Abstract
Steady-state irradiation under visible light of a covalent Ir(III)-photosensitized polyoxotungstate is reported. In the presence of a sacrificial electron donor, the photolysis leads to the very efficient photoreduction of the polyoxometalate. Successive formation of the one-electron and two-electron reduced species, which are unambiguously identified by comparison with spectroelectrochemical measurements, is observed with a significantly faster rate reaction for the formation of the one-electron reduced species. The kinetics of the photoreduction, which are correlated to the reduction potentials of the polyoxometalate (POM), can be finely tuned by the presence of an acid. Indeed light-driven formation of the two-electron reduced POM is considerably facilitated in the presence of acetic acid. The system is also able to perform photocatalytic hydrogen production under visible light without significant loss of performance over more than 1 week of continuous photolysis and displays higher photocatalytic efficiency than the related multi-component system, outlining the decisive effect of the covalent bonding between the POM and the photosensitizer. This functional and modular system constitutes a promising step for the development of charge photoaccumulation devices and subsequent photoelectrocatalysts for artificial photosynthesis.
Photoconversion of light into chemical fuels is emerging as a major scientific challenge.1-4 In the past decades, molecular approaches have mostly focused on one hand on the design of photosensitive systems displaying long-lived photo-induced charge separation states to permit further electron transfers5-10 and, on the other hand, on catalysts able to use these photogenerated charges for achieving either oxygen11-18 or hydrogen evolution.19-24 As these two reactions are multi-electronic processes while photosensitizers deliver electrons and holes sequentially, the charges need to be directed to a charge accumulation site.25 However, only a few molecular photoactive systems with a designed charge accumulation site have been described so far.26-30 Another requirement is crucial for efficient charge accumulation in such systems: when partially filled, the reservoir should not interfere with the photoactive moiety. Indeed, in classical donor-acceptor (D-A) systems, the electron acceptor, once reduced, potentially becomes an electron donor and often displays light-absorbing properties. Thus it may act, in a subsequent light-driven process, as a deleterious quencher of the excited donor D* by reverse charge transfer (equation 1) or energy transfer (equation 2).
| (1) |
| (2) |
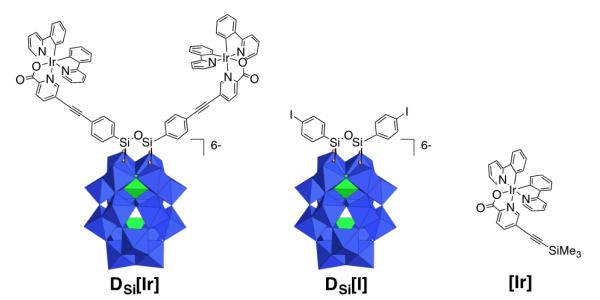
Since they can store several electrons with modest difference in potentials between successive reduction steps,31, 32 polyoxometallates (POMs) are attractive candidates to further illustrate charge photoaccumulation. While electrocatalytic reduction of protons by POMs has been thoroughly investigated,33-35 their activity in water photoreduction has been far less developed and only under UV-light excitation,36-38 with two recent notable exceptions.39, 40 This is in striking contrast with recent but extensive studies on light-driven water oxidation mediated by transition metal substituted POMs. In this context, we recently reported the synthesis and photophysical properties of a series of heteroleptic carbocyclometalated iridium(III)-polyoxometalate conjugates41, 42 following mild reaction conditions developed earlier by some of us.43-46 In such complexes, photoinduced charge-separated excited states of various lifetimes (ranging from nanoseconds to hundreds of nanoseconds) were observed by transient absorption spectroscopy. Most importantly, the functionalization of the heteroleptic cyclometalated iridium(III) on the picolinate ligand provides directionality to the photoinduced electron transfer by enhancing charge separation and delaying charge recombination, which is an asset for preventing reverse charge transfer from proceeding (equation 1). The kinetics of charge separation and charge recombination in these reported POM-[Ir] hybrids were correlated to the redox potential of the POM with the fastest electron transfer rates (both charge separation and charge recombination) observed for the POMs that are the most easily reduced. Among the different POM-[Ir] conjugates we selected the Dawson-type organosilyl hybrid [P2W17O61{O(SiC36H23N3O2Ir)2}]6−, named DSi[Ir], since it offers the best compromise between efficient charge separation and long-lived charge-separated state. This hybrid, isolated as a tetrabutyl ammonium salt, contains two heteroleptic cyclometalated iridium(III) units connected to the mono-lacunary site of the Dawson-type α2-[P2W17O61]10− through a Si-O-Si anchorage (Scheme 1). In this hybrid, the POM and the chromophore are poorly coupled electronically.
