Abstract
The epidemiology on human papillomavirus (HPV) among human immunodeficiency virus (HIV)-infected women in Korea is not well established. A retrospective study was conducted to determine the prevalence and genotype distribution of HPV infection among HIV-infected women in Korea. HPV DNA genotype and cervical cytology were examined in 60 HIV-positive women and 1,938 HIV-negative women. HPV genotypes were analyzed by using a HPV DNA chip. HIV-infected women had higher prevalence of high-risk HPV (hr-HPV) infection (30% vs 4.9%, adjusted odds ratio [AOR], 6.96; 95% confidence interval [CI], 3.63-13.34, P<0.001) and abnormal cervical cytology (18.3% vs 1.8%, AOR, 10.94; 95% CI, 5.18-23.1, P<0.001) compared with controls. The most common hr-HPV genotype detected in HIV-infected women was HPV 16 (10%), followed by 18 (6.7%) and 52 (5%). Prevalence of quadrivalent vaccine-preventable types (HPV 6, 11, 16, and 18) was 21.7% and 2.3% in HIV-positive women and HIV-negative women, respectively. Age was a significant risk factor for hr-HPV infection in HIV-infected women (P=0.039). The presence of hr-HPV was significantly associated with abnormal cervical cytology (P<0.001). These findings suggest that HPV testing for cervical cancer screening in HIV-infected women would be necessary, particularly among young age group.
Keywords: HIV, Women, Human Papillomavirus, Prevalence, Genotype
INTRODUCTION
Human immunodeficiency virus (HIV)-infected women have higher rates of human papillomavirus (HPV) infection than HIV-uninfected women, and are also at higher risk for persistent infection and progression to malignancy (1-4). In addition, HPV may be a cofactor in HIV acquisition (5, 6). Persistent infections with high risk HPV (hr-HPV) are known to be a necessary first step in the development of cervical cancer. The prevalence of hr-HPV infection and genotype distribution varied in different geographic areas (7). With the introduction of HPV vaccine, knowledge regarding hr-HPV genotype distribution has become increasingly important.
As of December 2010, a total of 623 women were diagnosed with HIV infection in Korea (8). Following the first case of HIV infection was reported in a foreign worker in 1985, active screening test for HIV infection was performed for commercial sex workers (CSWs) who might be contacted with the foreigners (9, 10). Eleven HIV-infected female CSWs were detected between 1985 and 1988. Since AIDS Prevention Law was enacted in 1987, mandatory screening for HIV infection was extended to all CSWs, overseas sailors, and people working in "hygiene related jobs" requiring health certificates, such as restaurants, coffee shops, barbershops, bars, and hotels. As a result, a sharp increase in the number of cases was detected among overseas sailors, who were responsible for 34% of people diagnosed with HIV infection until the mandatory screening test for this group ended in 1993 (9-11). HIV infection was also detected among their wives and other female sex partners. The proportion of women with HIV infection has remained stable and the man-to-woman ratio of patients infected with HIV was 11:1. A previous study showed that 67.6% of HIV-infected women in Korea had regular sexual partners; 26.1% were tested for HIV diagnosis because of their HIV-positive sex partners. These findings suggested that the majority of HIV-infected women were infected from their regular male sex partners, such as husband or person living together without being legally married (12).
There are limited data on the prevalence of HPV infection and hr-HPV genotypes among HIV-infected women in Korea, and little is known about their relationship with cervical cytology in this population. The objective of this study was to examine the prevalence and genotype distribution of HPV infection among HIV-infected women in Korea.
MATERIALS AND METHODS
Study population
We retrospectively reviewed the medical records for HIV-infected women attending Pusan National University Hospital between January 2009 and December 2012. The Hospital is a 1,220-bed university-affiliated teaching hospital and provides HIV care for HIV-infected patients in southeastern region of Korea. The study included HIV-infected ethnically Korean women who were aged 18 yr and older, and tested cervical cytology and HPV genotype in the study hospital. HIV-infected women who had current pregnancy or history of prior hysterectomy or conization were excluded. During that period, 78 HIV-infected women attended to the study hospital, and 60 (76.9%) were included in the study. For comparison, we used the data for women who visited Health Promotion Centers of Pusan National University Hospital and Yangsan Pusan National University Hospital for a regular medical check-up during the same period. Patients aged 18 yr and older who tested cervical cytology with HPV genotype were included, and HIV seropositive patients were excluded. Information obtained from database of the Health Promotion Centers included age, marital status, smoking history, parity, comorbidity, cervical cytology, HPV genotype, and serology for hepatitis B virus (HBV), hepatitis C virus (HCV) and syphilis. When multiple tests had been conducted from the same patient at different times, only the first test was used for assessing the prevalence and genotype distribution of HPV infection. Clinical categories were defined by the 1993 Centers for Disease Control and Prevention (CDC) classification criteria (13).
