Summary
The dogma that life without insulin is incompatible has recently been challenged by results showing viability of insulin-deficient rodents undergoing leptin mono-therapy. Yet, the mechanisms underlying these actions of leptin are unknown. Here, the metabolic outcomes of intracerebroventricular (icv) administration of leptin in mice devoid of insulin and lacking or re-expressing leptin receptors (LEPRs) only in selected neuronal groups were assessed. Our results demonstrate that concomitant re-expression of LEPRs only in hypothalamic γ-aminobutyric acid (GABA)ergic and pro-opiomelanocortin (POMC) neurons is sufficient to fully mediate the life-saving and anti-diabetic actions of leptin in insulin deficiency. Our analyses indicate that enhanced glucose uptake by brown adipose tissue and soleus muscle, as well as improved hepatic metabolism, underlie these effects of leptin. Collectively, our data elucidate a hypothalamic-dependent pathway enabling life without insulin and hence pave the way for developing better treatments for diseases of insulin deficiency.
Introduction
Insulin deficiency is caused by i) autoimmune-mediated destruction of pancreatic β-cells [as seen in type 1 diabetes mellitus (T1DM)], ii) metabolic-stress-induced pancreatic β-cell dysfunction and death or dedifferentiation [as seen in aging and type 2 diabetes mellitus (T2DM)], as well as complete pancreatectomy (Butler et al., 2007; Coppari and Bjorbaek, 2012; Talchai et al., 2012). If untreated, this defect leads to hyperglycemia, polyuria, ketoacidosis, and death. To date, insulin therapy is the only life-saving intervention available to several millions of people suffering from insulin deficiency. Thus, daily insulin administrations and frequent glucose monitoring are quotidian activities of these patients. Despite the undisputable fact that therapeutic insulin has converted a previously lethal defect into a life-compatible malady, this approach does not restore metabolic homeostasis and may even cause serious unwanted effects. For example, probably owing to the established lipogenic actions of the hormone (Horton et al., 2002), long-term insulin treatment is suspected to underlie the excessive ectopic lipid deposition (i.e.: in non-adipose tissues) and the extremely high incidence of coronary artery disease observed in diabetic subjects (Larsen et al., 2002; Orchard et al., 2003). Likely, these lipogenic actions of insulin promote a vicious cycle of lipid-induced insulin resistance in liver and skeletal muscle and hence lead to increased insulin requirements in the long-term management of diabetes (Shulman, 2000). Furthermore, due to the potent and fast-acting glycemia-lowering effects of insulin, intensive insulin therapy significantly increases the risk of hypoglycemia (an event that is disabling and can even be fatal) (Cryer, 2009). Thus, better therapies for the treatment of diseases characterized by insulin deficiency are needed.
A major barrier to the development of superior treatments has been the dogma that life without insulin is not possible. However, while insulin appears to be an absolute requirement for normal organismal development, the idea that insulin is also indispensable for survival in adulthood needs to be revised. Indeed, we and others have shown that leptin mono-therapy reverses several of the metabolic aberrancies and permits survival of adult rodents rendered insulin deficient. Of note, in this context leptin therapy does not cause hypoglycemia and exerts lipolytic actions (Fujikawa et al., 2010; Wang et al., 2010; Yu et al., 2008). Thus, exploiting the slow-acting glycemia-lowering effect of leptin and/or harnessing the component(s) underlying its effect may represent attractive alternative(s) or adjuvant(s) to insulin therapy.
In mammals able to produce insulin the glycemia-lowering action of leptin is mediated by its direct action on its cognate receptors expressed by hypothalamic neurons. For example, unilateral restoration of LEPRs signaling only in hypothalamic arcuate nucleus (ARC) normalizes hyperglycemia in mice otherwise deficient in LEPRs signaling (Coppari et al., 2005; Morton et al., 2005). Mechanistically, this action seems to require intact hypothalamic phosphatidylinositol 3-kinase (PI3K) signaling because delivery of PI3K inhibitors directly to the ARC impairs the ability of leptin to suppress hyperglycemia in these diabetic rodents (Morton et al., 2005). Recently, the biochemical identity of the ARC neurons underlying these actions of leptin has been unraveled. Indeed, re-expression of LEPRs only in POMC neurons has been shown to be sufficient to restore normoglycemia in mice otherwise deficient in LEPRs signaling (Berglund et al., 2012; Huo et al., 2009). LEPRs in the ventromedial hypothalamic nucleus (VMH) are also thought to be important for mediating the glucoregulatory actions of leptin. In fact, microinjection of leptin into the VMH of lean mice increases glucose uptake in skeletal muscle, heart and interscapular brown adipose tissue (iBAT) (Haque et al., 1999; Minokoshi et al., 1999). Together, these results support the notion that in animals able to produce insulin the glycemia-lowering action of leptin is mainly mediated by ARC and VMH neurons.
