 3=excellent, definitely localized staining (Fig. 2). The maximum diameter of the staining extent on the lung surface was measured. We calculated and compared scores and extent of staining between two materials.
Fig. 2.
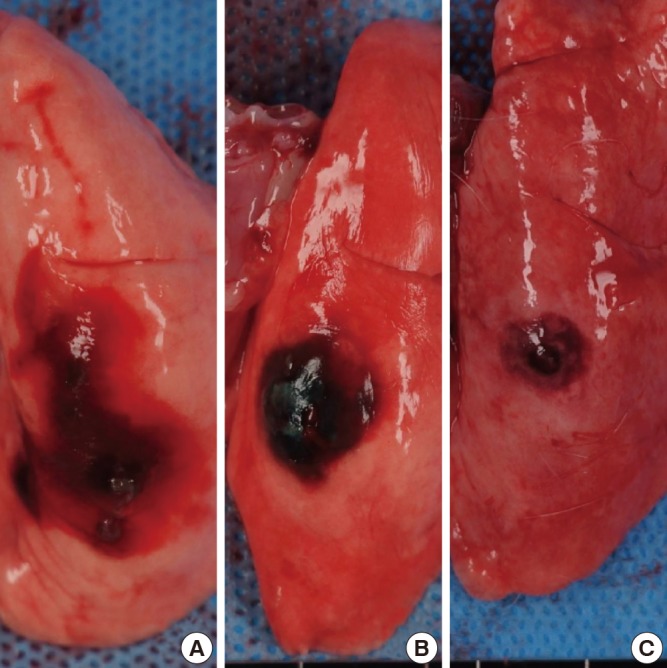
Examples of evaluation of staining on the lung surface. Photographs show (A) the extensive staining (score 1), (B) localized dispersion of staining (score 2), and (C) minimal dispersion of staining (score 3). The white lines on the bottom of the figure are markings of the ruler. The distance between two lines is one centimeter.
For the fluoroscopic findings, the radio-opacity of MLM was evaluated using 4-point scoring: 0=no detectable radio-opacity, 1=inappropriate, minimally increased opacity, 2=acceptable, low density of increased opacity, 3=excellent, compact nodular increased opacity (Fig. 3). We compared the average scores of initial and follow up fluoroscopic examinations.
Fig. 3.
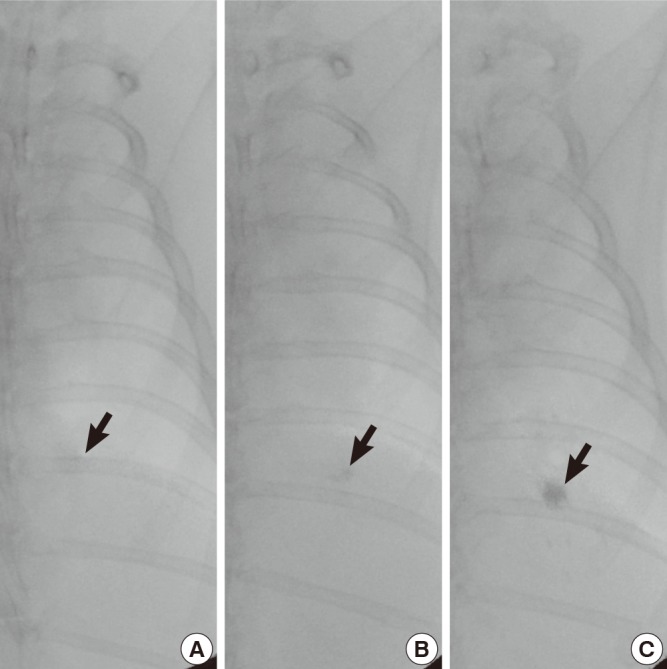
Examples of assessment of radio-opacity on the fluoroscopic examinations. The fluoroscopic images show (A) a minimally increased opacity (arrow) (score 1), (B) a low density of increased opacity (arrow) (score 2), and (C) a compact nodular increased opacity (arrow) (score 3).
We considered a score of 0 or 1 as inappropriate and a score of 2 or 3 as appropriate for localization for both staining and radio-opacity. We compared the number of appropriate or excellent localization between MLM and methylene blue.