Cervical cytology and human papillomavirus genotyping
Cervical samples for cytology and HPV genotyping were obtained by gynecologists as a routine care or a part of regular medical check-up. Pap smear results were categorized according to the 2001 Bethesda classification system terminology (14). HPV genotype was determined using an PCR based DNA microarray system, the HPV DNA chip (MyHPV Chip Kit®, BioMedLab, Seoul, Korea). This contains 24 type-specific probes detecting 13 types of high risk HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and 11 types of low risk HPV (6, 11, 34, 40, 42, 43, 44, 53, 54, 66, and 70).
Statistical analysis
Categorical variables were compared using Pearson's chi-square test or Fisher's exact test, whereas non-categorical variables were tested with the Mann-Whitney U-test. Comparison between HIV-positive women and HIV-negative women was conducted by using multiple logistic regression techniques while controlling for potential confounding variables. In multivariate analysis, variables were entered into the model simultaneously, not using an automatic model selection. All tests were considered statistically significant at P<0.05. The statistical analyses were conducted using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Ethics statement
This study protocol was approved by the institutional review board (IRB) of Pusan National University Hospital (IRB No. E-2013013) and Yangsan Pusan National University Hospital (IRB No. 05-2013-044), respectively. Informed consent was waived by the boards.
RESULTS
A total of 60 HIV-positive women and 1,938 HIV-negative women were included in this study. Their demographic characteristics are described in Table 1. Median age was 47 yr old (interquartile range 39-58) for HIV-positive women and 50 yr old (interquartile range 43-57) for HIV-negative women (P=0.092). The marital status, parity, smoking, comorbidity, HCV and syphilis serology showed significant difference between two groups. By using the multiple logistic regression model, we compared the prevalence of HPV infection and the results of cervical cytology between two groups (Table 2). Overall, HPV infection rate was significantly higher in HIV-positive women than HIV-negative women (46.7% vs 14.1%; adjusted odds ratio [AOR], 4.48; 95% confidence interval [CI], 2.56-7.83, P<0.001). Multiple HPV types were detected in 18.3% of HIV-positive women and 1.5% of HIV-negative women (AOR, 12.87; 95% CI, 5.43-30.54, P<0.001). HIV-positive women had a significantly greater hr-HPV infection rate than women without HIV infection (30% vs 4.9%; AOR, 6.96; 95% CI, 3.63-13.34, P<0.001). Multiple hr-HPV types were detected in 10% of HIV-positive women and 0.6% of HIV-negative women (AOR, 12.63; 95% CI, 3.75-42.56, P<0.001).
Table 1.
