Abstract
Increased FcεR1α expression with upregulated CD203c expression on peripheral basophils is seen in patients with chronic urticaria (CU). However, there has been no published report on the association between CD203c expression level and clinical disease activity in CU patients. To investigate whether the increase of basophil activation is associated with the disease activity of CU, we measured basophil CD203c expression using a tricolor flow cytometric method in 82 CU patients and 21 normal controls. The relationship between the percentage of CD203c-expressing basophils and clinical parameters was analyzed. The mean basophil CD203c expression was significantly higher in CU patients than in healthy controls (57.5% vs 11.6%, P < 0.001). The basophil CD203c expression in severe CU patients was significantly higher than in non-severe CU (66.5% ± 23.3% vs 54.0% ± 23.3%, P = 0.033). Multiple logistic regression analysis indicated that both ≥ 72% basophil CD203c expression and urticaria activity score (UAS)≥ 13 were significant predictors of severe CU (P = 0.005 and P = 0.032, respectively). These findings suggest that the quantification of basophil activation with CD203c at baseline may be used as a potential predictor of severe CU requiring another treatment option beyond antihistamines.
Keywords: Chronic Urticaria; Basophil Activation; CD203c Protein, Human
INTRODUCTION
Chronic urticaria (CU) is a common skin disorder characterized by persistent or recurrent itchy wheals that last for at least 6 weeks. The cause of CU is heterogeneous, as many triggering and aggravating factors are involved in its pathogenesis, including physical stimuli, drugs, infections, and autoimmune mechanisms. The clinical phenotypes, severity, and prognosis of CU differ from patient to patient, so that management strategies need to be personalized. Recent guideline suggests a stepwise treatment based on effective symptomatic control with non-sedating antihistamines up to four folds of dosage, which is often efficacious but may not reach well-controlled state in 5% to as many as 50% of CU patients (1, 2). The growing evidence that autoimmunity and altered basophil functions are involved in the pathogenesis of CU has accelerated the introduction of immunomodulating agents for refractory patients (1, 3-5). Accordingly, the need for safe and reliable in vitro diagnostic tools to link each mechanism with reasonable therapeutic approaches has increased. Although elevated plasma levels of metalloproteinase (MMP) 9, interlukin (IL) 6 and 18 and D-dimer have been reported as possible biomarkers in association with CU severity, those are not specific for the pathogenesis of CU (6-9). Approximately half of CU patients are characterized by an autoreactive state with autoantibodies specific for the high-affinity IgE receptor (FcεRIα) and IgE itself (10). Although both the sensitivity and specificity of the autologous serum skin test (ASST) are limited, it has been recommended for defining a subgroup with autoreactive CU (10). However, the association between ASST positivity and disease activity is inconsistent.
Basophils are rare granulocytes that resemble tissue mast cells, in that they express FcεRIα and release histamine and chemical mediators (11). In active CU patients, basophils infiltrate the urticarial wheals and there is relative basopenia (12-14). While peripheral basophils do not represent cutaneous basophils completely, basophil histamine release (BHR) using healthy donor basophils has been used to evaluate autoreactive CU (15, 16). The basophil activation test (BAT) using flow cytometry has been applied as an in vitro diagnostic method for various allergic diseases (17). CD203c (ectonucleotide pyrophosphatase [E-NPP3]) is a surface marker observed uniquely on basophils and mast cells that is upregulated by anti-IgE antibody and allergens (18). For predicting causative allergens in patients with food, insect sting and drug allergy, the measurement of basophil CD203c expression induced by various kinds of allergens is reported to be useful (17, 19).
To-date, few studies have examined the phenotypes and characteristics of basophils in CU patients (14, 20, 21). Although the expression of the basophil activation markers CD63 and CD69 is increased in CU patients (22), there is to our knowledge no report of the clinical relevance of CD203c-BAT in CU patients. Therefore, this study evaluated the association of basophil CD203c expression with urticarial activity and autoimmunity in CU patients.