Sacrifice and histopathologic examinations
Both freshly excised entire lungs were used as final specimens. The lung tissues were fixed in 10% neutral formalin, embedded in paraffin, and cut into 5 µm thick slices after we took photographs to record staining on the lung surface. We made 4 axial slices that covered the center of the staining. The slices were subjected to hematoxylin-eosin (H-E) stain to the evaluate lung parenchymal change. We evaluated the presence or absence of neutrophil infiltration, vasculitis, necrosis, hemorrhage, and foam cell in alveolus. The extent of each histopathologic finding was estimated using visual grading scores as 0 (no), 1 (focal), or 2 (diffuse). Localized parenchymal change (<50% of total area) surrounded by normal lung was defined as focal. Extensive lung parenchymal change (≥50% of total area) that replaced normal lung was defined as diffuse. An experienced pathologist with eight years of experience reviewed all slices. The overall severity of the lung parenchymal change was defined as a total score by adding visual grading scores for each histopathologic finding. We compared the overall severity score between MLM and methylene blue as well as between Group A and Group B.
Statistical analysis
All data are expressed as mean±standard deviation (SD) unless otherwise stated. Comparisons of the average scores were performed by two-tailed unpaired Student's t-test or Mann-Whitney test. We used a Fisher's exact test to compare the number of subjects in the subgroups. Linear by linear association evaluated the association of the extent of lung parenchymal change and materials or groups. Null hypotheses of no difference were rejected if the P values were less than 0.05. The statistical analysis was performed with commercially available statistical software, IBM SPSS Statistics version 20.0 (IBM Corp. in Armonk, NY, USA).
RESULTS
Subject characteristics, procedural records, time interval of injection and examinations
Among the 24 subjects included in our study, successful CT-guided percutaneous injections into the desired location of the lung were achieved in 21 subjects (11 in Group A and 10 in Group B). Three subjects died during anesthesia. Mean weight was 3.2±0.2 kg for Group A and 3.3±0.2 kg for Group B. Injection depth from visceral pleura to needle tip was 0.4±0.1 cm (range: 0.3-0.6 cm) for MLM and 0.4±0.1 cm (range: 0.3-0.7 cm) for methylene blue (P=0.43). Distance from skin to needle tip was 2.8±0.6 cm (range: 2.1-5.0 cm) for MLM and 2.8±0.3 cm (range: 2.2-3.5 cm) for methylene blue (P=0.83). Of 42 CT-guided percutaneous injections, total number of procedure related complications was 10 (24%), including 7 leakage (all in MLM) and 3 pneumothorax (2 in MLM, 1 in methylene blue). The complication rate in MLM was significantly higher than methylene blue (43% vs 5%) (P=0.004).
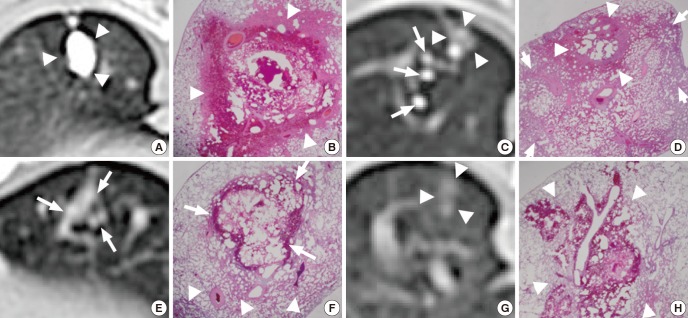
On post-procedural CT images, the extent of the radio-opacity of MLM was 1.3±0.4 cm (range: 0.7-2.0 cm) for Group A and 0.6±0.3 cm (range: 0.3-1.1 cm) for Group B. Discrete compact nodular opacity was achieved in 15 subjects (72%), scattered nodular opacities in 3 (14%) and small faint opacity in 3 (14%) (Fig. 4). The average value of radio-opacity of MLM was 1,415±856 HU (range: 307-2,768 HU).
Fig. 4.
CT and corresponding photomicrograph of lung specimen. MLM in Group B (A-D); (A) discrete and compact nodular opacity (arrowheads), (B) focal neutrophil infiltration, necrosis, and hemorrhage (arrowheads) (H&E, ×12.5), (C) scattered small nodular opacities of lipiodol (long arrows) and faint nodular opacity (arrowheads), (D) focal hemorrhage and necrosis (arrowheads) with diffuse neutrophil infiltration (short arrows) (H&E, ×12.5). MLM in Group A (E, F); (E) faint nodular lipiodol opacity (arrows), (F) focal hemorrhage (arrows) with diffuse neutrophil infiltration (arrowheads) (H&E, ×12.5). Methylene blue in Group A (G, H); (G) faint nodular opacity (arrowheads), and (H) focal extent of neutrophil infiltration, necrosis and hemorrhage (arrowheads) (H&E, ×12.5).