Scheme 1.
Molecular representation of the photoactive POM-based hybrid DSi[Ir], reference POM DSi[I] and reference iridium complex [Ir] described in this study. In the polyhedral representation, the WO6 octahedra are depicted with oxygen atoms at the vertices and metal cations buried inside. Color code: WO6 octahedra, blue; PO4 tetrahedra, green.
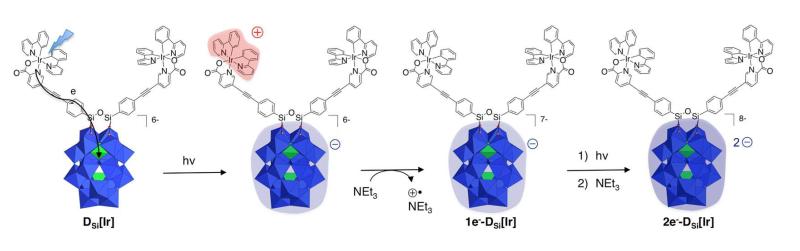
As a consequence, in DSi[Ir] the POM displays redox properties very similar to those of the hybrid precursor bearing two iodoaryl moieties, named DSi[I].47, 48 In the previously mentioned study, transient absorptions measurements only allowed for the characterization of the first photo-induced electron transfer. Charge photo-accumulation studies can be achieved in the presence of an additional electron donor in the solution that can irreversibly quench the charge separation state, regenerate the initial state of the photosensitizer and make a second photo-induced process possible. We herein provide unprecedented evidence of charge accumulation on a polyoxotungstate by visible-light. Furthermore, this system is able to perform direct photocatalytic hydrogen evolution, albeit at low pace, without noticeably decreasing over more than 1 week of continuous photolysis.
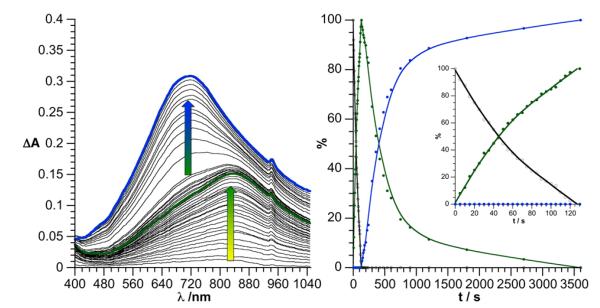
The photoreduction reaction of DSi[Ir] is easily monitored by electronic spectroscopy since, in their reduced forms, POMs display intense d-d and intervalence charge transfer transitions.49-52 For reference, spectroelectrochemical measurements carried out on the related DSi[I] are given in the Supporting Information: electrolysis was followed by rotating disk electrode voltamperometry and UV-Vis spectroscopy to provide the spectroscopic signature of the successively formed one- and two-electron reduced species. The evolution of the electronic spectrum of a degassed DMF solution of DSi[Ir] (0.2 mM) under visible light irradiation (λ > 400 nm) and in the presence of triethylamine (NEt3, 1 M) acting as sacrificial electron donor is shown in Figure 1. First, a large band with a maximum absorption at 840 nm, corresponding to the one-electron reduced POM (1e−-DSi[Ir]), grows.