MATERIALS AND METHODS
Subjects
This was a hospital-based, cross-sectional study including 21 healthy controls (NC) and 82 CU patients who had current urticarial symptoms almost daily for at least 6 weeks. CU disease activity was assessed using the urticaria activity score (UAS), which combines pruritus and four characteristics of wheals, including number, distribution range, mean diameter, and duration in total score range, 0-15, with higher scores indicating higher disease activity (23). After 3 months from the enrollment, we reviewed medical records and calculated the medication requirement of each CU patient enrolled. Those patients requiring more than a third level of medication according to the guidelines or systemic steroids to control urticaria were classified as severe CU (10).
Autologous serum skin test and measurements of total IgE and anti-thyroid antibody
Antihistamines and corticosteroids were withdrawn at least 1 week before blood sampling. Intradermal autologous serum skin test (ASST) was performed following the method by EAACI/GA2LEN task force consensus report (10). Serum-induced wheal of diameter greater by at least 1.5 mm than that of a control induced by saline at 30 min was accepted as positive. The levels of total IgE were measured by the ImmunoCAP system (Pharmacia Diagnostics, Uppsala, Sweden) according to the manufacturer's instructions. Anti-thyroglobulin and thyroid microsomal antibodies were detected by radioimmunoassay (BRAHMS Aktiengesellschaft, Hennigsdorf, Germany).
Sampling and assays
The basophil CD203c expression was measured by flow cytometry. Subjects did not take any medication yet or stopped medication for at least one week before blood sampling. Whole blood was collected in ethylene diamine tetra-acetic acid (EDTA) tubes and red blood cells (RBCs) were lysed with RBC lysis buffer (0.154 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2-7.4). After washing with phosphate-buffered saline (PBS), the resuspended cells were stained with phycoerythrin (PE)-conjugated antihuman CD203c (Beckman Coulter, Marseille, France), fluorescein isothiocyanate (FITC)-conjugated antihuman CD123 (BD PharMingen, San Jose, CA, USA), and allophycocyanin (APC)-conjugated antihuman human leukocyte antigen (HLA)-DR (BD PharMingen), or isotype-matched controls on ice in the dark for 30 min. After washing once with PBS, the cells were analyzed on a FACS Canto II flow cytometer (Becton Dickinson, San Jose, CA, USA). The cells were gated initially based on the dot plot defined by the forward and side scatter, and then a second gate of high FITC-CD123+ cells and low APC-HLA-DR was defined to select the basophil population, analyzing at least 500 basophils. The percent CD203c expression was defined as the percentage of basophils expressing more CD203c than the critical point located at 103 < × < 104 in the histogram, which was about 10% of the basophils incubated with buffer only in the normal control. This was determined by defining region M1 on the histogram analysis.
Statistical analysis
The receiver-operating characteristic (ROC) curve was used to determine the optimal cutoff of the percentage of CD203c-expressing basophils used to diagnose CU, and the area under the curve (AUC) with a 95% confidence interval (CI) was computed. Fisher's test and the Mann-Whitney U-test were used to compare the clinical characteristics of CU, including the positive rate of atopy, autoantibodies, and serum total IgE level. Spearman's rho was used for the correlation analysis. Logistic regression was used to examine the effect of various factors on the optimal cut-off of basophil CD203c expression and the risk for severe CU. P values < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS for Windows (ver. 12; SPSS, Chicago, IL, USA).
Ethics statement
All of the subjects gave written informed consent at the time of enrollment, and the study was approved by the institutional review board of Ajou University Hospital (AJIRB-GEN-GEN-09-140).
RESULTS
The positive ASST, anti-thyroid autoantibody, and atopy rates were 37.8%, 19.0%, and 39% in the CU patients, respectively (Table 1). The mean white blood cell counts and differential for basophils in peripheral blood were 7,297.5 ± 2,248.8/µL and 0.5% ± 0.2%, respectively. The 23 patients requiring at least third- level treatment according to the guidelines were classified as severe CU. The mean UAS was significantly higher in severe CU (11.5 ± 2.8 vs 9.8 ± 3.2, P = 0.037), while their ASST response, positive anti-thyroid autoantibody and atopy rates did not differ from non-severe CU patients.
Table 1.
