Abstract
Background
Cystic fibrosis (CF) is a complex, multi-system, life-shortening, autosomal recessive disease most common among Caucasians. Pulmonary pathology, the major cause of morbidity and mortality in CF, is characterized by dysregulation of cytokines and a vicious cycle of infection and inflammation. This cycle causes a progressive decline in lung function, eventually resulting in respiratory failure and death. The Th17 immune response plays an active role in the pathogenesis of CF pulmonary pathology, but it is not known whether the pathophysiology of CF disease contributes to a heightened Th17 response or whether CF naïve CD4+ T lymphocytes (Th0 cells) intrinsically have a heightened predisposition to Th17 differentiation.
Methods
To address this question, Th0 cells were isolated from the peripheral blood of CF mice, human CF subjects and corresponding controls. Murine Th0 cells were isolated from single spleen cell suspensions using fluorescence-activated cell sorting. Lymphocytes from human buffy coats were isolated by gradient centrifugation and Th0 cells were further isolated using a human naïve T cell isolation kit. Th0 cells were then assessed for their capacity to differentiate along Th17, Th1 or Treg lineages in response to corresponding cytokine stimulation. The T cell responses of human peripheral blood cells were also assessed ex vivo using flow cytometry.
Results
Here we identify in both mouse and human CF an intrinsically enhanced predisposition of Th0 cells to differentiate towards a Th17 phenotype, while having a normal propensity for differentiation into Th1 and Treg lineages. Furthermore, we identify an active Th17 response in the peripheral blood of human CF subjects.
Conclusions
We propose that these novel observations offer an explanation, at least in part, for the known increased Th17-associated inflammation of CF and the early signs of inflammation in CF lungs before any evidence of infection. Moreover, these findings point towards direct modulation of T cell responses as a novel potential therapeutic strategy for combating excessive inflammation in CF.
Keywords: Cystic fibrosis, Naïve T cells, Th17 phenotype
Background
Cystic Fibrosis (CF) is an autosomal recessive disorder caused by mutations within the CF transmembrane conductance regulator (CFTR) gene [1,2]. Initially identified in the apical membranes of epithelial cells, defects in CFTR expression associated with chloride channel defects [3] have also been found in circulating T lymphocytes [4]. Pulmonary disease, the major cause of morbidity and mortality in CF [5], is characterized by dysregulation of cytokines and a vicious cycle of infection and inflammation which causes a progressive decline in lung function, eventually resulting in respiratory failure and death (reviewed by [6,7]). The lung disease can be particularly exacerbated by P. aeruginosa infections [8]. Th17 is a recently identified helper T cell subset identified by production of interleukin (IL)-17 [9]; it has been linked to the pulmonary exacerbations and neutrophilia observed in CF [10,11], including neutrophilia very early in life [12]. CF patients with active P. aeruginosa infections have elevated levels of Th17 cytokines in their sputum and studies have identified the Th17 cytokine IL-23 as a major factor in orchestrating P. aeruginosa - induced pulmonary inflammation [10]. The pulmonary Th17 response, particularly IL-17 levels, predicts future acquisition of P. aeruginosa infections [13]. In a murine model of CF, the Th17 response has also been described as detrimental to clearance of A. fumigatus, a fungus which often exacerbates CF lung disease through the associated condition allergic bronchopulmonary aspergillosis [14]. Although studies have identified an active role of Th17 response in modulating CF pulmonary pathology, the underlying mechanism(s) specifically promoting a Th17 response are not understood. It is not known whether the pathophysiology of CF disease contributes to a heightened Th17 response, or whether naïve CF T cells are intrinsically prone to Th17 differentiation.
In this study, we identified an innate predisposition of naïve CF T cells in both humans and mice to selectively undergo differentiation into the Th17 lineage while retaining a normal predisposition to other helper T cell lineages. These findings identify an intrinsic defect in CF T cells, which independently of the cytokine milieu in the CF lung may initiate, contribute to, or perhaps even substantially account for, the detrimental Th17 response observed in CF patients.