Figure 1.
Left: evolution of the differential visible absorption spectrum of a solution of DSi[Ir] (0.2 mM) in DMF containing NEt3 (1 M) during photoirradiation under visible light (green t = 130 sec., blue t = 1 hour). For clarity purpose, the blank corresponds to the absorption of the initially yellow solution before photolysis. Right: distribution of the different reduction states of DSi[Ir] during the photolysis. Legend: non-reduced DSi[Ir] (colorless circle), 1e−-DSi[Ir] (green circle) and 2e−-DSi[Ir] (blue circles).
Then, under prolonged photolysis, an additional absorption appears at 710 nm, attributed to the two-electron reduced POM (2e−-DSi[Ir]). As luminescence of the iridium complex precursor [Ir] is not affected by the sacrificial donor under similar conditions, we assume that the active species responsible for the oxidation of the triethylamine is more likely the oxidized photosensitizer, in the charge-separated state, rather than the excited form of the photosensitizer itself (Scheme 2). The formation of 1e−-DSi[Ir] is very fast. We measured a half-reaction time constant of τ1/2 = 43 s with the setup shown in Figure S1 under continuous visible-light irradiation (400 nm < λ < 800 nm) and a quantum yield at 400 nm of ϕ400 nm = 10.5±1%. The second reduction to 2e−-DSi[Ir] is significantly slower (τ1/2 = 270 s, ϕ400 nm = 2.3±0.5%). This can be attributed to incomplete charge-separation in the already one-electron reduced species. Indeed, the second reduction process of DSi[Ir] occurs at a potential 400 mV more negative than the first, slowing down the second photoinduced electron transfer process. However the two-electrons photoreduction observed is much more efficient than that of a previously reported electrostatic [Ru(bpy)3]2[S2M18O62] system.53 If the quantitative photoreduction of [S2Mo18O62]4− at 420 nm has been nicely described, the corresponding tungstate [S2W18O62]4− led to reduced species that could only be poorly characterized after at least one hour of photolysis. Concomitantly, a decrease of the chromophore absorption was observed, suggesting a significant degradation of the system. By introducing a covalent link between the photosensitizer and the polyoxotungstate, we strongly favour direct charge injection to the POM, and prevent the system from degradation.
Scheme 2.
Charge photo-accumulation occurring in the DSi[Ir] dyad.
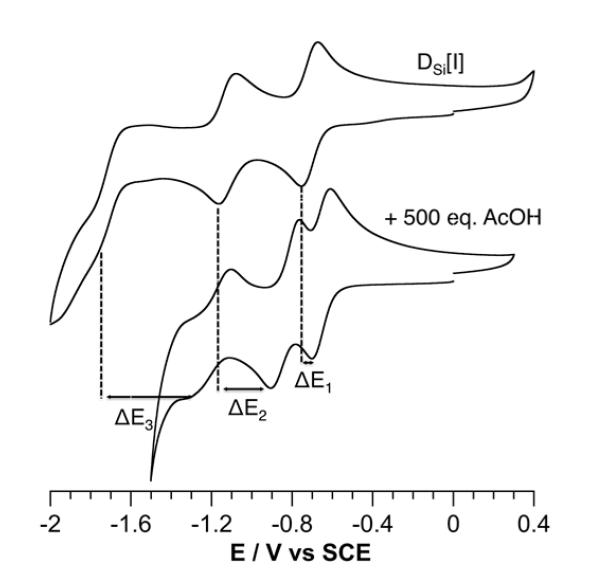
Multi-electron processes can be facilitated to a large extent in the presence of protons. In particular, the electrochemical behavior of POMs is drastically affected by the presence of acids.31 As shown in Figure 2, in the presence of acetic acid the waves of DSi[I] are shifted to higher potential, thus confering to the POM improved reservoir properties.