Clinical characteristics of study subjects
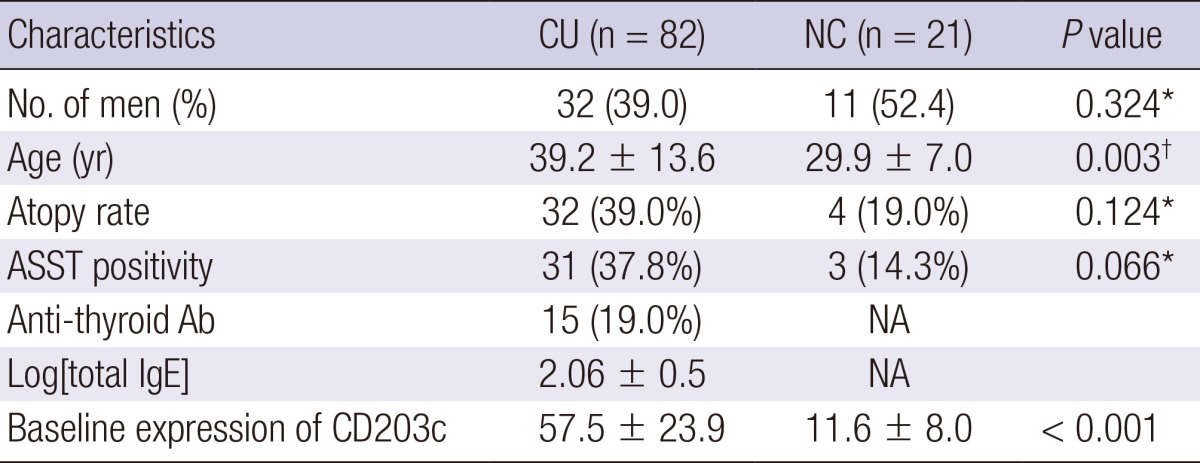
*Fisher's exact test, †Mann-Whitney test. ASST, autologous serum skin test; Log[total IgE], log-transformed serum total IgE levels; CU, chronic urticarial; NC, normal control; Ab, antibody; NA, not assessed.
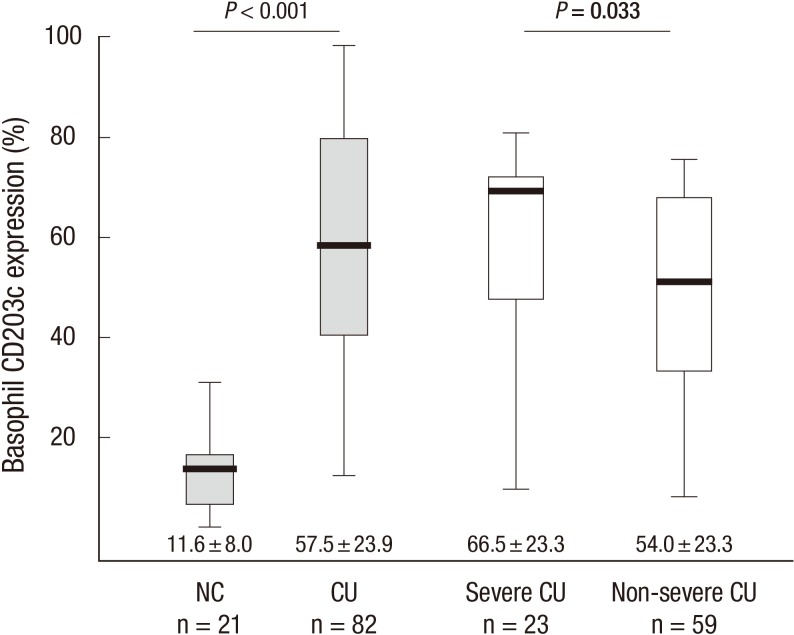
In CU patients, the mean percentage of basophil CD203c expression at baseline was 57.5% ± 23.9%, compared with an average activation of 11.6% ± 8.0% of basophils in normal controls (P < 0.001, Fig. 1). There was no significant difference in the baseline basophil CD203c expression according to gender or ASST, antithyroid autoantibodies, or atopy positivity rates among the CU patients. The leukocyte differential of basophils in peripheral blood was significantly correlated with CD203c expression (r=-0.220, P = 0.049) and serum total IgE level (r=-0.237, P = 0.033).