Because activation of either LEPRs or insulin receptor signaling partly impinges on the same molecules (e.g.: PI3K) (Fukuda et al., 2008; Hill et al., 2008) and leptin enhances insulin sensitivity in insulin-resistant rodents and humans (German et al., 2009; Oral et al., 2002; Shimomura et al., 1999), the glycemia-lowering effects of leptin have been thought to be due to synergistic actions between administered-leptin and endogenously-secreted insulin. However, in insulin-deficient rodents leptin administration is also very effective in ameliorating diabetes (Fujikawa et al., 2010; Wang et al., 2010; Yu et al., 2008). These latter findings may represent the groundwork for developing improved therapies for diseases of insulin deficiency. To reach this goal, a comprehensive understanding of the mechanisms (molecules and cell-types) underlying the life-saving and metabolic-improving actions of leptin in insulin deficiency is needed.
In this study, we used two different models of insulin deficiency: the streptozotocin (STZ)-induced (Fujikawa et al., 2010) and the diphtheria toxin (DT)-induced β-cell depleted models (Thorel et al., 2010). By determining the metabolic outcomes of icv leptin administration in these insulin-deficient models lacking or re-expressing LEPRs only in selected hypothalamic neurons, we established the identity of i) the neuronal populations and ii) peripheral components underlying the life-saving and metabolic-improving actions of leptin in the context of insulin deficiency.
Results
Brain LEPRs mediate the life-saving and metabolic actions of leptin in insulin deficiency
Experimental evidence suggests that LEPRs expressed by neurons within the central nervous system (CNS) underlie the beneficial effect of leptin administration in the context of insulin deficiency. Firstly, the glucoregulatory actions of leptin are not mediated by hepatic LEPRs as insulin-deficient mice lacking LEPRs only in liver respond normally to the hyperglycemia-lowering action of leptin administration (Denroche et al., 2011). Secondly, the idea that direct action of leptin on glucagon-secreting α-cells underlies the anti-T1DM action of leptin seems to be at odds with our data indicating that pancreatic α-cells do not express LEPR-B (the receptor isoform that mediates the majority of the biological actions of leptin) (Figures S1A–D). Thirdly, we have previously reported that icv delivery of leptin permits survival and normalizes hyperglycemia of STZ-induced insulin-deficient mice (Fujikawa et al., 2010). However, STZ-treated mice retain minuscule amount of pancreatic insulin and therefore it is unclear whether the residual insulin is required for the beneficial effect induced by leptin treatment (Fujikawa et al., 2010).
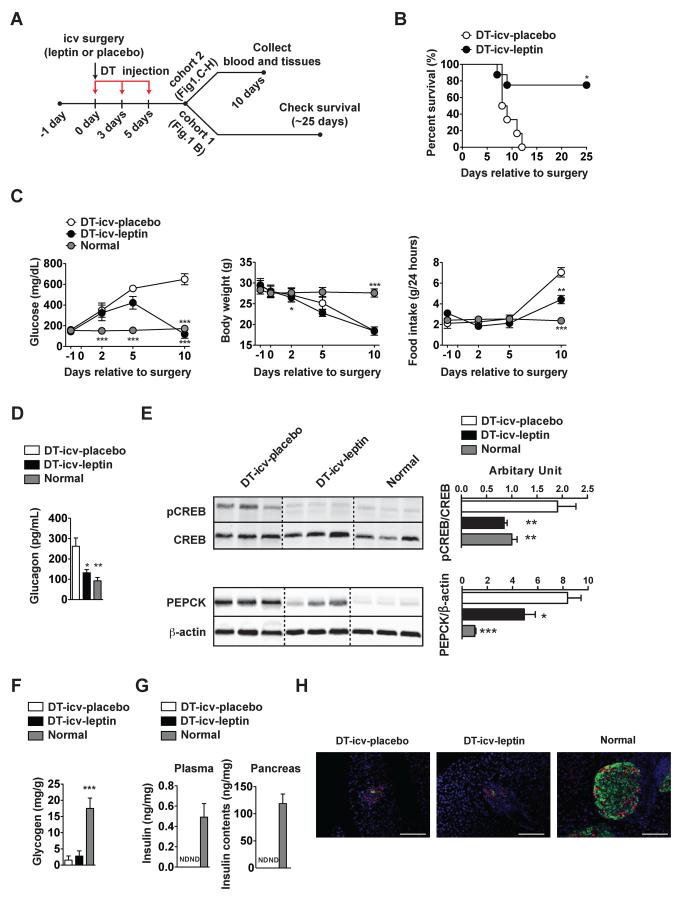