Figure 2.
Evolution of cyclic voltammograms of a 10−3 M solution of the reference POM hybrid DSi[I] in DMF containing 0.1 M TBAPF6 upon addition of 500 equiv AcOH. Working electrode : glassy carbon; reference electrode, SCE.
We then investigated the photoreduction reaction of DSi[Ir] in the presence of acetic acid (0.1 M). The study of the stability of the heteroleptic iridium(III) complex covalently linked to the POM showed that in DMF or MeCN solutions, the presence of strong acid (HCl, trifluoracetic acid) leads to an irreversible loss of picolinate ancillary ligands as recently described elsewhere.54 However, we checked that, even in large excess (500 equiv) of acetic acid (AcOH), DSi[Ir] is stable for few days (Figure S4). We note that AcOH (pKa =13.5 in DMF) is unable to protonate NEt3 (pKa =9.2 in DMF) under these conditions55 and thus does not significantly influence the apparent pH value of the reaction medium.56 The presence of the acid barely modifies the initial evolution of the absorption spectra. The initial one-electron reduced POM, has an absorption maximum at 845 nm and the absorption of the two-electron reduced POM is centred at 695 nm. While the formation of 1e−-DSi[Ir] is only slightly faster compared to the photoreduction in the absence of AcOH (τ1/2 = 37 s), formation of 2e−-DSi[Ir] is drastically accelerated (τ1/2 = 60 s) in the presence of acid. Again, the photoreduction kinetics seems to be correlated to the redox properties of the POM. Indeed, the presence of AcOH affects more significantly the redox potential of the second reduction of the POM than the first one (see Figure 2; ΔE1 = 60 mV, ΔE2 = 280 mV). As a consequence, in the presence of acetic acid, the light-driven formation of the two-electron reduced POM is considerably facilitated, while the kinetics of formation of the one-electron reduced POM is almost unchanged. We have previously described how the structure-related redox potentials of the POM impacts the kinetics of the charge separation/recombination steps.41 Further tunability is thus addressable through the acid dependence of the reduction processes. This makes POMs unique for the design of finely controlled molecular electron reservoirs.
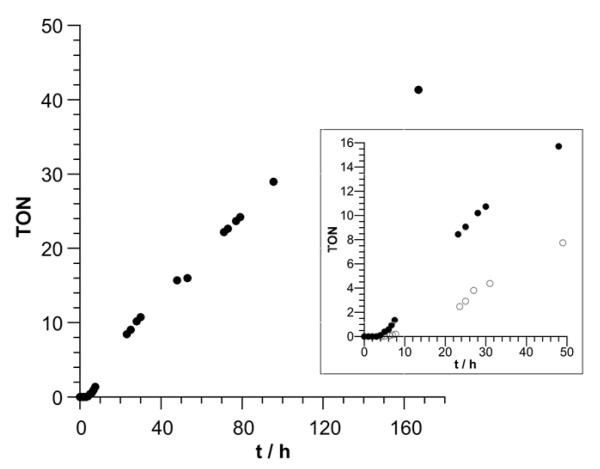
As POMs are known to catalyze proton reduction under certain conditions,31, 33, 34, 38 we thus wonder whether the POM moiety in DSi[Ir] could act both as an electron reservoir and as a H2-evolving catalyst under photolysis conditions. Gas chromatography monitoring of the photolysis of DSi[Ir] in the presence of NEt3 and acetic acid in DMF shows that H2 continuously accumulates in the headspace of the reaction vessel. After an initial induction period, H2 evolution was sustained for days with a turnover frequency (TOF) of ca. 0.25 h−1. Up to 41 turnovers (calculated vs the POM) are formed within a 7-days experiment (Figure 3). No H2 could be detected when NEt3 was omitted or when DSi[Ir] was replaced by the reference compound DSi[I]. Only traces of H2 were detected when a mononuclear carbocyclometalated picolinate iridium(III) complex, [Ir], was used instead of DSi[Ir]. Interestingly, the turnover frequency for hydrogen evolution is twice lower for the multicomponent system consisting of DSi[I] and 2 equiv of [Ir] under the same conditions, emphasizing again the beneficial effect of the covalent tether between the photosensitizer and the POM (See inset in Figure 3).