The interval between injection and sacrifice was 7.9±0.1 hr (range: 7.8-8.0 hr) for Group A and 23.5±0.1 hr (range: 23.4-23.7 hr) for Group B. Time from injection to initial and follow up fluoroscopy was 3.4±0.5 hr (range: 2.5-4.2 hr) and 6.8±0.4 hr (range: 6.3-7.7 hr) for Group A and 1.5±0.4 hr (range: 0.9-2.1 hr) and 22.6±0.4 hr (range: 21.9-23.2 hr) for Group B, respectively.
Scores and extent of staining and radio-opacity
Table 1 demonstrates the staining extent and localization ability of MLM and methylene blue. In total groups, the staining extent of MLM was significant smaller than methylene blue (0.6 cm vs 1.0 cm, P<0.001). MLM showed a significantly higher staining ability score than methylene blue (2.8 vs 2.2, P=0.010). Radio-opacity in the initial fluoroscopy was not significantly different from the follow up (2.0 vs 1.9, P=0.49).
Table 1.
Staining extent and localization ability of MLM versus methylene blue
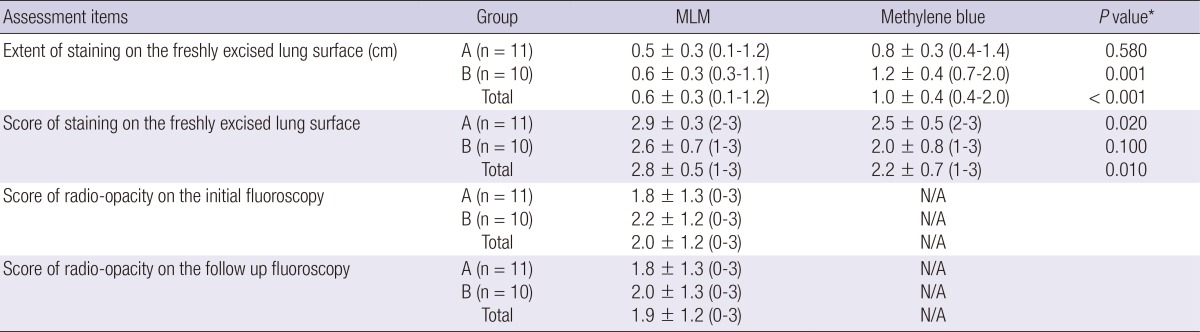
Data are mean±standard deviation. Numbers in parentheses are ranges. N/A indicates not available. *Non-parametric Mann-Whitney test was performed to compare the average score of MLM and methylene blue. MLM, mixture of lipiodol and methylene blue.
Table 2 showed the number of subjects in each score of localization ability of staining or radio-opacity. In Group A, appropriate staining was 100% for both MLM and methylene blue. In Group B, appropriate staining was 90% for MLM and 70% for methylene blue. Appropriate staining of MLM was not significantly different from that of methylene blue (95% vs 86%, P=0.61); however, excellent staining in MLM was significantly higher than methylene blue (81% vs 38%, P=0.011) (Table 3).
Table 2.
Localization ability score of staining and radio-opacity for MLM as well as methylene blue
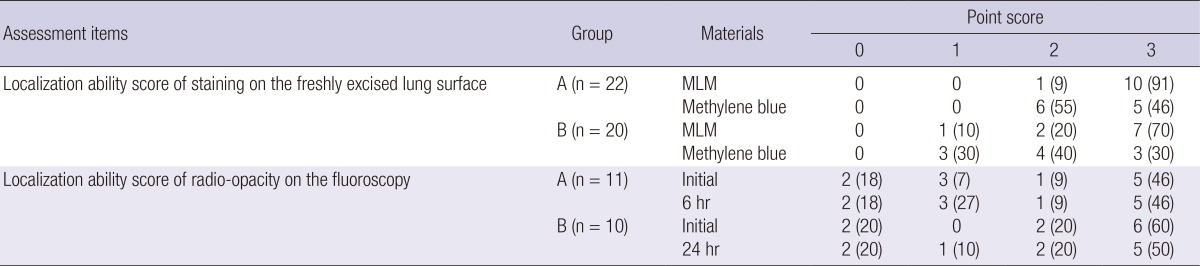
Data are numbers of subjects. Numbers in parentheses are percentages. MLM, mixture of lipiodol and methylene blue.