To address this issue, we used genetically-engineered mice that can be rendered completely insulin-deficient. RIP-DTR mice bear a rat insulin promoter (RIP)-DT receptor (DTR) allele cloned into the Hprt locus of the X chromosome. Three independent intraperitoneal (ip) DT administrations (at the dose of 0.5 μg/Kg of body weight each) in RIP-DTR mice have been shown to ablate virtually all of insulin-producing pancreatic β-cells (Thorel et al., 2010). Insulin deficiency in RIP-DTR mice was achieved by following this established protocol (Thorel et al., 2010) with a slight modification (Figure 1A). Accordingly, nearly all of pancreatic β-cells were ablated in DT-injected RIP-DTR mice (Figure S1E–G). Compared to normal mice, all of DT-injected RIP-DTR mice that underwent icv-placebo treatment (DT-icv-placebo) displayed overtly high hyperglycemia, reduced body weight, hyperphagia and hyperglucagonemia and inevitably succumbed within 15 days after the first DT administration (Figures 1B, 1C and 1D). However, the vast majority of DT-injected RIP-DTR mice that underwent icv-leptin treatment (DT-icv-leptin) were viable and had significantly improved hyperglycemia, hyperphagia and hyperglucagonemia albeit their body weight remained similar to DT-icv-pacebo mice (Figures 1B, 1C and 1D). In accordance with improved hyperglucagonemia, DT-icv-leptin mice had hepatic level of phosphorylated cAMP response element binding protein (pCREB; an established readout of glucagon receptor signaling) greatly reduced and indistinguishable compared to DT-icv-placebo and normal mice, respectively (Figure 1E). Hepatic phosphoenolpyruvate carboxykinase (PEPCK) protein expression levels were improved but not completely normalized by icv leptin administration (Figure 1E), and, in line with our previous report (Fujikawa et al., 2010) hepatic glycogen levels were not improved by icv leptin administration (Figure 1F). Importantly, circulating and pancreatic insulin levels were virtually abolished in both DT-icv-placebo and DT-icv-leptin mice compared to normal mice (Figure 1G). Also, immunohistological assays revealed minimal presence of pancreatic β-cells in both DT-icv-placebo and DT-icv-leptin mice compared to the normal group (Figure 1H). These data suggest that the aforementioned effects of icv leptin administration are not secondary to pancreatic β-cell regeneration. Collectively, our results establish that leptin administration permits survival and improves hyperglycemia of mice totally lacking insulin via CNS-dependent mechanisms.
Figure 1. icv leptin administration reverses lethality and improves hyperglycemia caused by complete insulin deficiency.
(A) Experimental design using RIP-DTR mice (Thorel et al., 2010). Leptin (25 ng/hour) or placebo (phosphate-buffered saline; PBS) was intracerebroventricularly (icv) administered starting at day 0 in DT-icv-leptin or DT-icv-placebo mice, respectively. DT-icv-leptin and DT-icv-placebo mice were rendered insulin deficient by intraperitoneal (ip) diphtheria toxin (DT) administration at day 0, 3, and 5. Age-matched, non-diabetic controls were used to gather parameters in surgically- and DT-untreated normal mice (normal group). (B) Kaplan-Meier survival analyses were performed on DT-icv-leptin and DT-icv-placebo mice; Statistical analyses were done using by Gehan-Breslow-Wilcoxon Test. ***P<0.001 versus DT-icv-placebo mice (numbers of mice at day 0 of DT-icv-leptin and DT-icv-placebo were 8 and 6, respectively). (C) Glucose levels in the blood, body weight, food intake, (D) glucagon in the plasma, (E) hepatic protein levels of pCREB and PEPCK, (F) glycogen in the liver, (G) insulin levels in the plasma and whole pancreas and (H) representative distribution of cells expressing insulin (green) and glucagon (red) in the pancreas of DT-icv-placebo, DT-icv-leptin and normal mice. Statistical analyses were done using one-way ANOVA (Tukey's Multiple Comparison Test). Values are mean ± S.E.M. (n = 4–6). ***P<0.001, **P<0.01, *P<0.05 versus DT-icv-placebo mice. ND = below the threshold of detection. Scale bar size = 100 μm. See also Figure S1