Methods
Subject characteristics
Five female CF subjects were diagnosed by repeated sweat testing using the method of Gibson and Cooke [15]. Each had two recognized disease - causing CFTR mutations: two were F508del homozygotes and the other three were compound heterozygotes, F508del/2183AA->G, F508del/2622+1G->A and G542X/R560T. All of these mutations are classified as severe mutations, producing very little or no functional CFTR. They were not receiving any systemic corticosteroids, were clinically stable, free of acute pulmonary exacerbation and free of signs of viral illness, and aged 15 to 22 years at the time of blood sampling. One was chronically infected with Pseudomonas aeruginosa, but the other four were not. Rather, their sputum cultures were positive for Staphylococcus aureus. The three healthy controls were two females aged 22 and 35, and one male aged 57 years. Human subject experiments were conducted according to the principles expressed in the Helsinki Declaration. All subjects (CF and controls) gave informed consent and the study was approved by the Research Ethics Board of The Hospital for Sick Children, Toronto, ON.
CF mice
Inbred congenic mice homozygous for the F508del CFTR mutation (CFTR-/-, CF knock-in mice) on a C57BL/6 background and littermate controls (CFTR+/+, wildtype) were obtained from Dr. Christine Bear at The Hospital for Sick Children Research Institute. The mice were handled according to the Guidelines of the Canadian Council on Animal Care in science, and the protocols of the mouse experiments were approved by the Animal Care Committee, Research Institute, The Hospital for Sick Children.
Isolation of T cells
Mouse naïve CD4+ T cells, defined as CD3+CD4+CD25- cells [16], were isolated by fluorescence-activated cell sorting (FACS Aria II, BD Biosciences, Mississauga, ON) from single spleen cell suspensions from CFTR+/+ and CFTR-/- mice. Lymphocytes from human buffy coats were isolated by gradient centrifugation in Lymphoprep (Axis-Shield, Oslo, Norway) following the manufacturer’s instructions. Human naïve T cells, defined as CD3+CD4+CD25-CD45RA+CD45RO- [17], were isolated using a human naïve T cell isolation kit (Miltenyi Biotec, Auburn, CA) following manufacturer’s instructions, with purity in excess of 95%. The isolation of naïve human T cells was performed in a two step process. The first step was a negative selection of non-CD4+ T cells along with CD45RO+ T cells, which negatively selected for both memory and effector T cells, and the second step was a positive selection for CD45RA+ T cells for isolation of naïve T cells.
Assessment of peripheral blood T cell response ex vivo
Mononuclear cells from human blood were cultured on plates (BD Biosciences) coated with CD3 antibody (clone HIT3a) in the presence of CD28 antibody (clone CD28.2, eBiosciences, San Diego, CA) for 4 days. On day 4, cells were treated with 50 ng/mL PMA and 1 μM Ionomycin (Sigma-Aldrich, Oakville, ON) for 6-8 hr followed by analysis for IL-17 and IFN-γ production by CD4+ T cells using flow cytometry [18].
In vitro differentiation of T cells
Naïve CD4+ T cells from CFTR+/+ and CFTR-/- mice were differentiated in vitro into IFN-γ- producing Th1 cells [19], into Foxp3+ regulatory T (Treg) cells [20] or into the IL-17- producing Th17 lineage as described previously [21]. Production of IFN-γ and IL-17 by differentiated mouse T cells was confirmed using respective ELISA kits following manufacturer’s instructions (R&D Systems, Minneapolis, MN). In vitro naïve human T cell differentiation was carried out by culturing cells in a plate coated with anti-CD3 antibody (5 μg/mL) for 6-7 days with anti-CD28 (2 μg/mL) in the presence of IL-6 (50 ng/mL), IL-23 (25 ng/mL), IL-1β (10 ng/mL), TGF-β1 (1 ng/mL; Peprotech, Rocky Hill, NJ), anti-IL-4 (clone MP4-25D2; 10 mg/mL) and anti-IFN-γ (10 mg/mL, clone NIB42; eBiosciences) for Th17 differentiation, or TGF-β1 (5 ng/mL; Peprotech) for Treg differentiation.
Statistical analysis
Student two-tailed t test was used for statistical analysis. A p value <0.05 was considered significant.