Table 3.
Comparison of localization ability between MLM and methylene blue in total subjects (n = 42)
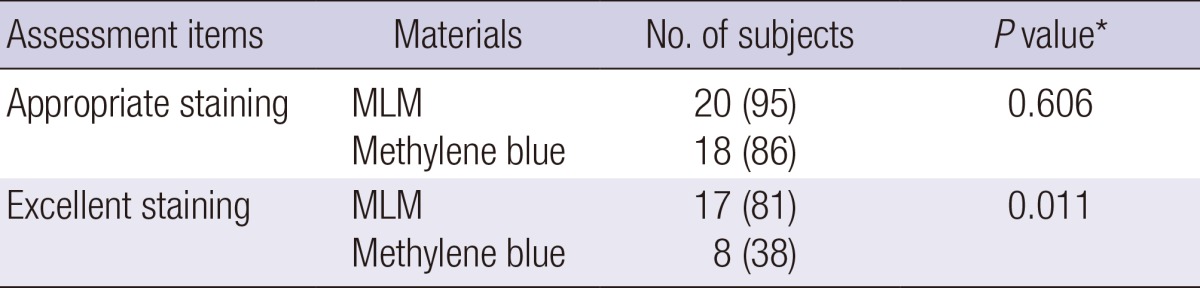
We considered a score of 2 or 3 as appropriate and 3 as excellent for localization, respectively. Numbers in parentheses are percentages. *Fisher's exact test compared the proportion of appropriate or excellent staining between the mixture and methylene blue. MLM, mixture of lipiodol and methylene blue.
Table 4 shows the localization ability of MLM regarding both staining ability and radio-opacity. There was no subject with a score of 0 or 1 in both radio-opacity and staining. MLM achieved appropriate staining or radio-opacity in 21 subjects (100%) with a dual localization feature.
Table 4.
Localization ability of MLM: Evaluation of radio-opacity and staining score
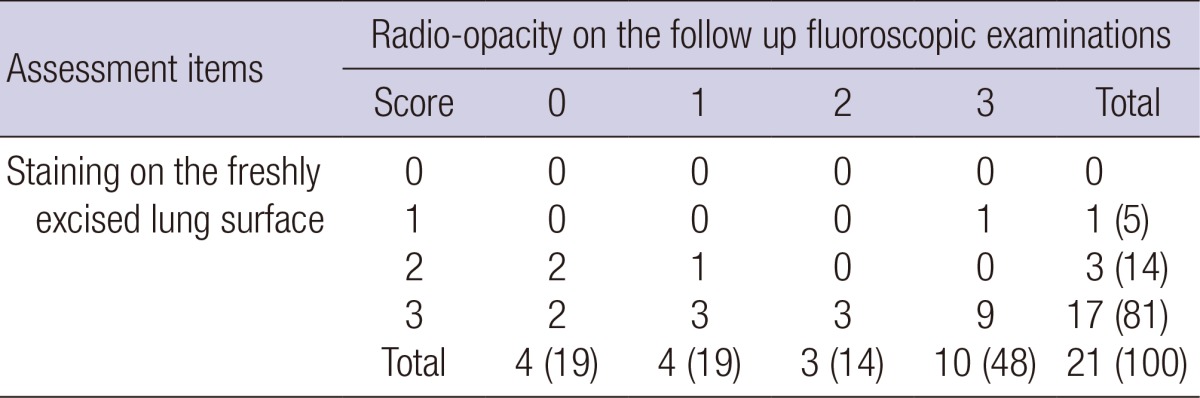
Data are given as numbers of subjects. Numbers in parentheses are percentages. MLM, mixture of lipiodol and methylene blue.
Histopathologic findings
Table 5 demonstrates the results of the histopathologic findings. In all lung specimens, both methylene blue and MLM showed acute lung parenchymal change that included neutrophil infiltration, hemorrhage, and foam cell in alveolus (Fig. 4). Comparing the two materials, the number of specimen having neutrophil infiltration, vasculitis, necrosis, hemorrhage, and foam cell in alveolus was similar in each extent. In terms of all features, the number of specimen that showed diffuse extent was more in Group B than Group A for both MLM and methylene blue. The extent of the histopathologic findings was not significantly associated with the materials for all histopathologic features (Table 5). Among the histopathologic findings, the extent of vasculitis was significantly associated with Group for both MLM and methylene blue (P=0.002 for both MLM and methylene blue). Focal or diffuse extent of vasculitis was more frequently found in Group A than Group B (P=0.001 for both MLM and methylene blue). The overall severity of lung parenchymal change was not different between MLM and methylene blue (5.6±1.6 vs 5.7±1.5, P=0.839); in addition, Group B showed a significantly higher overall severity score of lung parenchymal change than Group A (6.6±1.6 vs 4.7±0.9, P=0.005).