Marginal contribution of POMCLEPRs to the actions of leptin in insulin deficiency
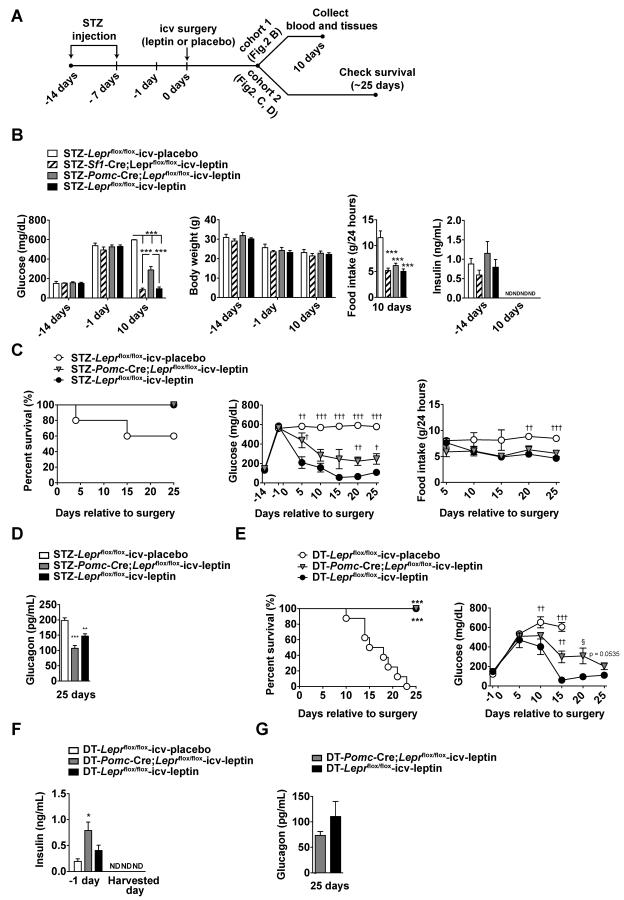
To determine the identity of the neuronal population(s) mediating the effect of leptin in insulin deficiency, we used genetically-engineered mice that enabled the deletion of LEPRs in a neuron-type-specific fashion. Because hypothalamic POMC and steroidogenic factor 1 (SF1) neurons have been shown to regulate glucose metabolism in insulin-intact mammals (Berglund et al., 2012; Huo et al., 2009; Ramadori et al., 2011), we directly tested whether LEPRs in POMC or SF1 neurons (POMCLEPRs or SF1LEPRs, respectively) are required for the effects of leptin in the context of insulin deficiency. Of note, SF1 is encoded by the Nr5a1 gene that is only expressed in the VMH within the CNS (Choi et al., 2013). Thus, SF1 neurons are a representative neuronal population of the VMH (Dhillon et al., 2006). By breeding Pomc-Cre or Sf1-Cre allele to Leprflox allele, we obtained LEPR-intact control (Leprflox/flox mice) and Pomc-Cre;Leprflox/flox or Sf1-Cre;Leprflox/flox mice that lack LEPRs only in POMC or SF1 neurons, respectively (Balthasar et al., 2004; Dhillon et al., 2006). Insulin deficiency in Pomc-Cre;Leprflox/flox, Sf1-Cre;Leprflox/flox, or Leprflox/flox mice was achieved by ip STZ administrations (Fujikawa et al., 2010) (Figure 2A). Compared to STZ-injected Leprflox/flox mice that underwent icv-placebo treatment (STZ-Leprflox/flox-icv-placebo), hyperglycemia was similarly reduced in STZ-injected Sf1-Cre;Leprflox/flox mice that underwent icv-leptin treatment (STZ-Sf1-Cre;Leprflox/flox-icv-leptin) and STZ-injected Leprflox/flox mice that underwent icv-leptin treatment (STZ-Leprflox/flox-icv-leptin) (Figure 2B). However, STZ-injected Pomc-Cre;Leprflox/flox mice that underwent icv-leptin treatment (STZ-Pomc-Cre;Leprflox/flox-icv-leptin) displayed a blunted response to the hyperglycemia-lowering action of the treatment (Figure 2B). Because food intake and body weight were comparable between STZ-Pomc-Cre;Leprflox/flox-icv-leptin, STZ-Sf1-Cre;Leprflox/flox-icv-leptin, and STZ-Leprflox/flox-icv-leptin mice, the diminished action of leptin on hyperglycemia in the STZ-Pomc-Cre;Leprflox/flox-icv-leptin group was not secondary to hyperphagia (Figure 2B). Similarly, the dampened action of the hormone was not due to impaired leptin delivery because phosphorylated signal transducer and activator of transcription 3 (pSTAT3; an established readout of leptin-induced LEPRs signaling) was readily detectable in the brain of STZ-Pomc-Cre;Leprflox/flox-icv-leptin mice that displayed hyperglycemia (Figure S2). Of note, 10 days after icv surgery circulating insulin was below the threshold of detection in all groups (Figure 2B) suggesting that all these mice were devoid of circulating insulin.