Figure 3.
Photochemical production of hydrogen from DMF solutions (10 mL) of NEt3 (1 M) and AcOH (0.1 M) catalyzed by DSi[Ir] (0.2 mM). Comparison with the multicomponent system consisting of DSi[I] (0.2 mM) and 2 equiv. of [Ir] (0.4 mM) is shown in the inset (colorless dots). In these experiments, one turnover corresponds to 2 μmol (48 μL) H2 evolved.
We have thus demonstrated that upon steady-state visible irradiation and in the presence of a sacrificial electron donor, the carbocyclometalated iridium(III)-polyoxometalate conjugate DSi[Ir] is capable of photo-accumulating two electrons on the POM in a very efficient manner. This all-integrated system offers unique advantages for charge photo-accumulation since it fulfills the previously mentioned requisites.
(1) The iridium complex, because of its heteroleptic character, favors charge transfer to the POM and slows down backward electron transfer. This prevents the reduced POM from acting as a deleterious quencher of the excited iridium complex by reverse charge transfer (i.e. equation 1). (2) The absorbance of both one-electron and two-electron reduced POMs, although important in the lower energy part of the visible spectrum is modest to weak at ca. 510 nm (emission maximum wavelength of the iridium complex)57 disfavoring energy transfer from the excited iridium complex to the reduced POM (i.e. equation 2). (3) The presence of acid leads to proton-assisted reduction of the POM, which provides the POM with improved reservoir properties and enhances the rate of photoproduction of the two-electron reduced POM. Finally, the system displays promising photocatalytic activity for hydrogen evolution. Further studies are required to establish the catalytic mechanism at work and eventually identify the catalytic active species formed in situ during the induction period.58 While the catalytic rate is modest, the deceleration over seven days of continuous photolysis is very low, which establishes the robustness of the system upon turn-over. Indeed, monitoring of the 31P NMR signals of DSi[Ir] under irradiation indicates that the POM framework of the hybrid is maintained (Figure S4). We are currently working on the improvement of photocatalytic activity for hydrogen evolution of the system. This implies optimization of the photocatalytic conditions (solvent, electron donor…), improvement of the robustness of the photoactive complex towards acid and design of multicomponent POM-[Ir]/electrocatalyst systems.
Supplementary Material
Acknowledgment
The authors gratefully thank Géraldine Lorin (CEA, Institut Liten, DTNM/L2CE) for the calibration of the optical bench for quantum yield measurements. They acknowledge support from the CNRS and the Ministère de la Recherche et de l’Enseignement Supérieur for a PhD fellowship to BM. The COST Action CM1202 PERSPECT-H2O and funding from the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement n.306398 are also acknowledged.