Table 5.
Histopathologic findings of lung specimens after percutaneous injections
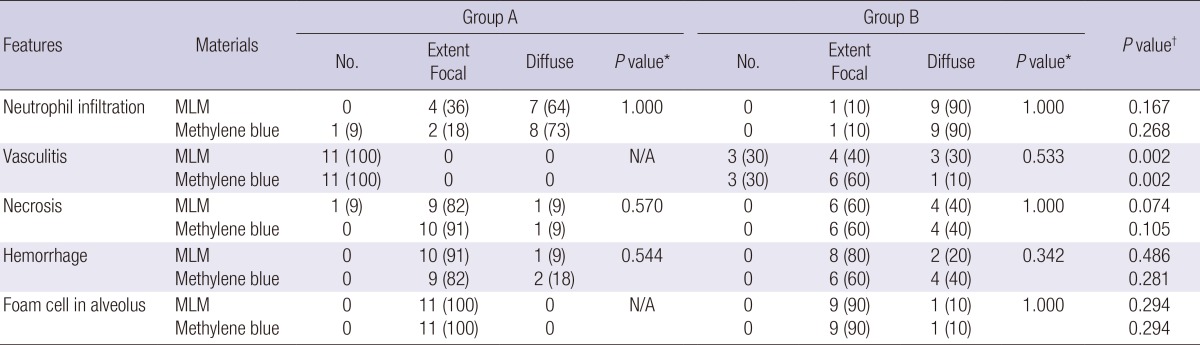
Data are numbers of subjects. Numbers in parentheses are percentages. N/A indicates not available. *Linear by linear association was performed between material and the extent of the histopathologic findings. †Linear by linear association was performed between groups and the extent of the histopathologic findings. MLM, mixture of lipiodol and methylene blue.
DISCUSSION
The results of this study show that MLM is a useful percutaneous injection material for a successful localization in the lung. The average staining score of MLM was significantly higher than methylene blue (2.8±0.5 vs 2.2±0.7, P=0.010). In terms of staining, the appropriate localization rate (acceptable or excellent staining) in our study was 95% using MLM. The result was in close agreement with previous studies that showed a high success performance rate of lipiodol localization (99%-100%) (21-23). An appropriate localization rate (acceptable or excellent staining) of methylene blue injection was 86% in our study. This is lower than the results found in previous studies where the success rate of methylene blue injection was 96%-100% (18, 20). We found that an acceptable (or excellent staining rate) of MLM and methylene blue was not significantly different (95% vs 86%, P=0.610). However, MLM showed excellent staining for localization in 17 (81%) of 21 subjects and was significantly higher than methylene blue (38%) (P=0.011). The results indicate that lipiodol reduced the spread of methylene blue. This is the first study to indicate that MLM is an available percutaneous injection material for localization with superior staining ability compared to methylene blue.
The complication rate was 43% in MLM and 5% in the methylene blue (P=0.004). Possible complications after percutaneous injection for pulmonary localization include pneumothorax, leakage, hemorrhage, pain, hemoptysis, hemothorax, and embolism. Previous studies reported that the complication rate was 17-29% for lipiodol and 33% for methylene blue (20, 23, 24). The complication rate of MLM in the current study was higher than the results of previous studies mainly due to the leakage of MLM into the pleural cavity (n=9). This difference was probably because the distance from the pleura to the injecting needle tip (0.4±0.1 cm for MLM) was inadequate to avoid leakage into the pleural cavity. In the previous studies of lipiodol marking for localization, the mean distance from the pleura to the target nodule was 1.0-1.9 cm (22-24), more than twice our study. The results indicate that the high complication rate of our study is associated with the inserting procedure of the needle rather than MLM itself.