Results and discussion
Naïve CFTR-/- CD4+ T cells preferentially undergo Th17 differentiation
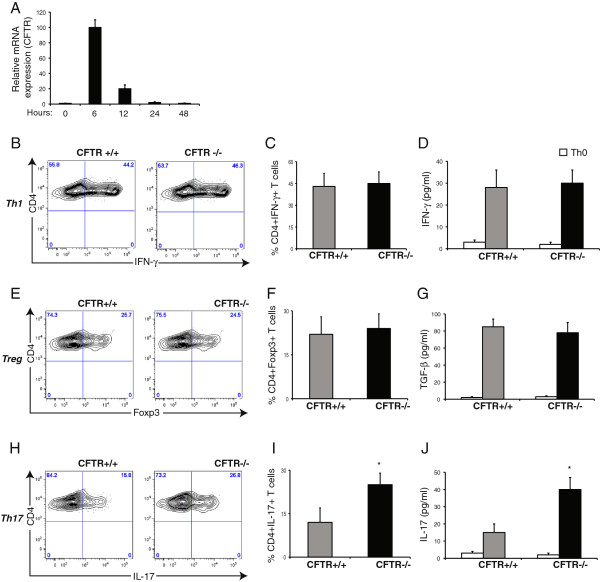
Initially, CFTR expression was thought to be restricted to epithelial cells [22,23], but later studies confirmed that CFTR is also expressed in lymphocytes [4]. Moreover, T cell clones derived from CF subjects show a defective c-AMP regulated chloride current, pointing towards a functional role of CFTR in regulating T cell function [4]. Differentiation of naïve CD4+ T cells into different effector lineages, such as Th17 cells, is directed primarily by the local cytokine environment in the presence of T cell receptor activation and co-activation of co-stimulatory molecules [24,25]. We assessed the kinetics of CFTR expression during naïve CD4+ T cell differentiation. Naïve CD4+ T cells were isolated from CFTR+/+ mice and stimulated in vitro with antibodies to drive T cell receptor activation. On quantitative polymerase chain reaction analysis, CFTR expression is induced up to 100 fold following 6 hours of stimulation, subsequently returning to basal levels within 48 hr (Figure 1A). By contrast, we failed to observe inducibility of CFTR mRNA expression in T cells isolated from CFTR-/- mice (data not shown). The inducibility of CFTR expression in naïve T cells following antibody stimulation led us to hypothesize that defective CFTR may affect T cell differentiation into different lineages. Naïve CD4+ T cells from CFTR+/+ and CFTR-/- mice were differentiated into IFN-γ- producing Th1 cells [19]in vitro (Figure 1B-D). Similar proportions of naïve CD4+ T cells underwent Th1 differentiation, which was further confirmed by using ELISA to measure levels of IFN-γ released into the media (Figure 1B-D). Similar results were identified following differentiation of naїve CD4+ T cells into Foxp3+ regulatory T cells [20], whereby both CFTR+/+ and CFTR-/- naïve CD4+ T cells displayed comparable propensities to undergo Treg differentiation, by analysis of Foxp3 expression (Figure 1E, F) as well as levels of TGF-β production (Figure 1G). However, when cells were differentiated along the IL-17-producing Th17 lineage [21], 16% of naïve T cells from CFTR+/+ mice differentiated into IL-17 producing cells, whereas Th17 differentiation of naïve CFTR -/- T cells was almost 2 fold higher with approximately 25-30% cells undergoing differentiation into IL-17-producing cells (Figure 1H, I). This was further confirmed by measuring IL-17 production using ELISA, which identified that in vitro differentiated CFTR-/- T cells produce significantly higher amounts of IL-17 than CFTR+/+ T cells differentiated along a Th17 lineage (Figure 1J). Therefore, data from mice identified an intrinsic predisposition of naïve CFTR-/- T cells to preferentially undergo Th17 differentiation.
Figure 1.
CFTR-/- T cells are intrinsically predisposed to differentiate along the Th17 lineage. (A) Relative mRNA expression of CFTR in naїve CD4+ T cells from CFTR+/+ mice following stimulation with anti-CD3 and anti-CD28 antibodies at different time points. (B-J) Naїve CD4+ T cells from CFTR+/+ and CFTR-/- mice were differentiated into Th1, Treg and Th17 lineages respectively in the presence of inducing cytokines. (B) Flow cytometry plots of IFN-γ production by CD4+ T cells. (C) Proportions of IFN-γ producing cells generated under Th1 inducing conditions. (D) Levels of IFN-γ released by differentiated cells. (E) Flow cytometry plots of Foxp3 expression by CD4+ T cells. (F) Proportions of Foxp3+ cells generated under Treg inducing conditions. (G) Levels of TGF-β released by differentiated cells. (H) Flow cytometry plots of IL-17 production by differentiated cells. (I) Proportions of IL-17 producing cells generated under Th17 inducing conditions. (J) Levels of IL-17 released by differentiated cells. n = 4-6 mice per group, representative of 2-3 independent experiments. Mean ± SEM, * p < 0.05 vs CFTR +/+.