Notes and references
- 1.Tran PD, Wong LH, Barber J, Loo JSC. Energy Environ. Sci. 2012;5:5902–5918. [Google Scholar]
- 2.Andreiadis ES, Chavarot-Kerlidou M, Fontecave M, Artero V. Photochem. Photobiol. 2011;87:946–964. doi: 10.1111/j.1751-1097.2011.00966.x. [DOI] [PubMed] [Google Scholar]
- 3.Thapper A, Styring S, Saracco G, Rutherford AW, Robert B, Magnuson A, Lubitz W, Llobet A, Kurz P, Holzwarth A, Fiechter S, de Groot H, Campagna S, Braun A, Bercegol H, Artero V. Green. 2013;3:43–57. [Google Scholar]
- 4.Benniston AC, Harriman A. Materials Today. 2008;11:26–34. [Google Scholar]
- 5.Imahori H, Guldi DM, Tamaki K, Yoshida Y, Luo CP, Sakata Y, Fukuzumi S. J. Am. Chem. Soc. 2001;123:6617–6628. doi: 10.1021/ja004123v. [DOI] [PubMed] [Google Scholar]
- 6.Borgström M, Shaikh N, Johansson O, Anderlund MF, Styring S, Åkermark B, Magnuson A, Hammarström L. J. Am. Chem. Soc. 2005;127:17504–17515. doi: 10.1021/ja055243b. [DOI] [PubMed] [Google Scholar]
- 7.Di Valentin M, Bisol A, Agostini G, Liddell PA, Kodis G, Moore AL, Moore TA, Gust D, Carbonera D. J. Phys. Chem. B. 2005;109:14401–14409. doi: 10.1021/jp051345c. [DOI] [PubMed] [Google Scholar]
- 8.Gust D, Moore TA, Moore AL, Gao F, Luttrull D, Degraziano JM, Ma XCC, Makings LR, Lee SJ, Trier TT, Bittersmann E, Seely GR, Woodward S, Bensasson RV, Rougee M, Deschryver FC, Vanderauweraer M. J. Am. Chem. Soc. 1991;113:3638–3649. [Google Scholar]
- 9.Wasielewski MR, Gaines GL, Wiederrecht GP, Svec WA, Niemczyk MP. J. Am. Chem. Soc. 1993;115:10442–10443. [Google Scholar]
- 10.Le Pleux L, Pellegrin Y, Blart E, Odobel F, Harriman A. J. Phys. Chem. A. 2011;115:5069–5080. doi: 10.1021/jp2012182. [DOI] [PubMed] [Google Scholar]
- 11.Gersten SW, Samuels GJ, Meyer TJ. J. Am. Chem. Soc. 1982;104:4029–4030. [Google Scholar]
- 12.Concepcion JJ, Jurss JW, Templeton JL, Meyer TJ. J. Am. Chem. Soc. 2008;130:16462–16463. doi: 10.1021/ja8059649. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Concepcion JJ, Jurss JW, Cardolaccia T, Templeton JL, Meyer TJ. Inorg. Chem. 2008;47:1727–1752. doi: 10.1021/ic701249s. [DOI] [PubMed] [Google Scholar]
- 14.Geletii YV, Botar B, Koegerler P, Hillesheim DA, Musaev DG, Hill CL. Angew. Chem., Int. Ed. 2008;47:3896–3899. doi: 10.1002/anie.200705652. [DOI] [PubMed] [Google Scholar]
- 15.Yin QS, Tan JM, Besson C, Geletii YV, Musaev DG, Kuznetsov AE, Luo Z, Hardcastle KI, Hill CL. Science. 2010;328:342–345. doi: 10.1126/science.1185372. [DOI] [PubMed] [Google Scholar]
- 16.Sartorel A, Carraro M, Scorrano G, De Zorzi R, Geremia S, McDaniel ND, Bernhard S, Bonchio M. J. Am. Chem. Soc. 2008;130:5006–5007. doi: 10.1021/ja077837f. [DOI] [PubMed] [Google Scholar]
- 17.Sala X, Romero I, Rodriguez M, Escriche L, Llobet A. Angew. Chem., Int. Ed. 2009;48:2842–2852. doi: 10.1002/anie.200802659. [DOI] [PubMed] [Google Scholar]