The dispersion of methylene blue throughout the lung parenchyma may lead to unnecessarily large wedge resections; in addition, some have reported instances of the dispersion of methylene blue throughout the entire pleural surface or intraoperative identification failure due to severe anthracosis of the visceral pleura. The failure rate was reported to be 0%-13% with the use of methylene blue (18, 19, 25). The results are similar to our study and indicate that inappropriate staining on the lung surface was 14% in methylene blue. In this study, we found that the dispersion of methylene blue in MLM through the lung parenchyma was significantly smaller than methylene blue (0.6±0.3 cm vs 1.0±0.4 cm, P<0.05). The result implies that lipiodol reduces the spread of methylene blue in lung parenchyma.
Regarding the score of radio-opacity, 38% of MLM showed non-visualization or minimally increased opacity on the fluoroscopic examinations. It means the proportion of lipiodol in MLM at the time of the percutaneous injection was too small to be detected. Post-procedural CT images also revealed that 3 subjects had small faint radio-opacity after the injection of MLM. It suggests that the uneven blending of lipiodol and methylene blue occurred during the preparation of MLM. Water-insolubility of lipiodol would result in the uneven mixing of water soluble methylene blue after mechanical blending of the two materials. Further research is required to reduce non-homogeneity of MLM at the time of injection.
Previous studies reported the availability of a mixture of methylene blue with other materials such as collagen or autologous blood (15, 16). They performed VATS resection on the same day as localization. In our study, we evaluated the localization ability of MLM on the same day of localization (6 hr) as well as 24 hr after injection. Localization is usually performed on the day of surgery. This requires the simultaneous use of the CT and the operating room, which is not always available. Surgeries on the next day of localization were reported in several published articles (26, 27). MLM shows a prolonged localization ability of up to 24 hr in terms of staining ability and radio-opacity. Stable localization ability is the advantage of MLM in our study. Due to uneven blending of MLM, one subject (10%) showed inappropriate staining and appropriate radio-opacity and required an intraoperative fluoroscopic examination to detect MLM. Possible radiation exposure is a drawback of MLM. We would like to justify the use of intraoperative fluoroscopy because the operator can avoid radiation exposure with a lead apron. In regards to the risk-benefit for patients, lowering the risk of detection failure is thought to be more important than radiation exposure.
Histopathologic examinations showed lung parenchymal changes in all specimens. Both methylene blue and MLM induced acute lung injury that included neutrophil infiltration, vasculitis, necrosis, hemorrhage, and foam cell in alveolus (Table 5). The results of our study are similar to those of a previous study by Kwon et al. (28) that showed that lipiodol led to acute lung injury. They described that lipiodol creates the histopathologic feature of acute lung injury such as peripheral endothelial cell damage, neutrophil infiltration, necrosis, hemorrhage, alveolar wall destruction, vasculitis, emboli (or thrombi in arteriole), and macrophages in the alveolar space (28). In our results, the extent of lung parenchymal change was not associated with the materials for all histopathologic features. In addition, the overall severity score of lung parenchymal change in MLM was not different from methylene blue (5.6 and 5.7, P=0.839). This suggests that MLM shows similar histopathologic effects in the lung parenchyma to methylene blue. The overall severity score of parenchymal change was higher in Group B (follow up interval of 24 hr) than Group A (follow up interval of 6 hr) (6.6 vs 4.7, P=0.005). The extent of lung parenchymal change depends on the time interval.
Acute lung injury after the percutaneous injection of lipiodol or methylene blue was reported in animal studies (28, 29); however, there are no clinical results that show the adverse effect of acute lung injury in human lungs. Injection material (such as barium) can potentially complicate the pathologic diagnosis of the target lesion due to acute inflammation (29, 30). To our knowledge, no study has indicated that lipiodol or methylene blue hinders the histopathologic diagnosis of target lesions in human lungs. The small amount of material injection in human lungs might not create a significant parenchymal change or disrupt underlying lung disease. It is necessary to avoid directly injecting materials into the target lesion in human lungs in order to avoid the adverse effect of injection materials on underlying lung disease (especially ground glass opacity nodule or potential benign lesion).
There were several limitations in our study. First, we included only a small number of subjects. Second, the overall localization success rate was low and the complication rate was high (compared to the results of previous studies) due to the difficulty in an accurate percutaneous injection at the desired location and depth in the small sized rabbit lung. Third, we used a 1 mL syringe with manual administration to inject materials in the lung parenchyma and there were possible individual difference in the administering volume of materials. Fourth, we could not evaluate complications such as intractable pain, material related anaphylaxis or embolism. Fifth, we could not evaluate if the histopathologic changes had any effect on underlying lung disease because the lung parenchyma of the experimental rabbits were normal. Finally, we did not evaluate a successful localization for the true target lesion in lung parenchyma. The criteria for appropriate staining and radio-opacity were subjective. We expect that further clinical studies might provide an answer to if MLM can be a useful percutaneous injection material for localization in the human lung.