Fig. 1.
Mean percentages of basophil CD203c expression (%) at baseline in normal controls (NC) and patients with chronic urticaria (CU) classified into severe and non-severe groups. P values were determined using the Mann-Whitney U-test.
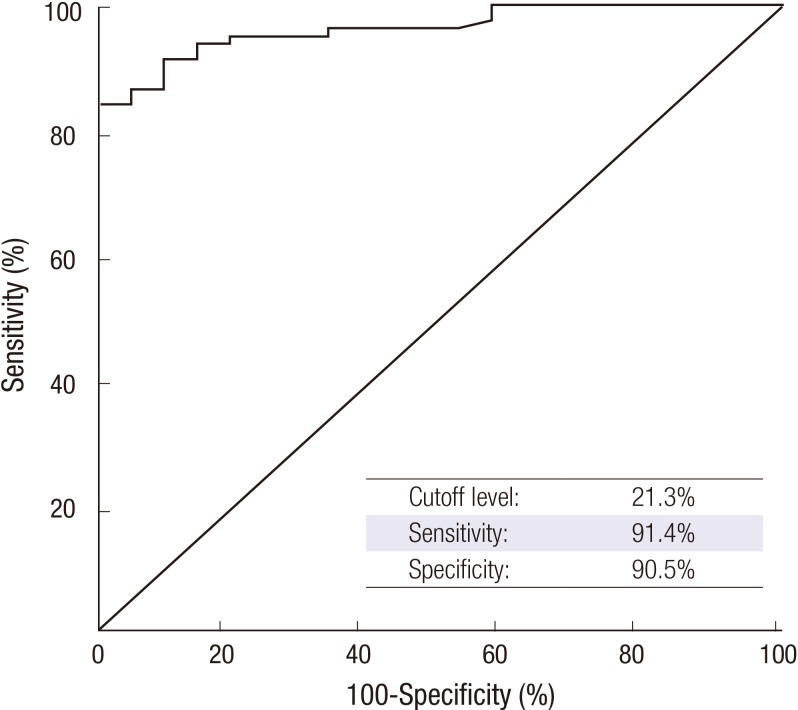
Taking a cutoff of 21.3% CD203c+ basophils as the positivity threshold yielded a sensitivity of 91.4% and a specificity of 90.5% with an AUC value of 0.9663 (95% CI, 0.931-0.995, P < 0.001). After adjusting for age, gender, and atopy, the logistic regression analysis revealed that the odds ratio for a 21.3% basophil CD203c expression at baseline for detecting CU patients was 166.04 (95% CI, 19.804-1392.071, P < 0.001, Fig. 2).
Fig. 2.
Receiver-operating characteristic (ROC) curve analysis yielded 21.3% CD203c-expressing basophils as the optimal cut-off for discriminating chronic urticaria (CU) from normal controls (NC). The sensitivity and specificity were 91.4% and 90.5%, respectively, with an AUC value of 0.963 (95% CI, 0.932-0.995; P < 0.001). The sensitivity and specificity were calculated according to the identified optimal cutoffs, and P values were adjusted for age and gender.
Severe CU patients had significantly higher CD203c expression than the non-severe group (66.5% ± 23.3% vs 54.0% ± 23.3%, P = 0.033, Fig. 1). Using the ROC analysis of CD203c expression to determine severe CU, a cutoff of 72.0% CD203+ basophils was chosen as the threshold of severe CU. However, the sensitivity (65.2%) and specificity (71.7%) were not remarkable (95% CI, 0.512-0.782). CD203c-expressing basophils ≥ 72% (P = 0.005) and a UAS ≥ 13 (P = 0.032) were significant, independent predictors of severe CU after adjusting for other factors, including age, gender, atopy, and ASST results (Table 2).
Table 2.