- 18.Surendranath Y, Kanan MW, Nocera DG. J. Am. Chem. Soc. 2010;132:16501–16509. doi: 10.1021/ja106102b. [DOI] [PubMed] [Google Scholar]
- 19.Chao TH, Espenson JH. J. Am. Chem. Soc. 1978;100:129–133. [Google Scholar]
- 20.Artero V, Fontecave M. Coord. Chem. Rev. 2005;249:1518–1535. [Google Scholar]
- 21.Razavet M, Artero V, Fontecave M. Inorg. Chem. 2005;44:4786–4795. doi: 10.1021/ic050167z. [DOI] [PubMed] [Google Scholar]
- 22.Baffert C, Artero V, Fontecave M. Inorg. Chem. 2007;46:1817–1824. doi: 10.1021/ic061625m. [DOI] [PubMed] [Google Scholar]
- 23.Helm ML, Stewart MP, Bullock RM, DuBois MR, DuBois DL. Science. 2011;333:863–866. doi: 10.1126/science.1205864. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Chen L, Sun LC. Energy Environ. Sci. 2012;5:6763–6778. [Google Scholar]
- 25.Pellegrin Y, Odobel F. Coord. Chem. Rev. 2011;255:2578–2593. [Google Scholar]
- 26.Harriman A, Elliott KJ, Alamiry MAH, Le Pleux L, Severac M, Pellegrin Y, Blart E, Fosse C, Cannizzo C, Mayer CR, Odobel F. J. Phys. Chem. C. 2009;113:5834–5842. [Google Scholar]
- 27.Karlsson S, Boixel J, Pellegrin Y, Blart E, Becker HC, Odobel F, Hammarström L. J. Am. Chem. Soc. 2010;132:17977–17979. doi: 10.1021/ja104809x. [DOI] [PubMed] [Google Scholar]
- 28.Konduri R, Ye HW, MacDonnell FM, Serroni S, Campagna S, Rajeshwar K. Angew. Chem., Int. Ed. 2002;41:3185–3187. doi: 10.1002/1521-3773(20020902)41:17<3185::AID-ANIE3185>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Oneil MP, Niemczyk MP, Svec WA, Gosztola D, Gaines GL, Wasielewski MR. Science. 1992;257:63–65. doi: 10.1126/science.257.5066.63. [DOI] [PubMed] [Google Scholar]
- 30.Huang P, Magnuson A, Lomoth R, Abrahamsson M, Tamm M, Sun L, van Rotterdam B, Park J, Hammarström L, Åkermark B, Styring S. J. Inorg. Biochem. 2002;91:159–172. doi: 10.1016/s0162-0134(02)00394-x. [DOI] [PubMed] [Google Scholar]
- 31.Sadakane M, Steckhan E. Chem. Rev. 1998;98:219–237. doi: 10.1021/cr960403a. [DOI] [PubMed] [Google Scholar]
- 32.Hijazi A, Kemmegne-Mbouguen JC, Floquet S, Marrot J, Mayer CR, Artero V, Cadot E. Inorg. Chem. 2011;50:9031–9038. doi: 10.1021/ic201239y. [DOI] [PubMed] [Google Scholar]
- 33.Keita B, Nadjo L. J. Electroanal. Chem. 1987;217:287–304. [Google Scholar]
- 34.Keita B, Kortz U, Holzle LRB, Brown S, Nadjo L. Langmuir. 2007;23:9531–9534. doi: 10.1021/la7016853. [DOI] [PubMed] [Google Scholar]
- 35.Keita B, Nadjo L. J. Mol. Catal. A: Chem. 2007;262:190–215. [Google Scholar]
- 36.Yamase T, Cao XO, Yazaki S. J. Mol. Catal. A: Chem. 2007;262:119–127. [Google Scholar]
- 37.Yamase T. Catal. Surv. Asia. 2003;7:203–217. [Google Scholar]
- 38.Ioannidis A, Papaconstantinou E. Inorg. Chem. 1985;24:439–441. [Google Scholar]
- 39.Zhang ZY, Lin QP, Zheng ST, Bu XH, Feng PY. Chem. Commun. 2011;47:3918–3920. doi: 10.1039/c0cc04697c. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Li YX, Peng SQ, Lu GX, Li SB. Int. J. Hydrogen Energy. 2012;37:12150–12157. [Google Scholar]
