Abstract
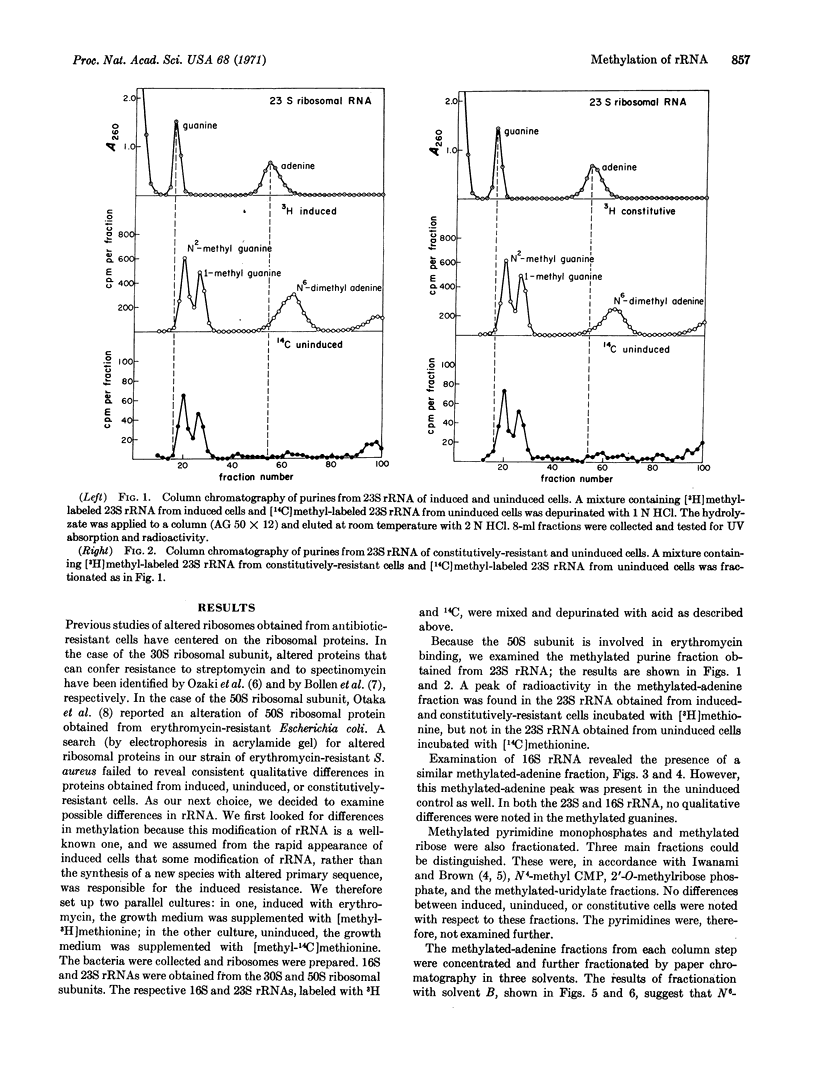
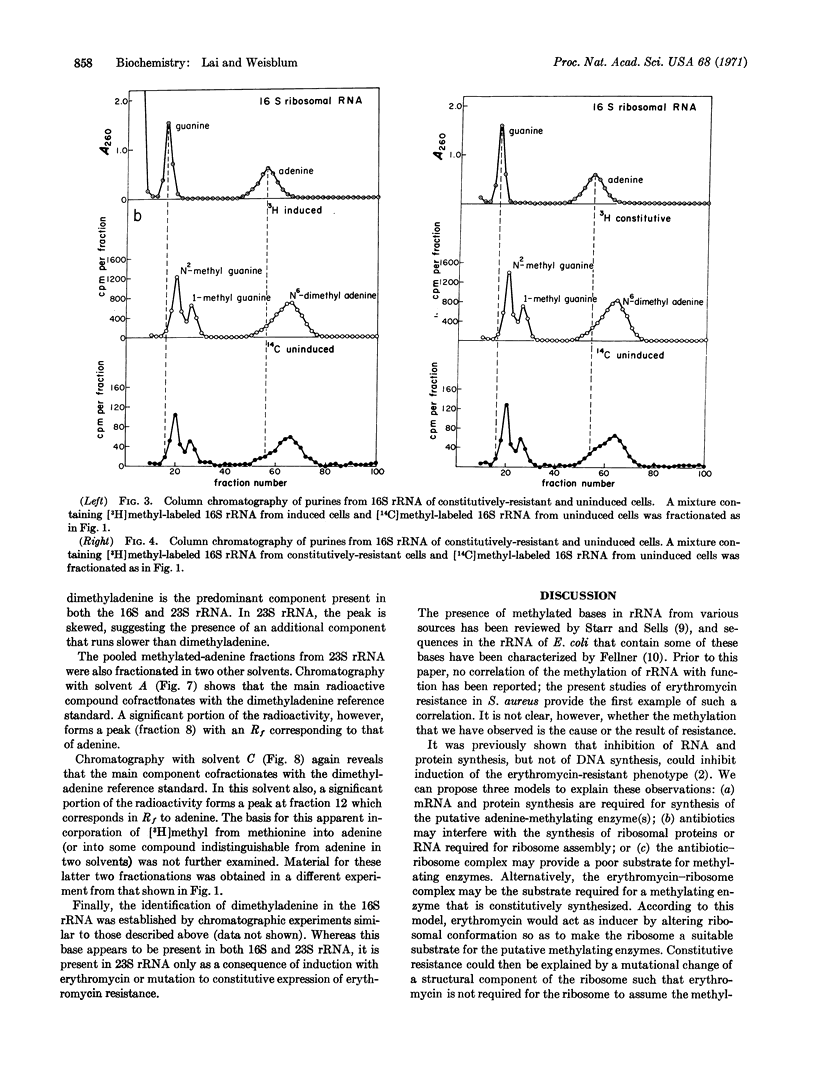
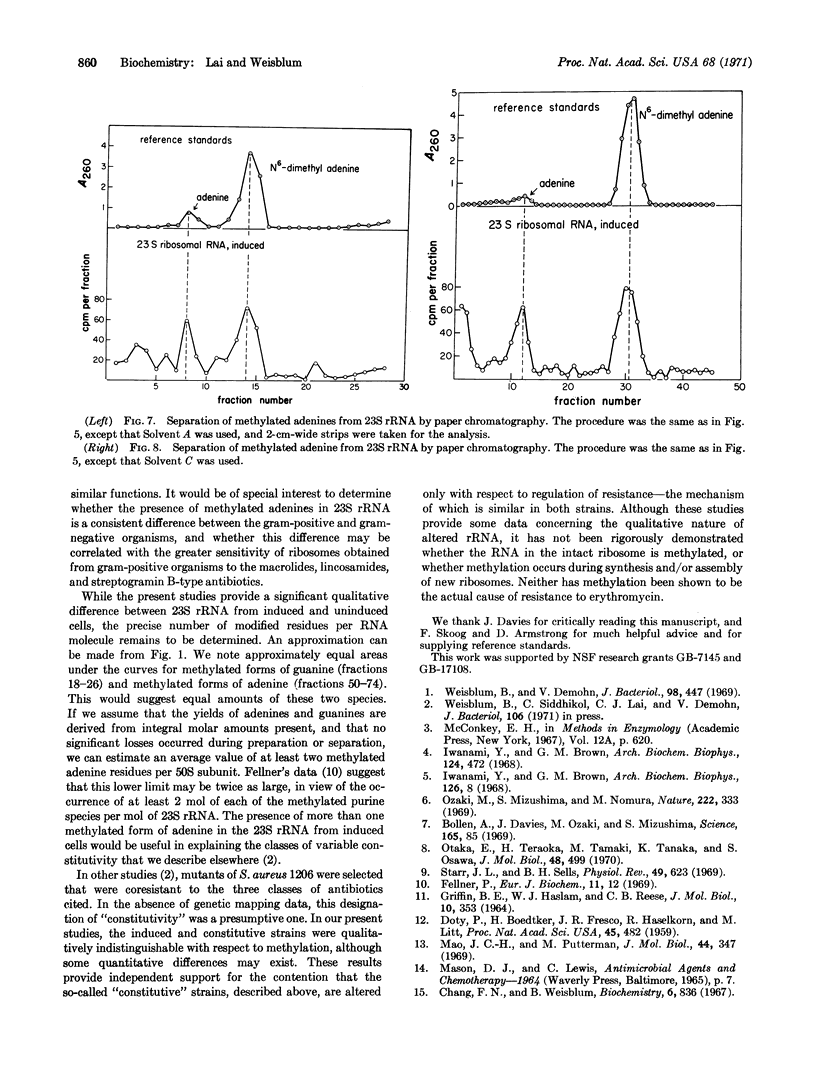
In certain strains of Staphylococcus aureus, a concentration of erythromycin between 10-8 and 10-7 M can induce resistance to concentrations of this drug as high as 10-4 M. In one such strain studied, S. aureus (1206), N6-dimethyladenine is not normally present in 23S rRNA; however, a compound presumptively identified (on the basis of paper chromatography in three different solvents) as N6-dimethyladenine appears in the 23S rRNA of growing cells that have been incubated in a medium containing 10-7 M erythromycin. It has been shown previously that the induction of the erythromycin-resistant phenotype that occurs under these conditions requires 10-8-10-7 M erythromycin for maximal expression within 1 hr and that induction results in modified 50S ribosomal subunits, which are then unable to bind erythromycin or lincomycin. Methylated adenine is also found in the 16S rRNA from the strain of S. aureus studied; however, in contrast to the situation with 23S