Figure 2. LEPRs in POMC neurons are required to mediate a marginal component of anti-diabetic action of leptin in the context of insulin deficiency.
(A) Experimental design using streptozotocin (STZ)-treated mice (Fujikawa et al., 2010). To induce insulin deficiency, STZ was ip administered to mice lacking LEPRs selectively in POMC neurons (Pomc-Cre;Leprflox/flox), or SF1 neurons (Sf1-Cre;Leprflox/flox), and littermate control mice (Leprflox/flox). Leptin (25 ng/hour) was delivered icv to Pomc-Cre;Leprflox/flox, Sf1-Cre;Leprflox/flox and Leprflox/flox mice (STZ-Pomc-Cre;Leprflox/flox-icv-leptin, STZ-Sf1-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin group, respectively). Placebo (PBS) was delivered icv to Leprflox/flox mice (STZ-Leprflox/flox-icv-placebo). (B) Glucose levels in the blood, body weight, food intake and insulin levels in the plasma of STZ-Leprflox/flox-icv-placebo, STZ-Sf1-Cre;Leprflox/flox-icv-leptin, STZ-Pomc-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin mice. (C) Kaplan-Meier survival analyses were performed on STZ-Leprflox/flox-icv-placebo, STZ-Pomc-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin mice; Statistical analyses were done using by Log-rank Test (numbers of mice at day of all group were 5), glucose levels in the blood, food intake and (D) glucagon levels in the plasma of STZ-Leprflox/flox-icv-placebo, STZ-Pomc-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin mice. (E) Kaplan-Meier survival analyses were performed on DT-Leprflox/flox-icv-placebo, DT-Pomc-Cre;Leprflox/flox-icv-leptin and DT-Leprflox/flox-icv-leptin mice; Statistical analyses were done using Log-rank Test (among all groups) followed by Gehan-Breslow-Wilcoxon Test (each group versus DT-Leprflox/flox-icv-placebo). ***P<0.001. (numbers of mice at day 0 of DT-Leprflox/flox-icv-placebo, DT-Pomc-Cre;Leprflox/flox-icv-leptin and DT-Leprflox/flox-icv-leptin were 8, 9 and 6, respectively). Glucose levels in the blood, food intake and (F) glucagon levels in the DT-Leprflox/flox-icv-placebo, DT-Pomc-Cre;Leprflox/flox-icv-leptin and DT-Leprflox/flox-icv-leptin mice. Harvested day means the date of death of succumbed DT-Leprflox/flox-icv-placebo mice and 25 days after icv leptin administration in DT-Pomc-Cre;Leprflox/flox-icv-leptin and DT-Leprflox/flox-icv-leptin mice. Statistical analyses were done using one-way ANOVA (Tukey's or Dunnett's Multiple Comparison Test). Values are mean ± S.E.M. (n = 3–9). ***P<0.001, **P<0.01 versus STZ- or DT-Leprflox/flox-icv-placebo or STZ- or DT-Pomc-Cre;Leprflox/flox-icv-leptin mice. †††P<0.001, ††P<0.01, †P<0.05 versus STZ- or DT-Leprflox/flox-icv-leptin. ND = below the threshold of detection. See also Figure S2
As the hormonal effects on glycemia were slightly dampened, we used a second cohort to assess whether the action of leptin on survival was also altered in STZ-Pomc-Cre;Leprflox/flox-icv-leptin mice. Similarly to results obtained with the first cohort, food intake and body weight were comparable between STZ-Pomc-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin mice (Figure 2C and data not shown). Also, glycemia was again moderately higher in the former compared to the latter group (Figure 2C). Interestingly, no difference in percent survival between STZ-Pomc-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin mice was noted (Figure 2C). Noteworthy, at 25 days into the treatment circulating glucagon levels in STZ-Pomc-Cre;Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-leptin mice were similarly reduced compared to STZ-Leprflox/flox-icv-placebo mice (Figure 2D).