In conclusion, MLM is available for percutaneous injection for the pulmonary localization. The results of this study showed that MLM provides superior ability for appropriate localization than that of methylene blue. Further research on human lungs can clarify the availability of MLM as a CT guided percutaneous injection material.
Footnotes
This study was supported by grant from the Seoul National University College of Medicine Research Fund 2012 (800-20120036).
We have no potential conflicts of interest or commercial involvement to disclose.
References
- 1.Nakashima S, Watanabe A, Obama T, Yamada G, Takahashi H, Higami T. Need for preoperative computed tomography-guided localization in video-assisted thoracoscopic surgery pulmonary resections of metastatic pulmonary nodules. Ann Thorac Surg. 2010;89:212–218. doi: 10.1016/j.athoracsur.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Zhou J, Zhang J, Hu H, Luo X, Zhang Y, Chen H. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc. 2011;25:1723–1729. doi: 10.1007/s00464-010-1502-3. [DOI] [PubMed] [Google Scholar]
- 3.Ciriaco P, Negri G, Puglisi A, Nicoletti R, Del Maschio A, Zannini P. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg. 2004;25:429–433. doi: 10.1016/j.ejcts.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki K, Nagai K, Yoshida J, Ohmatsu H, Takahashi K, Nishimura M, Nishiwaki Y. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115:563–568. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 5.Seo JM, Lee HY, Kim HK, Choi YS, Kim J, Shim YM, Lee KS. Factors determining successful computed tomography-guided localization of lung nodules. J Thorac Cardiovasc Surg. 2012;143:809–814. doi: 10.1016/j.jtcvs.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Gossot D, Miaux Y, Guermazi A, Celerier M, Friga J. The hook-wire technique for localization of pulmonary nodules during thoracoscopic resection. Chest. 1994;105:1467–1469. doi: 10.1378/chest.105.5.1467. [DOI] [PubMed] [Google Scholar]
- 7.Pittet O, Christodoulou M, Pezzetta E, Schmidt S, Schnyder P, Ris HB. Video-assisted thoracoscopic resection of a small pulmonary nodule after computed tomography-guided localization with a hook-wire system: experience in 45 consecutive patients. World J Surg. 2007;31:575–578. doi: 10.1007/s00268-006-0343-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Chen L, Yang S, Chen Z, Qian G, Zhang S, Jing J. A novel technique for localization of small pulmonary nodules. Chest. 2007;131:1526–1531. doi: 10.1378/chest.06-1017. [DOI] [PubMed] [Google Scholar]
- 9.Bernard A. Resection of pulmonary nodules using video-assisted thoracic surgery: the Thorax Group. Ann Thorac Surg. 1996;61:202–204. doi: 10.1016/0003-4975(95)01014-9. [DOI] [PubMed] [Google Scholar]
- 10.Martin AE, Chen JY, Muratore CS, Mayo-Smith WW, Luks FI. Dual localization technique for thoracoscopic resection of lung lesions in children. J Laparoendosc Adv Surg Tech A. 2009;19:S161–S164. doi: 10.1089/lap.2008.0143.supp. [DOI] [PubMed] [Google Scholar]
- 11.Kawanaka K, Nomori H, Mori T, Ikeda K, Ikeda O, Tomiguchi S, Yamashita Y. Marking of small pulmonary nodules before thoracoscopic resection: injection of lipiodol under CT-fluoroscopic guidance. Acad Radiol. 2009;16:39–45. doi: 10.1016/j.acra.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Yamagami T, Miura H, Yoshimatsu R, Tanaka O, Ono S, Iehara T, Hosoi H, Nishimura T. Experience of fluoroscopy-aided thoracoscopic resection of pulmonary nodule localised with Lipiodol in a child. J Med Imaging Radiat Oncol. 2011;55:401–403. doi: 10.1111/j.1754-9485.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki Y, Nagata K, Yuba T, Hosogi S, Kohno K, Ohsugi S, Kuwahara H, Takemura Y, Yokomura I. Fluoroscopy-guided barium marking for localizing small pulmonary lesions before video-assisted thoracic surgery. Respir Med. 2005;99:285–289. doi: 10.1016/j.rmed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida J, Nagai K, Nishimura M, Takahashi K. Computed tomography-fluoroscopy guided injection of cyanoacrylate to mark a pulmonary nodule for thoracoscopic resection. Jpn J Thorac Cardiovasc Surg. 1999;47:210–213. doi: 10.1007/BF03217996. [DOI] [PubMed] [Google Scholar]