Baseline characteristics of HIV-positive women and HIV-negative controls
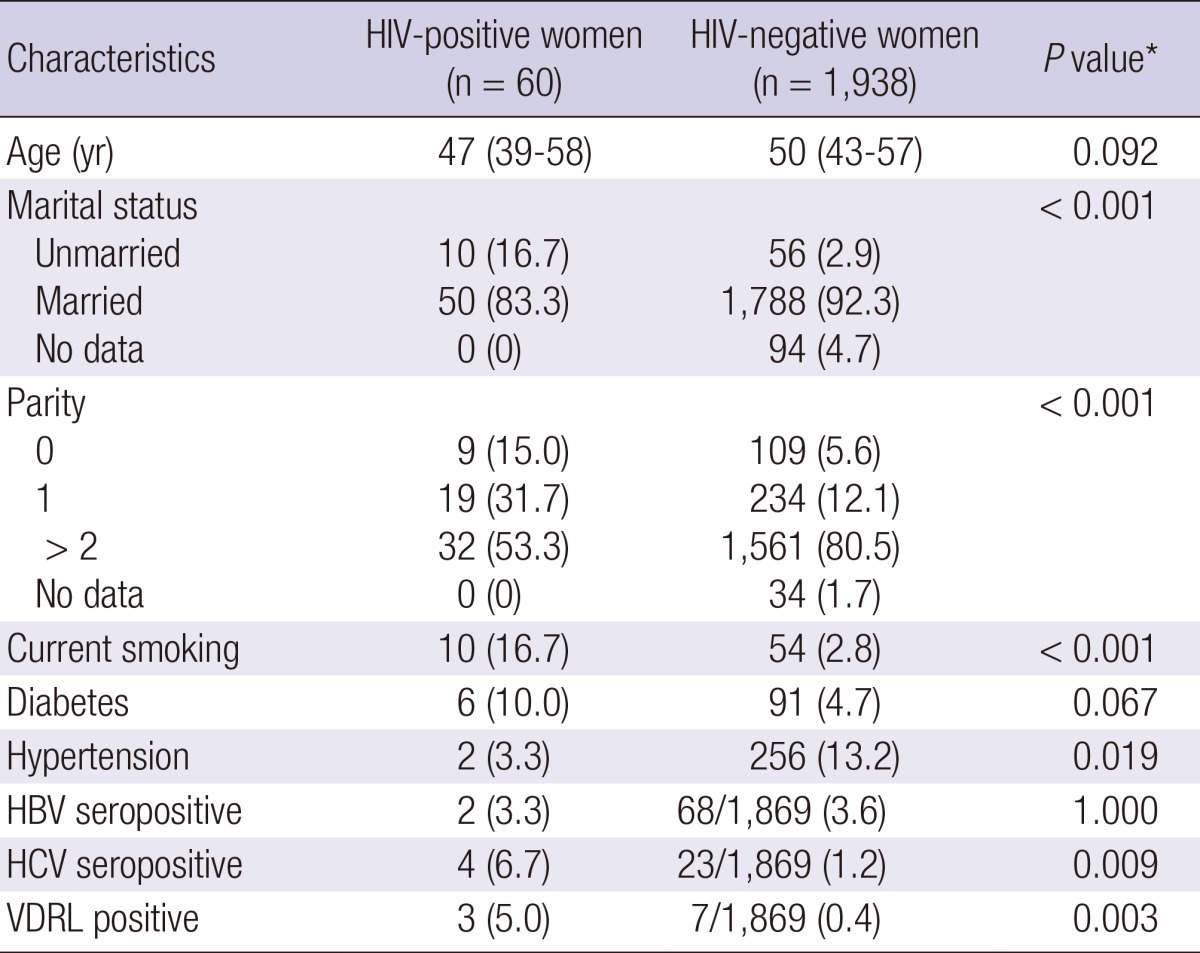
Data are number (%) of patients, unless otherwise indicated. *Calculated using chi-square test, Fisher's exact test, or the Mann-Whitney U-test. HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; VDRL, venereal disease research laboratory.
Table 2.
Prevalence of human papillomavirus infection and abnormal cervical cytology in 60 HIV-positive women and 1,938 HIV-negative women, and adjusted odds ratios and 95% confidence intervals determined by multiple logistic regression model using HIV status as an independent variable
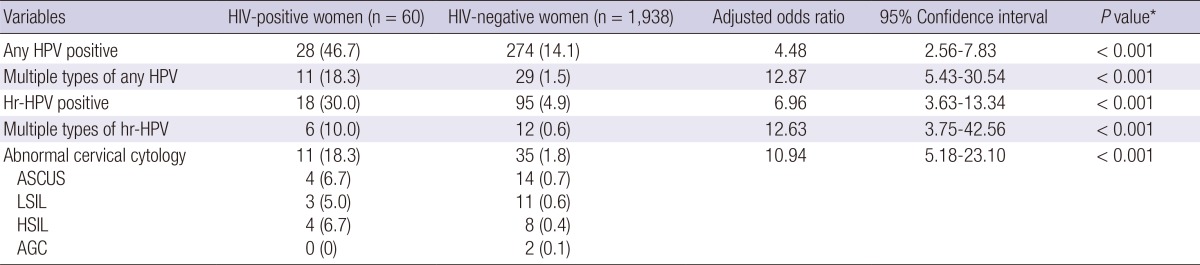
Data are number (%) of patients, unless otherwise indicated. *Calculated using multivariate logistic regression analysis. HIV, human immunodeficiency virus; HPV, humanpapilloma virus; Hr, high risk; ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions; AGC, atypical glandular cells.