- 41.Matt B, Xiang X, Kaledin AL, Han N, Moussa J, Amouri H, Alves S, Hill CL, Lian T, Musaev DG, Izzet G, Proust A. Chem. Sci. DOI: 10.1039/c3sc21998d. [Google Scholar]
- 42.Matt B, Moussa J, Chamoreau LM, Afonso C, Proust A, Amouri H, Izzet G. Organometallics. 2012;31:35–38. [Google Scholar]
- 43.Damas A, Ventura B, Moussa J, Esposti AD, Chamoreau LM, Barbieri A, Amouri H. Inorg. Chem. 2012;51:1739–1750. doi: 10.1021/ic202021w. [DOI] [PubMed] [Google Scholar]
- 44.Damas A, Moussa J, Rager MN, Amouri H. Chirality. 2010;22:889–895. doi: 10.1002/chir.20882. [DOI] [PubMed] [Google Scholar]
- 45.Moussa J, Rager MN, Chamoreau LM, Ricard L, Amouri H. Organometallics. 2009;28:397–404. [Google Scholar]
- 46.Waern JB, Desmarets C, Chamoreau LM, Amouri H, Barbieri A, Sabatini C, Ventura B, Barigelletti F. Inorg. Chem. 2008;47:3340–3348. doi: 10.1021/ic702327z. [DOI] [PubMed] [Google Scholar]
- 47.Matt B, Renaudineau S, Chamoreau LM, Afonso C, Izzet G, Proust A. J. Org. Chem. 2011;76:3107–3112. doi: 10.1021/jo102546v. [DOI] [PubMed] [Google Scholar]
- 48.Matt B, Coudret C, Viala C, Jouvenot D, Loiseau F, Izzet G, Proust A. Inorg. Chem. 2011;50:7761–7768. doi: 10.1021/ic200906b. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez C, Livage J, Launay JP, Fournier M. J. Am. Chem. Soc. 1983;105:6817–6823. [Google Scholar]
- 50.Varga GM, Papaconstantinou E, Pope MT. Inorg. Chem. 1970;9:662–667. [Google Scholar]
- 51.Papaconstantinou E, Pope MT. Inorg. Chem. 1970;9:667–669. [Google Scholar]
- 52.Baffert C, Boas JF, Bond AM, Kogerler P, Long DL, Pilbrow JR, Cronin L. Chem. Eur. J. 2006;12:8472–8483. doi: 10.1002/chem.200501450. [DOI] [PubMed] [Google Scholar]
- 53.Fay N, Hultgren VM, Wedd AG, Keyes TE, Forster RJ, Leane D, Bond AM. Dalton Trans. 2006:4218–4227. doi: 10.1039/b605663f. [DOI] [PubMed] [Google Scholar]
- 54.Baranoff E, Curchod BFE, Frey J, Scopelliti R, Kessler F, Tavernelli I, Rothlisberger U, Gratzel M, Nazeeruddin MK. Inorg. Chem. 2012;51:215–224. doi: 10.1021/ic202162q. [DOI] [PubMed] [Google Scholar]
- 55.Izutsu K, International Union of Pure and Applied Chemistry Commission on Electroanalytical Chemistry. Acid-base dissociation constants in dipolar aprotic solvents. Blackwell Scientific Publications; Oxford: 1990. Distributors, USA, Publishers’ Business Services; Boston Brookline Village, Mass. [Google Scholar]
- 56.Probst B, Rodenberg A, Guttentag M, Hamm P, Alberto R. Inorg. Chem. 2010;49:6453–6460. doi: 10.1021/ic100036v. [DOI] [PubMed] [Google Scholar]
- 57.Luminescence of the iridium complex precursor will be described elesewhere.
- 58.Artero V, Fontecave M. Chem. Soc. Rev. 2013 doi: 10.1039/c2cs35334b. DOI: 10.1039/C1032CS35334B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.