rRNA, the amount in 16S rRNA is not affected by erythromycin. These findings provide the first example of a correlation between the methylation of rRNA and altered ribosomal function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollen A., Davies J., Ozaki M., Mizushima S. Ribosomal Protein Conferring Sensitivity to the Antibiotic Spectinomycin in Escherichia coli. Science. 1969 Jul 4;165(3888):85–86. doi: 10.1126/science.165.3888.85. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Weisblum B. The specificity of lincomycin binding to ribosomes. Biochemistry. 1967 Mar;6(3):836–843. doi: 10.1021/bi00855a025. [DOI] [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner P. Nucleotide sequences from specific areas of the 16S and 23S ribosomal RNAs of E. coli. Eur J Biochem. 1969 Nov;11(1):12–27. doi: 10.1111/j.1432-1033.1969.tb00733.x. [DOI] [PubMed] [Google Scholar]
- GRIFFIN B. E., HASLAM W. J., REESE C. B. SYNTHESIS AND PROPERTIES OF SOME METHYLATED POLYADENYLIC ACIDS. J Mol Biol. 1964 Nov;10:353–356. doi: 10.1016/s0022-2836(64)80055-3. [DOI] [PubMed] [Google Scholar]
- Iwanami Y., Brown G. M. Methylated bases of ribosomal ribonucleic acid from HeLa cells. Arch Biochem Biophys. 1968 Jul;126(1):8–15. doi: 10.1016/0003-9861(68)90553-5. [DOI] [PubMed] [Google Scholar]
- Iwanami Y., Brown G. M. Methylated bases of transfer ribonucleic acid from HeLa and L cells. Arch Biochem Biophys. 1968 Mar 20;124(1):472–482. doi: 10.1016/0003-9861(68)90355-x. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Putterman M. The intermolecular complex of erythromycin and ribosome. J Mol Biol. 1969 Sep 14;44(2):347–361. doi: 10.1016/0022-2836(69)90180-6. [DOI] [PubMed] [Google Scholar]
- Otaka E., Teraoka H., Tamaki M., Tanaka K., Osawa S. Ribosomes from erythromycin-resistant mutants of Escherichia coli Q13. J Mol Biol. 1970 Mar;48(3):499–510. doi: 10.1016/0022-2836(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- Starr J. L., Sells B. H. Methylated ribonucleic acids. Physiol Rev. 1969 Jul;49(3):623–669. doi: 10.1152/physrev.1969.49.3.623. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol. 1969 May;98(2):447–452. doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