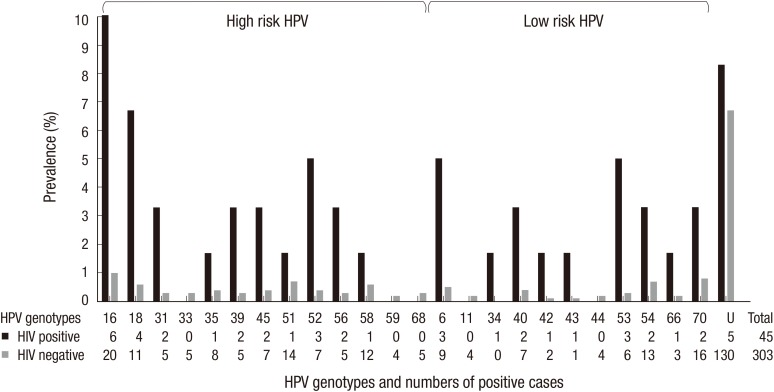
Fig. 1 shows the type-specific HPV prevalence of both HIV-positive and HIV-negative women. The most common hr-HPV genotype detected in HIV-positive women was HPV 16 (10%), followed by 18 (6.7%) and 52 (5%). Other common hr-HPV types detected in HIV-positive women were HPV 31 (3.3%), 39 (3.3%), 45 (3.3%), and 56 (3.3%). In HIV-negative women, the most prevalent hr-HPV was HPV 16 (1.0%), followed by 51 (0.7%), 58 (0.6%), and 18 (0.6%). Other commonly detected hr-HPV types in HIV-negative women were HPV 35 (0.4%), 45 (0.4%), and 52 (0.4%). Commonly encountered non-oncogenic viruses were HPV 70 (0.8%), 54 (0.7%), 6 (0.5%), 40 (0.4%), and 53 (0.3%) in HIV-negative women, and HPV 6 (5%), 53 (5%), 40 (3.3%), 54 (3.3%), and 70 (3.3%) in HIV-positive women. Five samples (8.3%) in HIV-positive women and 130 (6.7%) in HIV-negative women were unable to type. Prevalence of quadrivalent vaccine-preventable types (HPV 6, 11, 16, and 18) was 21.7% (13/60) and 2.3% (44/1,938) in HIV-positive women and HIV-negative women, respectively.
Fig. 1.
Human papillomavirus (HPV) genotypic distribution in 60 human immunodeficiency virus (HIV)-positive and 1,938 HIV-negative women. U, un-typeable HPV.
A total of 11 HIV-positive women (18.3%) and 35 HIV-negative women (1.8%) had abnormal cytology (AOR, 10.94; 95% CI, 5.18-23.1, P<0.001) (Table 2). HIV-infected women with hr-HPV were more likely to have abnormal cytology than those without hr-HPV (P<0.001) (Table 3). All 7 HIV-positive women with high-grade squamous intraepithelial lesion (HSIL) or low-grade squamous intraepithelial lesion (LSIL) tested positive for hr-HPV and had multiple HPV types, five of which also had multiple hr-HPV. A total of 12 hr-HPV were detected in 7 HIV-positive women with HSIL or LSIL, and the most frequently detected hr-HPV was HPV 16 (3/7). Other detected hr-HPV were 18, 31, 35, 39, 45, 51, 52, 56, and 58 (by 1 occurrence for each).
Table 3.