Predictors of severe chronic urticaria in the multiple logistic regression analysis
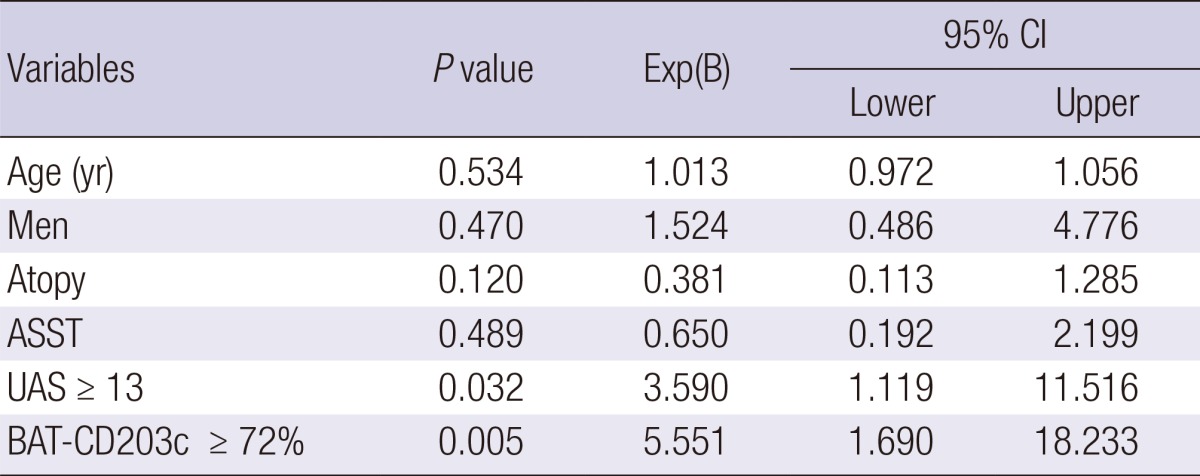
95% CI, 95% confidence interval; ASST, autologous serum skin test; UAS, urticaria activity score; BAT-CD203c, percentage of CD203c-expressing basophils.
DISCUSSION
This study confirmed previous reports (14, 21) that patients with CU show increased basophil CD203c expression at baseline and suggested that a cutoff basophil CD203c expression of 21.3% be used for determining basophil activation in CU patients, with a very high odds ratio of 166.04. Moreover, this study is the first to suggest that patients with severe CU have higher levels of basophil activation.
We found a significant negative correlation between the number of peripheral basophils and both the percentage of CD203c-expressing basophils and total IgE level in CU patients. Previous investigators suggested that basophils migrate from the circulation into wheals according to the urticarial activity (12). It has been demonstrated that serum IgE upregulates the expression of FcεRI on human basophils both in vitro and in vivo (24). In response to cross-linking of IgE and FcεRI, human basophils produce IL-3, which plays a crucial role in their development, survival, and priming for greater expression of activation markers and histamine release in an autocrine manner (14, 25). Various surface markers expressed on human basophils, such as CD63, CD69, and CD203c, have been used to demonstrate the role of basophils in vivo and in vitro (11, 12). Compared with other activation markers, the regulation of CD203c is not limited to the FcεRI-mediated reaction, but is also induced by IL-3. CD203c is thought to be the most useful marker of basophil activation and differentiation (18, 26, 27). The measurement of basophil CD203c expression has been demonstrated to be useful for detecting causative allergenic components in patients with wheat allergy and wheat dependent exercise-induced anaphylaxis, insect sting and amoxicillin allergy (17, 19). If peripheral basophils of CU patients were sensitized by autoantibodies, inflammatory mediators and other extrinsic stimulants during the active urticaria, they would express a high amount of IgE bound to FcεRIα on their surface. And then, as like basophils from other allergic diseases are triggered by different stimulants (19), they upregulate several activation markers including CD203c on their surface. Notably, basophil CD203c expression was significantly higher in severe CU patients whose symptoms were not completely controlled with antihistamines.