Active Th17 response in the peripheral blood of CF subjects
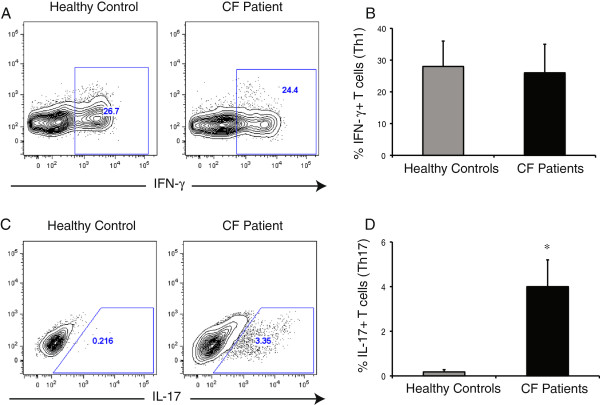
Although studies have identified an active Th17 profile in the lungs of CF patients [13,26], an active Th17 response has not been demonstrated in the peripheral blood. Based on our findings in the above CF mouse studies suggesting a predisposition in CFTR-/- T cells, we postulated that an intrinsic predisposition to undergo Th17 differentiation should lead to the presence of an identifiable Th17 response in the peripheral blood of CF subjects. Peripheral blood mononuclear cells were isolated from CF subjects and healthy individuals and subsequently stimulated in vitro. Analysis of the Th1 response, as assessed by IFN-γ production, revealed similar proportions of IFN-γ- producing T cells in the peripheral blood of healthy individuals as well as CF subjects (Figure 2A, B). However, healthy individuals showed an extremely low frequency of IL-17- producing T cells (0.2%), which was almost 17 fold higher in CF subjects with approximately 3-4% of T cells showing IL-17 production (Figure 2C, D). These findings clearly identified an active Th17 response in the peripheral blood of CF subjects. An active Th17 response in the lung has been identified as predictive of P. aeruginosa infection, which promotes exacerbation of pulmonary disease [13]. Although such a predictive biomarker would be useful, it is limited by the requirement for bronchoalveolar lavage (BAL) [13]. It remains to be determined if prediction of lung infection with P. aeruginosa infection can be achieved using the Th17 profile in peripheral blood, bypassing the need for BAL.
Figure 2.
CF subjects have an active Th17 response, which can be detected in peripheral blood. (A) Representative flow cytometry plots of IFN-γ production by peripheral blood T cells from healthy controls and CF subjects. (B) Proportions of IFN-γ producing Th1 cells in peripheral blood. (C) Representative flow cytometry plots of IL-17 production by peripheral blood T cells from healthy controls and CF subjects. (D) Proportions of IL-17- producing Th17 cells in peripheral blood. 5 CF subjects and 3 healthy controls, representative of 3-4 independent experiments. Mean ± SEM. *p < 0.05.