Comparison of demographic characteristics and cervical cytology results between 18 high risk HPV positive HIV-infected women and 42 high risk HPV negative counterparts
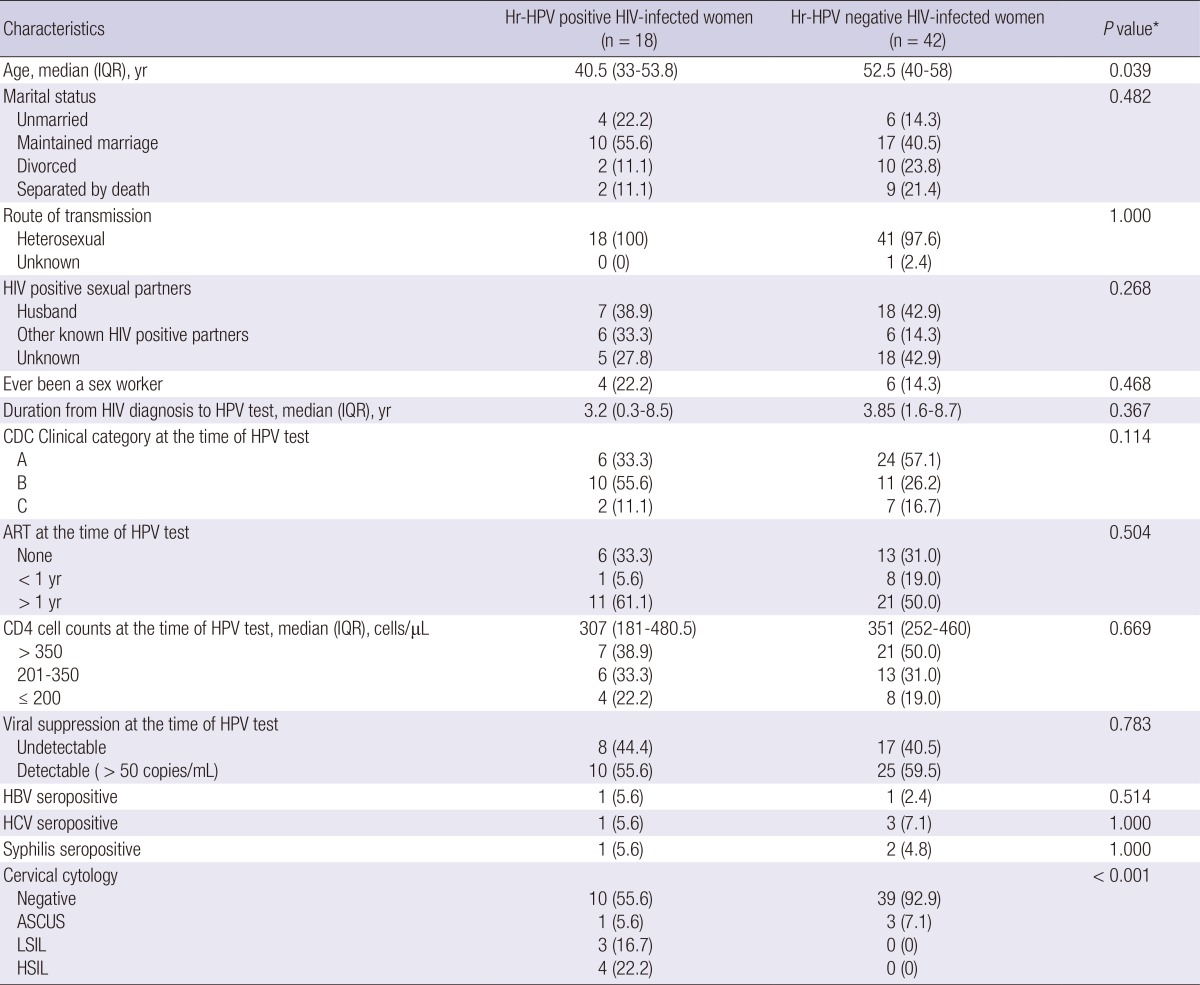
Data are number (%) of patients, unless otherwise indicated. *Calculated using chi-square test, Fisher's exact test, or the Mann-Whitney U-test. HIV, human immunodeficiency virus; Hr, high risk; HPV, humanpapilloma virus; IQR, interquartile range; CDC, Centers for Disease Control and Prevention; ART, anti-retroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; ASCUS, atypical squamous cells of undetermined significance; LSIL, low-grade squamous intraepithelial lesions; HSIL, high-grade squamous intraepithelial lesions.
When we compared the predictors for hr-HPV infection between hr-HPV positive and hr-HPV negative HIV-infected women, age was a significant risk factor for hr-HPV infection (P=0.039). Other factors were not associated with hr-HPV detection (Table 3).