There are no convincing predictors of urticarial development after antihistamine treatment or when patients can stop taking immunomodulators. The guidelines emphasize using the UAS in clinical practice to determine disease activity and the response to treatment of CU (10). We also found that a UAS ≥ 13 was a significant determinant of severe CU. However, as one recent study demonstrated (14), the ASST response was not reflected in the basophil CD203c expression or the clinical severity of the CU patients in our series. Flow cytometric BAT for CU patients is usually performed to detect the serologic factors in the patients that trigger histamine release from donor basophils. Recently, several serological markers, such as MMP-9, IL-6, IL-18, C-reactive protein and D-dimer, in association with the clinical severity of CU have been suggested (6-9). Since the coagulation factors, complement, and cytokines associated with chronic low-grade inflammation, in addition to the IgE- and FcεRI-dependent pathways, may activate basophils, a variety of diagnostic tools should be used to further our understanding of CU. This study enrolled 82 CU patients and obtained the optimal cutoff for determining basophil activation in CU patients and identifying severe CU using ROC analysis, although a validation study is needed in a second cohort. Therefore, BAT using CD203c expression can be extended to assess the degree of basophil activation for predicting the clinical severity of and treatment response to CU.
In conclusion, we suggest that the quantification of basophil CD203c expression at baseline is useful for predicting severe CU patients, who may need further treatment beyond antihistamines.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, Grattan CE, Kapp A, Maurer M, Merk HF, et al. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–1443. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 2.Morgan M, Khan DA. Therapeutic alternatives for chronic urticaria: an evidence-based review, part 1. Ann Allergy Asthma Immunol. 2008;100:403–411. doi: 10.1016/S1081-1206(10)60462-0. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan AP. Treatment of chronic spontaneous urticaria. Allergy Asthma Immunol Res. 2012;4:326–331. doi: 10.4168/aair.2012.4.6.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grattan CE, O'Donnell BF, Francis DM, Niimi N, Barlow RJ, Seed PT, Kobza Black A, Greaves MW. Randomized double-blind study of cyclosporin in chronic 'idiopathic' urticaria. Br J Dermatol. 2000;143:365–372. doi: 10.1046/j.1365-2133.2000.03664.x. [DOI] [PubMed] [Google Scholar]
- 5.Nam YH, Kim JH, Jin HJ, Hwang EK, Shin YS, Ye YM, Park HS. Effects of omalizumab treatment in patients with refractory chronic urticaria. Allergy Asthma Immunol Res. 2012;4:357–361. doi: 10.4168/aair.2012.4.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessel A, Bishara R, Amital A, Bamberger E, Sabo E, Grushko G, Toubi E. Increased plasma levels of matrix metalloproteinase-9 are associated with the severity of chronic urticaria. Clin Exp Allergy. 2005;35:221–225. doi: 10.1111/j.1365-2222.2005.02168.x. [DOI] [PubMed] [Google Scholar]
- 7.Kasperska-Zajac A, Sztylc J, Machura E, Jop G. Plasma IL-6 concentration correlates with clinical disease activity and serum C-reactive protein concentration in chronic urticaria patients. Clin Exp Allergy. 2011;41:1386–1391. doi: 10.1111/j.1365-2222.2011.03789.x. [DOI] [PubMed] [Google Scholar]
- 8.Tedeschi A, Lorini M, Suli C, Asero R. Serum interleukin-18 in patients with chronic ordinary urticaria: association with disease activity. Clin Exp Dermatol. 2007;32:568–570. doi: 10.1111/j.1365-2230.2007.02450.x. [DOI] [PubMed] [Google Scholar]
- 9.Triwongwaranat D, Kulthanan K, Chularojanamontri L, Pinkaew S. Correlation between plasma D-dimer levels and the severity of patients with chronic urticaria. Asia Pac Allergy. 2013;3:100–105. doi: 10.5415/apallergy.2013.3.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, Grattan CE, Kapp A, Merk HF, Rogala B, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1417–1426. doi: 10.1111/j.1398-9995.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 11.Falcone FH, Knol EF, Gibbs BF. The role of basophils in the pathogenesis of allergic disease. Clin Exp Allergy. 2011;41:939–947. doi: 10.1111/j.1365-2222.2011.03781.x. [DOI] [PubMed] [Google Scholar]