To further assess the role of POMCLEPRs on the life-saving and hyperglycemia-lowering actions of leptin in the absence of insulin, we used our DT model of insulin deficiency. By following the breeding strategy described in the Experimental Procedures section, the RIP-DTR allele was introduced into mice lacking LEPRs only in POMC neurons and their controls. Insulin deficiency in these mice was achieved by ip DT injections as described above and shown in Figure 1A. Data presented in Figure 2E indicate that icv leptin administration exerted similar survival effects in DT-treated RIP-DTR mice lacking LEPRs only in POMC neurons compared to their LEPR-intact controls. However, also in this model of insulin deficiency icv-leptin-treated mice lacking LEPRs in POMC neurons were slightly hyperglycemic compared to icv-leptin-treated LEPR-intact controls (Figure 2E). Of note, circulating insulin was undetectable in all groups (Figure 2F). In line with data shown in Figure 2D, POMCLEPRs were dispensable for the effects of icv leptin administration on glucagon also in the DT-induced model of insulin deficiency, as at 25 days into the treatment circulating glucagon levels in DT-Pomc-Cre;Leprflox/flox-icv-leptin and DT-Leprflox/flox-icv-leptin mice were indistinguishable (Figure 2G). Collectively, our results from experiments conducted on two different mouse models of insulin deficiency establish that POMCLEPRs are i) required for mediating only a marginal component of the hyperglycemia-lowering effect and ii) dispensable for the life-saving, hyperphagia-suppressing, and hyperglucagonemia-lowering actions of leptin in the context of insulin deficiency.
POMCLEPRs are not sufficient for mediating the actions of leptin in insulin deficiency
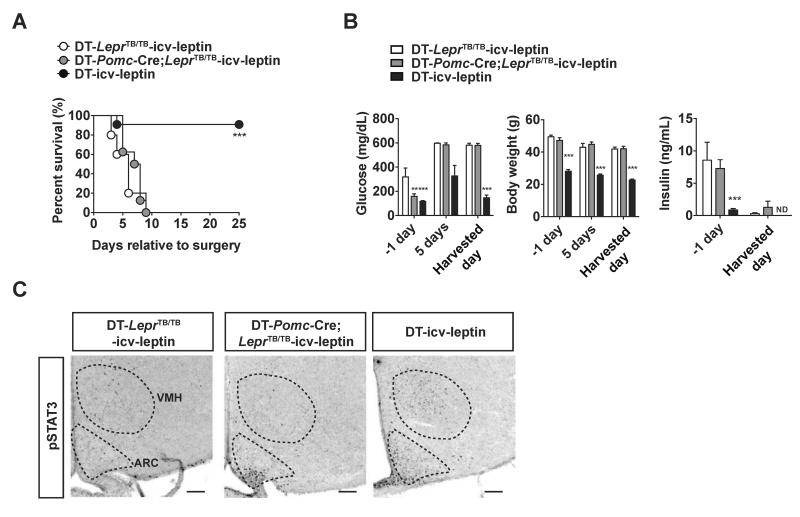
Although our data shown in Figure 2 indicate that POMCLEPRs are marginally important they do not establish whether POMCLEPRs are sufficient to mediate the anti-T1DM effects of leptin. To directly test this hypothesis, mice expressing LEPRs only in POMC neurons were generated. The Pomc-Cre allele was bred to a Cre-conditional Lepr null reactivatable (LeprTB) allele that allows for the re-expression of endogenous LEPRs upon Cre-mediated excision of a loxP-flanked transcriptional blocking cassette (Berglund et al., 2012). Importantly, Pomc-Cre;LeprTB/TB mice express LEPRs only in POMC neurons (Berglund et al., 2012). By following the breeding strategy described in the Experimental Procedures section, the RIP-DTR allele was introduced into Pomc-Cre;LeprTB/TB mice and their controls. Insulin deficiency in these mice was achieved by ip DT injections as described above and in Figure 1A. In agreement with data presented in Figures 1B and 1C, icv leptin administration reversed mortality and improved hyperglycemia in the vast majority of DT-injected RIP-DTR mice (DT-icv-leptin) (Figures 3A and 3B). However, it failed to do so in DT-injected RIP-DTR mice expressing LEPRs only in POMC neurons (DT-Pomc-Cre;LeprTB/TB-icv-leptin) and DT-injected RIP-DTR mice deficient in LEPRs (DT-LeprTB/TB-icv-leptin) both of which had similar body weights (Figures 3A and 3B). Of note, the recalcitrant response to treatment displayed by DT-LeprTB/TB-icv-leptin and DT-Pomc-Cre;LeprTB/TB-icv-leptin mice occurred in the presence of minuscule amount of circulating insulin (Figure 3B). Of note, while icv leptin administration induced pSTAT3 in several hypothalamic sites (including ARC and VMH) of DT-icv-leptin mice it failed to exert this action in DT-LeprTB/TB-icv-leptin mice (Figure 3C), hence proving that the latter are indeed LEPR-deficient mice. Noteworthy, pSTAT3 was detected only in ARC (the site in which POMC neurons are located) of DT-Pomc-Cre;LeprTB/TB-icv-leptin mice (Figure 3C). These results further bolster the idea that these mutants express functional LEPRs only in POMC neurons (Berglund et al., 2012).