DISCUSSION
Several studies have consistently shown that HIV-infected women are at increased risk for HPV infection, although the prevalence and genotype distribution varied in a different geographic area (15-21). This is the first report of prevalence of HPV among HIV-infected women in Korea to our knowledge. In this study, we found significantly higher prevalence of HPV infection in HIV-infected women, 46.7% for any HPV and 30% for hr-HPV, compared to HIV-negative controls, 14.1 and 4.9%, respectively. Hr-HPV was about 7 times more commonly detected among HIV-infected patients than among HIV-negative controls when adjusted with other demographic factors. The frequency of abnormal cervical cytology was about 11 times higher in HIV-infected women than in HIV-negative women. Our findings are in line with the results from other studies in other countries that reported hr-HPV prevalence rates ranging from 35.3% to 56% in HIV-infected women (15-21).
The prevalence of hr-HPV in HIV-negative women in our study is slightly lower than those reported in previous population based studies conducted in Busan, where it has been reported between 6% and 9%, 6.3% in the general population, 8.1% in the women attending cervical cancer screening program, and 8.6% in female university students (22-25). The differences between the studies are probably due to differences in the study population, detection method, and the definition of hr-HPV type.
In this study, HPV 16 was the most prevalent hr-HPV type detected in both HIV-positive and HIV-negative women. HPV 18, 52, 31, 39, 45, and 56 were also common among HIV-infected women with prevalence ranging from 3.3% to 6.7%. Other commonly detected hr-HPV types in HIV-negative women were 51, 58, 18, 35, 45, and 52 with prevalence ranging from 0.4% to 0.7%. There were some differences in the distribution of HPV genotypes between HIV-positive and HIV-negative women except for HPV 16. In a previous study evaluating the concordance of HPV infection among heterosexually active couples and the impact of HIV coinfection on the prevalence of HPV, HIV coinfection in one partner had a significant impact on the prevalence of HPV infection in the other partner (26). Concordance of the same HPV genotypes was more commonly found among couples where one or both partners were HIV-infected, compared with HIV-uninfected couples (26). The majority of HIV-infected women were infected by their male sexual partners in Korea (12), and our HIV-positive women had regular sexual partners in 61.7%. Therefore, HPV type may be diverse depending on types of their regular sexual partners. Although there is no study regarding HPV types of HIV-positive regular sexual partners, this may explain some differences in diverse type distribution of HPV infection between HIV-positive and HIV-negative women. In the previous studies conducted with CSWs in Korea, HPV 16, 18, and 58 were the most frequently detected hr-HPV types (27, 28). In our study, about 17% of HIV-infected women had ever been a CSW, and this also may contribute some differences in the distribution of HPV genotypes between HIV-positive and HIV-negative women.
Quadrivalent HPV vaccine was immunogenic and well tolerated in HIV-infected young women in a recent study (29). HPV vaccine is recommended by the Advisory Committee on Immunization Practices (ACIP) for HIV-infected individuals through age 26 yr for those who did not get any or all doses when they were younger (30). Prevalence of vaccine-preventable types (HPV 6, 11, 16, and 18) in our HIV-infected women was 21.7%, suggesting that HPV DNA or serologic screening would be needed before immunization in HIV-infected women in Korea, although the ACIP does not recommend HPV DNA or serologic screening before immunization (30).
We also investigated risk factors for carrying hr-HPV in HIV-infected women. Age was a significantly important risk factor of hr-HPV infection in HIV-infected women. Sexually active young HIV-infected women tend to have hr-HPV more frequently. However, other socio-demographic factors including marital status, route of transmission, presence of HIV-positive regular sex partners, history of female CSW, duration from HIV diagnosis to test, CDC clinical category, CD4 cell counts, ART, viral suppression, syphilis, and HBV/HCV coinfection were not significantly associated with the presence of hr-HPV in HIV-infected women. The presence of hr-HPV was significantly associated with abnormal findings on Pap smear. All HIV-infected women with LSIL or worse on Pap smear reacted positive for hr-HPV in our study.
This study has some limitations. First, this study was a retrospective cross-sectional study. The longitudinal follow-up data were not available, and the behavioral risk factors, such as the number of lifetime sex partners, were not considered in more detail. Second, our study was conducted in the southeastern region of Korea, and the number of HIV-infected women was relatively small, therefore our findings may not be generalized to other region of the country. Third, we did not test all genotypes of HPV; the proportion of persons with un-typeable HPV was relatively high, particularly in HIV-negative controls (31). Even with these limitations, our study could reflect the overall situation of HPV infection among HIV-infected women in Korea.