- 12.Grattan CE, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy. 2003;33:337–341. doi: 10.1046/j.1365-2222.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66:1107–1113. doi: 10.1111/j.1398-9995.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 14.Lourenço FD, Azor MH, Santos JC, Prearo E, Maruta CW, Rivitti EA, Duarte AJ, Sato MN. Activated status of basophils in chronic urticaria leads to interleukin-3 hyper-responsiveness and enhancement of histamine release induced by anti-IgE stimulus. Br J Dermatol. 2008;158:979–986. doi: 10.1111/j.1365-2133.2008.08499.x. [DOI] [PubMed] [Google Scholar]
- 15.Asero R, Lorini M, Chong SU, Zuberbier T, Tedeschi A. Assessment of histamine-releasing activity of sera from patients with chronic urticaria showing positive autologous skin test on human basophils and mast cells. Clin Exp Allergy. 2004;34:1111–1114. doi: 10.1111/j.1365-2222.2004.01997.x. [DOI] [PubMed] [Google Scholar]
- 16.Gentinetta T, Pecaric-Petkovic T, Wan D, Falcone FH, Dahinden CA, Pichler WJ, Hausmann OV. Individual IL-3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol. 2011;128:1227.e5–1234.e5. doi: 10.1016/j.jaci.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Chirumbolo S. Basophil activation test in allergy: time for an update. Int Arch Allergy Immunol. 2012;158:99–114. doi: 10.1159/000331312. [DOI] [PubMed] [Google Scholar]
- 18.Boumiza R, Monneret G, Forissier MF, Savoye J, Gutowski MC, Powell WS, Bienvenu J. Marked improvement of the basophil activation test by detecting CD203c instead of CD63. Clin Exp Allergy. 2003;33:259–265. doi: 10.1046/j.1365-2222.2003.01594.x. [DOI] [PubMed] [Google Scholar]
- 19.Chinuki Y, Kaneko S, Dekio I, Takahashi H, Tokuda R, Nagao M, Fujisawa T, Morita E. CD203c expression-based basophil activation test for diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2012;129:1404–1406. doi: 10.1016/j.jaci.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 20.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956–1963. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 21.Yasnowsky KM, Dreskin SC, Efaw B, Schoen D, Vedanthan PK, Alam R, Harbeck RJ. Chronic urticaria sera increase basophil CD203c expression. J Allergy Clin Immunol. 2006;117:1430–1434. doi: 10.1016/j.jaci.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Vasagar K, Vonakis BM, Gober LM, Viksman A, Gibbons SP, Jr, Saini SS. Evidence of in vivo basophil activation in chronic idiopathic urticaria. Clin Exp Allergy. 2006;36:770–776. doi: 10.1111/j.1365-2222.2006.02494.x. [DOI] [PubMed] [Google Scholar]
- 23.Ye YM, Park JW, Kim SH, Choi JH, Hur GY, Lee HY, Lee EH, Park HS. Clinical evaluation of the computerized chronic urticaria-specific quality of life questionnaire in Korean patients with chronic urticaria. Clin Exp Dermatol. 2012;37:722–728. doi: 10.1111/j.1365-2230.2012.04414.x. [DOI] [PubMed] [Google Scholar]
- 24.Saini SS, MacGlashan D. How IgE upregulates the allergic response. Curr Opin Immunol. 2002;14:694–697. doi: 10.1016/s0952-7915(02)00404-1. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009;182:2432–2438. doi: 10.4049/jimmunol.0801782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gernez Y, Tirouvanziam R, Yu G, Ghosn EE, Reshamwala N, Nguyen T, Tsai M, Galli SJ, Herzenberg LA, Herzenberg LA, et al. Basophil CD203c levels are increased at baseline and can be used to monitor omalizumab treatment in subjects with nut allergy. Int Arch Allergy Immunol. 2011;154:318–327. doi: 10.1159/000321824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bühring HJ, Streble A, Valent P. The basophil-specific ectoenzyme E-NPP3 (CD203c) as a marker for cell activation and allergy diagnosis. Int Arch Allergy Immunol. 2004;133:317–329. doi: 10.1159/000077351. [DOI] [PubMed] [Google Scholar]