Figure 3. LEPRs in POMC neurons are notsufficient to mediate the anti-diabetic action of leptin in the context of insulin deficiency.
(A) Kaplan-Meier survival analyses were performed on insulin-deficient mice expressing LEPRs selectively in POMC neurons, LEPR-intact control (Lepr+/+ and Pomc-Cre;Lepr+/+) and LEPR-null littermates that received icv leptin (25 ng/hour) administration (DT-Pomc-Cre;LeprTB/TB-icv-leptin, DT-icv-leptin, DT-LeprTB/TB-icv-leptin mice, respectively); Statistical analyses were done using Log-rank Test (among all groups) followed by Gehan-Breslow-Wilcoxon Test (each group versus DT-LeprTB/TB-icv-leptin). ***P<0.001. (numbers of mice at day 0 of DT-LeprTB/TB-icv-leptin, DT-Pomc-Cre;LeprTB/TB-icv-leptin and DT were 11, 8 and 5, respectively). (B) Glucose levels in the blood, body weight, insulin levels in the plasma and (C) representative distribution of cells expressing phosphorylated STAT3 (pSTAT3) in the mediobasal hypothalamus of DT-LeprTB/TB-icv-leptin, DT-Pomc-Cre;LeprTB/TB-icv-leptin and DT-icv-leptin mice. Harvested day means the date of death of succumbed DT-Pomc-Cre;LeprTB/TB-icv-leptin and DT-icv-leptin mice and 25 days after icv leptin administration in DT-icv-leptin mice. Statistical analyses were done using one-way ANOVA (Tukey's Multiple Comparison Test). Values are mean ± S.E.M. (n = 5–11). ***P<0.001, **P<0.01 versus DT-LeprTB/TB-icv-leptin. ND = below the threshold of detection. Scale bar size = 100 μ m. ARC, arcuate nucleus; VMH, ventromedial hypothalamic nucleus. See also Figure S3
The refractory response to leptin administration displayed by DT-Pomc-Cre;LeprTB/TB-icv-leptin mice could be due to i) failure to delivering the hormone into the brain of these mice, and/or ii) DT injections caused ablation of POMC neurons and/or iii) non-POMC neurons that are crucial components of this neurocircuitry. The first possibility is ruled out by data showing pSTAT3 staining in ARC of DT-Pomc-Cre;LeprTB/TB-icv-leptin mice (Figure 3C). The likelihood that DT injections caused ablation of POMC neurons is also ruled out because hypothalamic Pomc mRNA levels in RIP-DTR mice did not change 24 hours after DT injection whereas pancreatic preproinsulin mRNA level drastically decreased in these mice at the same time point (Figures S3A and S3B). As expected, glucose levels in the blood increased 48 hours after DT injection (Figure S3C). Also, the anatomical distribution of β-endorphin (a product of POMC) in the hypothalamus was comparable between RIP-DTR mice treated with or without DT (Figures S3D–I). On the other hand, the anatomical distribution of pancreatic β-cells was dramatically different between these 2 groups (Figures S3D–I). The possibility that DT injections caused ablation of non-POMC neurons that are crucial components of this neurocircuitry is also unlikely. In fact, our previously published results (Fujikawa et al., 2010) and our data shown in Figure 1 strongly indicate that icv leptin administration exerts very similar survival and anti-diabetic effects in the DT-induced and STZ-induced models of insulin deficiency. Nevertheless, Dtr was detected in pancreas and also hypothalamus of RIP-DTR mice (Figure S3J). Thus, we cannot rule out the possibility that DT injections ablated other types of cells within this brain structure. Taken together our results indicate that even if DT administration killed some hypothalamic cells in mice bearing the RIP-DTR allele, these cells are likely dispensable for mediating the beneficial effects of leptin administration. Collectively, our results shown in Figures 2, 3, S2 and S3 establish that POMCLEPRs exert only a minor role in mediating the beneficial effects of leptin administration in the context of insulin deficiency.