Naïve CD4+ T cells in human CF preferentially undergo Th17 differentiation
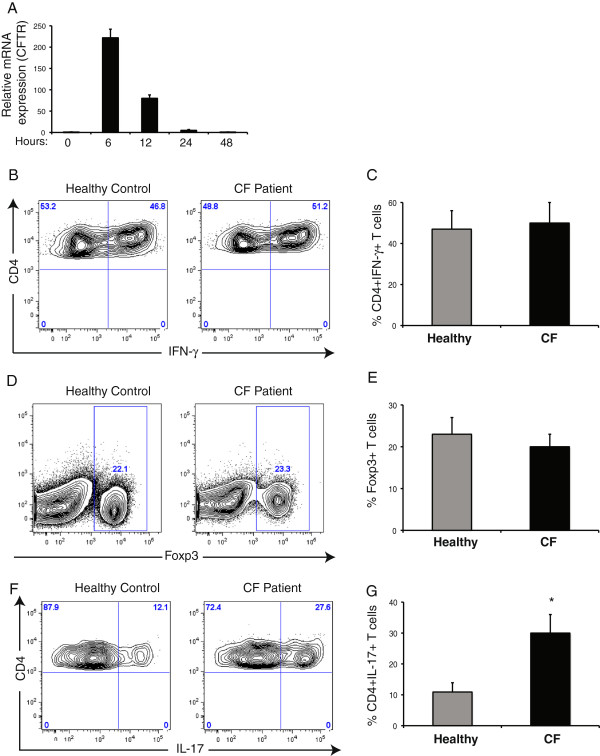
There have been clear differences documented between murine and human CF in terms of pulmonary inflammation, and thus observations from murine CF may not always translate to human CF. Therefore, we wanted to confirm inducibility of human CFTR in naïve human T cells following antibody stimulation, as was observed in murine CF. CFTR was induced approximately 200 fold at the mRNA level following 6 hr of stimulation of naïve human CD4+ T cells and normalized to basal levels within 48 hr (Figure 3A), consistent with our mouse CF observations (Figure 1A). Next we wanted to identify whether, similar to mouse CF, T cells from human CF subjects are intrinsically predisposed towards Th17 differentiation whilst having a normal propensity for Th1 and Treg differentiation. Under conditions known to selectively drive Th1 differentiation, approximately 50% of naïve T cells both from CF subjects and from healthy individuals differentiated into IFN-γ- producing Th1 cells (Figure 3B, C). Similarly, in the presence of TGF-β [27], T cells from both healthy individuals and CF patients showed a similar propensity to Treg generation with almost 20% of the cells differentiating into Foxp3+ Tregs [27,28] (Figure 3D, E). Although only 12% of naïve T cells from healthy individuals differentiated into IL-17- producing Th17 cells, this proportion was roughly doubled in CF subjects, with approximately 27% of naïve CD4+ T cells undergoing differentiation into Th17 cells (Figure 3F, G). These findings confirm our observations from CF mice and clearly indicate the preferential predisposition of naïve T cells in CF to undergo differentiation along a Th17 lineage.
Figure 3.
Naїve T cells from CF subjects are intrinsically predisposed to Th17 differentiation. (A) Relative mRNA expression levels of CFTR on naїve human CD4+ T cells following antibody stimulation at different time-points. (B) Representative flow cytometry plots of IFN-γ production by naїve T cells from healthy controls and CF subjects following Th1 differentiation. (C) Proportions of IFN-γ- producing Th1 cells. (D) Representative flow cytometry plots of Foxp3 expression by naїve T cells from healthy controls and CF subjects following differentiation into Tregs. (E) Proportions of Foxp3+ Tregs. (F) Representative flow cytometry plots of IL-17 production by naїve T cells from healthy controls and CF subjects following Th17 differentiation. (G) Proportions of IL-17- producing Th17 cells. 5 CF subjects and 3 healthy controls, representative of 3-4 independent experiments. Mean ± SEM. *p < 0.05.
Contamination of naïve CD4+ T cells with memory T cells could affect our results. Our strategy for isolation was based on depletion of CD45RO+ cells. CD45RO is not expressed exclusively on memory T cells, but is also expressed on effector T cells [29]. Therefore, depletion of CD45RO+ cells leads to depletion of both the memory as well as effector T cell subsets. Lack of CD45RO+ cells was employed to confirm the lack of memory T cells in isolated naïve CD4+ T cells. Moreover, the proportions of IL-17- producing T cells in the peripheral blood were in the range of 0.2-3%, whereas following differentiation of naïve T cells into IL-17- producing T cells, the proportions ranged between 10-30%. Polyclonal T cell activation was used for differentiation of naïve T cells into IL-17- producing T cells as well as for activation of peripheral T cells. If contamination with memory T cells had been affecting the data in our experiments, we would then expect the proportions of IL-17- producing T cells should have been comparable between peripheral blood and following naïve T cell differentiation. However, the 10-fold higher proportions of IL-17- producing T cells following naïve T cell differentiation indicates that these were likely the naïve T cells which underwent differentiation into Th17 cells and not just memory T cells, because if memory T cells underwent proliferation then the proportions of IL-17-producing cells should have been comparable between the two experiments.