- 15.Nomori H, Horio H. Colored collagen is a long-lasting point marker for small pulmonary nodules in thoracoscopic operations. Ann Thorac Surg. 1996;61:1070–1073. doi: 10.1016/0003-4975(96)00024-0. [DOI] [PubMed] [Google Scholar]
- 16.McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg. 2002;37:1729–1731. doi: 10.1053/jpsu.2002.36707. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Zhang C, Sun L. Localization of small pulmonary nodules for videothoracoscopic surgery. ANZ J Surg. 2006;76:649–651. doi: 10.1111/j.1445-2197.2006.03790.x. [DOI] [PubMed] [Google Scholar]
- 18.Wicky S, Mayor B, Cuttat JF, Schnyder P. CT-guided localizations of pulmonary nodules with methylene blue injections for thoracoscopic resections. Chest. 1994;106:1326–1328. doi: 10.1378/chest.106.5.1326. [DOI] [PubMed] [Google Scholar]
- 19.Vandoni RE, Cuttat JF, Wicky S, Suter M. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998;14:265–270. doi: 10.1016/s1010-7940(98)00160-2. [DOI] [PubMed] [Google Scholar]
- 20.Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol. 1994;163:297–300. doi: 10.2214/ajr.163.2.7518642. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda K, Nomori H, Mori T, Kobayashi H, Iwatani K, Yoshimoto K, Kawanaka K. Impalpable pulmonary nodules with ground-glass opacity: success for making pathologic sections with preoperative marking by lipiodol. Chest. 2007;131:502–506. doi: 10.1378/chest.06-1882. [DOI] [PubMed] [Google Scholar]
- 22.Nomori H, Horio H, Naruke T, Suemasu K. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg. 2002;74:170–173. doi: 10.1016/s0003-4975(02)03615-9. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K, Nomori H, Ohtsuka T, Kaji M, Naruke T, Suemasu K. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg. 2006;132:320–324. doi: 10.1016/j.jtcvs.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Kim YD, Jeong YJ, I H, Cho JS, Lee JW, Kim HJ, Lee SH, Kim DH. Localization of pulmonary nodules with lipiodol prior to thoracoscopic surgery. Acta Radiol. 2011;52:64–69. doi: 10.1258/ar.2010.100307. [DOI] [PubMed] [Google Scholar]
- 25.Mayo JR, Clifton JC, Powell TI, English JC, Evans KG, Yee J, McWilliams AM, Lam SC, Finley RJ. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology. 2009;250:576–585. doi: 10.1148/radiol.2502080442. [DOI] [PubMed] [Google Scholar]
- 26.Lee NK, Park CM, Kang CH, Jeon YK, Choo JY, Lee HJ, Goo JM. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol. 2012;13:694–701. doi: 10.3348/kjr.2012.13.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamiyoshihara M, Ishikawa S, Morishita Y. Pulmonary cryptococcosis diagnosed by video-assisted thoracoscopic surgery with CT-guided localization: report of a case. Kyobu Geka. 2000;53:795–797. [PubMed] [Google Scholar]
- 28.Kwon WJ, Kim HJ, Jeong YJ, Lee CH, Kim KI, Kim YD, Lee JH. Direct lipiodol injection used for a radio-opaque lung marker: stability and histopathologic effects. Exp Lung Res. 2011;37:310–317. doi: 10.3109/01902148.2011.566672. [DOI] [PubMed] [Google Scholar]
- 29.Jang HS. Effect of drugs for preoperative localization of thoracoscopy to histopathologic change in rabbit lung. Seoul: the Catholic University of Korea; 2000. p. 27. Dissertation. [Google Scholar]
- 30.Okumura T, Kondo H, Suzuki K, Asamura H, Kobayashi T, Kaneko M, Tsuchiya R. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg. 2001;71:439–442. doi: 10.1016/s0003-4975(00)02378-x. [DOI] [PubMed] [Google Scholar]