In conclusion, this study demonstrated that hr-HPV infection was common among HIV-infected women in Korea. The most frequent hr-HPV was HPV 16, followed by HPV 18 and 52. Age was a significantly important risk factor of hr-HPV infection in HIV-infected women. The presence of hr-HPV was significantly associated with abnormal cervical cytology. These findings suggest that HPV testing for cervical cancer screening in HIV-infected women would be necessary in Korea, particularly among young age group.
Footnotes
This study was supported by Medical Research Institute Grant (2005-5), Pusan National University.
The authors have no conflicts of interest to disclose.
References
- 1.Ellerbrock TV, Chiasson MA, Bush TJ, Sun XW, Sawo D, Brudney K, Wright TC., Jr Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283:1031–1037. doi: 10.1001/jama.283.8.1031. [DOI] [PubMed] [Google Scholar]
- 2.Hawes SE, Critchlow CW, Faye Niang MA, Diouf MB, Diop A, Touré P, Aziz Kasse A, Dembele B, Salif Sow P, Coll-Seck AM, et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis. 2003;188:555–563. doi: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- 3.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D Clinical Epidemiology Group of the FHDH-ANRS CO4 Cohort. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 4.Abraham AG, D'Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, Saag MS, Rourke SB, Rachlis A, Napravnik S, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013;62:405–413. doi: 10.1097/QAI.0b013e31828177d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lissouba P, Van de Perre P, Auvert B. Association of genital human papillomavirus infection with HIV acquisition: a systematic review and meta-analysis. Sex Transm Infect. 2013;89:350–356. doi: 10.1136/sextrans-2011-050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houlihan CF, Larke NL, Watson-Jones D, Smith-McCune KK, Shiboski S, Gravitt PE, Smith JS, Kuhn L, Wang C, Hayes R. Human papillomavirus infection and increased risk of HIV acquisition: a systematic review and meta-analysis. AIDS. 2012;26:2211–2222. doi: 10.1097/QAD.0b013e328358d908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, Clifford GM. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 8.Korea Centers for Disease Control and Prevention. Annual report on the notified HIV/AIDS in Korea. [accessed on 1 July 2013]. Available at http://stat.mw.go.kr.
- 9.Shin Y, Kee M. Health care systems in transition II. Korea, part II. the current status of HIV-AIDS in Korea. J Public Health Med. 1998;20:47–51. [PubMed] [Google Scholar]
- 10.Oh MD, Choe K. Epidemiology of HIV infection in the Republic of Korea. J Korean Med Sci. 1999;14:469–474. doi: 10.3346/jkms.1999.14.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe PG, Park WB, Song JS, Kim NH, Park JY, Song KH, Park SW, Kim HB, Kim NJ, Oh MD. Late presentation of HIV disease and its associated factors among newly diagnosed patients before and after abolition of a government policy of mass mandatory screening. J Infect. 2011;63:60–65. doi: 10.1016/j.jinf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Lee EJ, Kim SS, Nam JG, Whang J, Kee MK. Epidemiological characteristics of HIV-infected women in the Republic of Korea: a low HIV prevalence country. J Public Health Policy. 2009;30:342–355. doi: 10.1057/jphp.2009.16. [DOI] [PubMed] [Google Scholar]
- 13.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 14.Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, Jr, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuijzen NJ, Braunstein SL, Vyankandondera J, Ingabire C, Ntirushwa J, Kestelyn E, Tuijn C, Wit FW, Umutoni A, Uwineza M, et al. The epidemiology of human papillomavirus infection in HIV-positive and HIV-negative high-risk women in Kigali, Rwanda. BMC Infect Dis. 2011;11:333. doi: 10.1186/1471-2334-11-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dartell M, Rasch V, Kahesa C, Mwaiselage J, Ngoma T, Junge J, Gernow A, Ejlersen SF, Munk C, Iftner T, et al. Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the PROTECT study. Sex Transm Dis. 2012;39:201–208. doi: 10.1097/OLQ.0b013e31823b50ad. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie ND, Kobetz EN, Hnatyszyn J, Twiggs LB, Lucci JA., 3rd Women with HIV are more commonly infected with non-16 and -18 high-risk HPV types. Gynecol Oncol. 2010;116:572–577. doi: 10.1016/j.ygyno.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 18.Luque AE, Hitti J, Mwachari C, Lane C, Messing S, Cohn SE, Adler D, Rose R, Coombs R. Prevalence of human papillomavirus genotypes in HIV-1-infected women in Seattle, USA and Nairobi, Kenya: results from the Women's HIV Interdisciplinary Network (WHIN) Int J Infect Dis. 2010;14:e810–e814. doi: 10.1016/j.ijid.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar K, Pal R, Bal B, Saha B, Bhattacharya S, Sengupta S, Mazumdar PP, Chakraborti S. Oncogenic HPV among HIV infected female population in West Bengal, India. BMC Infect Dis. 2011;11:72. doi: 10.1186/1471-2334-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dols JA, Reid G, Brown JM, Tempelman H, Bontekoe TR, Quint WG, Boon ME. HPV type distribution and cervical cytology among HIV-positive Tanzanian and South African women. ISRN Obstet Gynecol. 2012;2012:514146. doi: 10.5402/2012/514146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mane A, Nirmalkar A, Risbud AR, Vermund SH, Mehendale SM, Sahasrabuddhe VV. HPV genotype distribution in cervical intraepithelial neoplasia among HIV-infected women in Pune, India. PLoS One. 2012;7:e38731. doi: 10.1371/journal.pone.0038731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clifford GM, Shin HR, Oh JK, Waterboer T, Ju YH, Vaccarella S, Quint W, Pawlita M, Franceschi S. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16:1874–1879. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 23.Oh JK, Franceschi S, Kim BK, Kim JY, Ju YH, Hong EK, Chang YC, Rha SH, Kim HH, Kim JH, et al. Prevalence of human papillomavirus and Chlamydia trachomatis infection among women attending cervical cancer screening in the Republic of Korea. Eur J Cancer Prev. 2009;18:56–61. doi: 10.1097/CEJ.0b013e328305a0a6. [DOI] [PubMed] [Google Scholar]
- 24.Shin HR, Lee DH, Herrero R, Smith JS, Vaccarella S, Hong SH, Jung KY, Kim HH, Park UD, Cha HS, et al. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int J Cancer. 2003;103:413–421. doi: 10.1002/ijc.10825. [DOI] [PubMed] [Google Scholar]
- 25.Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, Kong HJ, Rha SH, Jung SI, Kim JI, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–476. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 26.Mbulawa ZZ, Coetzee D, Marais DJ, Kamupira M, Zwane E, Allan B, Constant D, Moodley JR, Hoffman M, Williamson AL. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J Infect Dis. 2009;199:1514–1524. doi: 10.1086/598220. [DOI] [PubMed] [Google Scholar]
- 27.Yun H, Park J, Choi I, Kee M, Choi B, Kim S. Prevalence of human papillomavirus and herpes simplex virus type 2 infection in Korean commercial sex workers. J Microbiol Biotechnol. 2008;18:350–354. [PubMed] [Google Scholar]
- 28.Rhee JE, Shin MY, Kim CM, Kee HY, Chung JK, Min SK, Kim SJ, Jang DH, Kim SS, Choi BS. Prevalence of human papillomavirus infection and genotype distribution among high-risk Korean women for prospecting the strategy of vaccine development. Virol J. 2010;7:201. doi: 10.1186/1743-422X-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn JA, Xu J, Kapogiannis BG, Rudy B, Gonin R, Liu N, Wilson CM, Worrell C, Squires KE. Immunogenicity and safety of the human papillomavirus 6, 11, 16, 18 vaccine in HIV-infected young women. Clin Infect Dis. 2013;57:735–744. doi: 10.1093/cid/cit319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bridges CB, Woods L, Coyne-Beasley T Centers for Disease Control and Prevention (CDC), ACIP Adult Immunization Work Group. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older: United States, 2013. MMWR Surveill Summ. 2013;62:9–19. [PubMed] [Google Scholar]
- 31.Lee EH, Um TH, Chi HS, Hong YJ, Cha YJ. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012;27:1091–1097. doi: 10.3346/jkms.2012.27.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]