Previous studies have identified an active Th17 response in the CF lung, thought likely to be orchestrated by the local pulmonary environment [12-14]. In accord with these findings, we report a Th17 response in the peripheral blood of CF subjects, providing a potential biomarker of the severity of CF disease through measurement of Th17 in the peripheral blood.
Dysregulation of cytokines has been reported in CF airway epithelial cells, recently reviewed by Cohen-Cymberknoh et al., [30] and, in distinct patterns, in the neutrophils of the CF lung and circulating blood [31]. To the best of our knowledge, none of the studies conducted to date have looked at the differentiation ability of naïve human CF CD4+ T cells to helper T cell lineages compared to cells from healthy subjects. At a time before the Th17 subset of T helper cells was recognized, Moss et al.[32] reported activated, freshly isolated, peripheral CD4+ T lymphocytes had decreased IFN-γ secretion in CF compared to control cells, prompting them to suggest a link between CF genotype and T cell cytokine dysregulation. However, it is important to note that the T cells isolated in that study were CD4+ T cells which had already differentiated within the subjects into cytokine-producing cells [32]. Hence, differences in cytokine production by those cells compared to cells from healthy controls likely reflected the differences in the ongoing T cell response in CF subjects and did not directly implicate a role of CF genotype in mediating T cell function. In our present study, naïve CD4+ T cells from CF subjects differentiated in vitro into IFN-γ- producing Th1 cells to an extent similar to naïve CD4+ T cells from healthy controls, further indicating that the findings reported by Moss et al. were not indicative of a role of CF genotype in regulating overall T cell function.
The human controls in our present study were older than the CF subjects (22, 35 and 57 years compared to 15-22 years). Li et al.[33] identified a decline in miR-181a microRNA as being responsible for reduced CD4+ T cell function observed with aging. However, the difference was seen in T cells from individuals who were over 70 years old and in our study, none of the CF subjects or the controls met this criterion. Moreover, the levels of IFN-γ-producing Th1 cells as well as Foxp3+ regulatory T cells were similar amongst both CF subjects as well as controls in our study and it was only the Th17 response which was selectively exaggerated in CF. Our murine data also indicated that only the Th17 response was affected in CF. In the unlikely scenario that our observations were age dependent, we would have expected an age-dependent effect on the overall T cell response and not just the selective Th17 response that was in fact observed. Furthermore, we did not observe outliers amongst the 22, 35 and 57 year old control individuals in the ability of their T cells to produce IFN-γ or IL-17 following ex vivo stimulation and/or ex vivo differentiation.
Recent evidence suggests IL-17+ cells may be important very early in CF lung disease. Increased numbers of Th17 cells have been demonstrated in the submucosa of endobronchial biopsies of newly diagnosed CF infants and young children [12]. Furthermore, the numbers of total IL-17+ cells (including Th17, IL-17+ neutrophils, γδT and NKT cells) are increased in newly diagnosed CF subjects with bronchoalveolar lavage fluid neutrophilia in the absence of IL-8 [12]. We have identified a phenotype unique to naïve helper T cells from CF subjects, attributable to loss of CFTR expression, which predisposes them selectively towards Th17 differentiation while retaining a normal propensity to differentiate into other lineages such as Th1 and Treg. Our findings suggest that the observations of a Th17 response during the early onset of CF lung disease may be attributable to preferential differentiation of naïve T cells along the Th17 lineage. Moreover, the present study confirms a role of CFTR in modulating T cell biology by regulating differentiation along the Th17 lineage. However, the molecular pathways by which CFTR regulates Th17 differentiation remain to be elucidated.
Conclusions
Taken together, our findings are to our knowledge the first to indicate an intrinsic phenotype of CF CD4+ T cells, attributable to loss of CFTR expression, which predisposes them towards selective Th17 differentiation while retaining a normal ability to undergo differentiation into Th1 and Treg lineages. These findings may account for the initiation of the Th17 response, amplified by the local cytokine milieu, that is associated with a clinical exacerbation of CF pulmonary pathology [11,13,26]. Therefore, direct modulation of T cells may hold potential as a novel therapeutic strategy in CF.
Abbreviations
CF: Cystic fibrosis; CFTR: CF transmembrane conductance regulator; IL: Interleukin; Th0 cells: Naїve helper T lymphocytes.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RK designed and conducted the immunological analysis of the T cell responses in the peripheral blood of mice and human subjects, collected and interpreted data, and wrote most of the first draft of the paper. SG made substantial contributions to the acquisition of data. NBS drafted parts of the discussion. All authors were involved in revising the manuscript critically for important intellectual content, and read and approved the final manuscript.
Contributor Information
Rahul Kushwah, Email: kushwah@mcmaster.ca.
Stéphane Gagnon, Email: stephane.gagnon@sickkids.ca.
Neil B Sweezey, Email: neil.sweezey@sickkids.ca.
Acknowledgements
The authors thank Prof. F. Ratjen for helpful discussions, Dr. Christine Bear for providing mice used in this study, and the CF and control subjects whose donation of blood made the human part of the study possible. This work was supported by grants from Cystic Fibrosis Canada and the Irwin Family Fund to NBS. RK is supported by a CIHR-Banting Postdoctoral Fellowship.
References
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL. et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;14:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Cutting GR, Kasch LM, Rosenstein BJ, Zielenski J, Tsui LC, Antonarakis SE, Kazazian HH Jr. A cluster of cystic fibrosis mutations in the first nucleotide-binding fold of the cystic fibrosis conductance regulator protein. Nature. 1990;14:366–369. doi: 10.1038/346366a0. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;14:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Dong YJ, Chao AC, Kouyama K, Hsu YP, Bocian RC, Moss RB, Gardner P. Activation of CFTR chloride current by nitric oxide in human T lymphocytes. EMBO J. 1995;14:2700–2707. doi: 10.1002/j.1460-2075.1995.tb07270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;14:1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- Chmiel JF, Konstan MW. Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin Chest Med. 2007;14:331–346. doi: 10.1016/j.ccm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;14:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;14:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;14:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;14:L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ. et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;14:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;14:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, Dehlink E, Nachbaur E, Horak F, Jaksch P. et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;14:621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- Iannitti RG, Carvalho A, Cunha C, De LA, Giovannini G, Casagrande A, Zelante T, Vacca C, Fallarino F, Puccetti P. et al. Th17/Treg imbalance in murine cystic fibrosis is linked to indoleamine 2,3-dioxygenase deficiency but corrected by kynurenines. Am J Respir Crit Care Med. 2013;14:609–620. doi: 10.1164/rccm.201207-1346OC. [DOI] [PubMed] [Google Scholar]
- Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by ionophoresis. Pediatr. 1959;14:545–549. [PubMed] [Google Scholar]
- Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;14:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, Dalod M, Soumelis V, Amigorena S. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;14:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;14:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K. et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;14:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;14:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;14:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, Ostedgaard L, Klinger KW, Welsh MJ, Smith AE. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990;14:382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;14:358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Chambers CA. The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 2001;14:217–223. doi: 10.1016/S1471-4906(01)01868-3. [DOI] [PubMed] [Google Scholar]
- Tsoukas CD, Landgraf B, Bentin J, Valentine M, Lotz M, Vaughan JH, Carson DA. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J Immunol. 1985;14:1719–1723. [PubMed] [Google Scholar]
- Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A. et al. Patients with cystic fibrosis have inducible IL-17 + IL-22+ memory cells in lung draining lymph nodes. J Allergy Clin Immunol. 2013;14:1117–1129. doi: 10.1016/j.jaci.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4 + CD25- naive T cells to CD4 + CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;14:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;14:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- Mackay CR. Dual personality of memory T cells. Nature. 1999;14:659–660. doi: 10.1038/44309. [DOI] [PubMed] [Google Scholar]
- Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013. [DOI] [PubMed]
- Corvol H, Fitting C, Chadelat K, Jacquot J, Tabary O, Boule M, Cavaillon JM, Clement A. Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;14:L997–1003. doi: 10.1152/ajplung.00156.2002. [DOI] [PubMed] [Google Scholar]
- Moss RB, Hsu YP, Olds L. Cytokine dysregulation in activated cystic fibrosis (CF) peripheral lymphocytes. Clin Exp Immunol. 2000;14:518–525. doi: 10.1046/j.1365-2249.2000.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;14:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]