Importance of concomitant expression of LEPRs in GABAergic and POMC neurons
To determine the identity of the important neurons in the neurocircuitry engaged by leptin we took advantage of several lines of evidence. Firstly, our data shown in Figure 2 excluded a role for LEPRs expressed by SF1 neurons that represent a large population of leptin-responsive glutamatergic neurons (Vong et al., 2011). Secondly, our data shown in Figures 2, 3, S2 and S3 establish that LEPRs on POMC neurons (also in part glutamatergic) (Vong et al., 2011) play only a marginal role in mediating the effects of leptin in insulin deficiency. Thus, LEPRs on glutamatergic neurons are unlikely to exert a major function in this pathway. Therefore, we investigated the role of LEPRs expressed by GABAergic neurons. To directly test the possibility that LEPRs on GABAergic neurons are the crucial mediators of the effects of leptin in insulin deficiency, we assessed the consequence of icv leptin administration in insulin-deficient mice expressing LEPRs only in GABAergic neurons. The vesicular GABA transporter (VGAT) is encoded by the gene Slc32a1 and biochemically characterizes GABAergic neurons. By breeding Vgat-ires-Cre allele (known to drive Cre-mediated recombination of loxP-flanked alleles in all GABAergic neurons) (Vong et al., 2011) to LeprTB allele mice expressing LEPRs only in GABAergic neurons (Vgat-ires-Cre;LeprTB/TB mice) were obtained. By following the breeding strategy described in the Experimental Procedures section, the RIP-DTR allele was introduced into Vgat-ires-Cre;LeprTB/TB mice and their controls. Insulin deficiency in these mice was achieved by ip DT injections as described above and shown in Figure 1A.
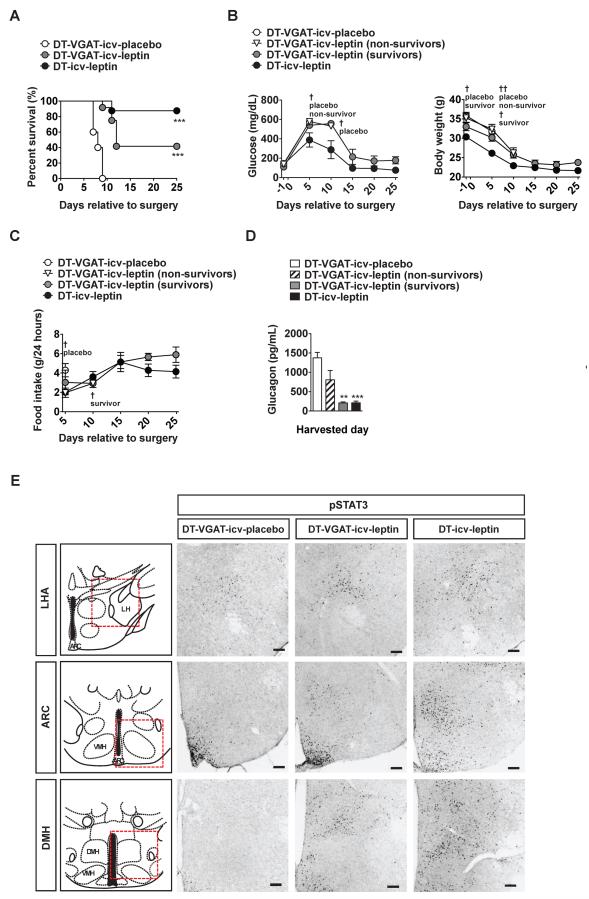
As expected, none of DT-treated Vgat-ires-Cre;LeprTB/TB;RIP-DTR mice undergoing icv placebo administration (DT-VGAT-icv-placebo) survived for longer than 10 days into treatment (Figure 4A). In line with our results shown in Figure 1, 7 out of 8 DT-icv-leptin controls survived 25 days into treatment (Figure 4A). Of note, 5 out of 12 DT-treated Vgat-ires-Cre;LeprTB/TB;RIP-DTR mice undergoing icv leptin administration (DT-VGAT-icv-leptin) also survived 25 days into treatment (Figure 4A). Remarkably, all of these survivors displayed significant hyperglycemic improvements albeit these were not as complete as the ones shown by DT-icv-leptin controls that have intact LEPRs (Figure 4B). Insulin in the plasma was undetectable in both DT-VGAT-icv-leptin survivors and DT-icv-leptin controls, hence supporting the notion that DT-VGAT-icv-leptin survivors were truly insulin deficient (data not shown). Food intake was also similar between DT-VGAT-icv-leptin survivors and DT-icv-leptin controls while body weight tended to be slightly higher in the former compared to the latter group (Figures 4B and 4C). Interestingly, the ability of icv leptin administration to suppress hyperglucagonemia was fully intact and only partial in DT-VGAT-icv-leptin survivors and non-survivors, respectively, compared to DT-icv-leptin controls (Figure 4D).
Figure 4. LEPRs in GABAergic neurons are sufficient to largely mediate the survival and anti-diabetic action of leptin in the context of insulin deficiency.
(A) Kaplan-Meier survival analyses were performed on DT-treated mice expressing LEPRs selectively in GABAergic neurons that received icv leptin (25 ng/hour) administration (DT-VGAT-icv-leptin), or placebo (PBS) administration (DT-VGAT-icv-placebo) and LEPR-intact control (composed of Vgat-ires-Cre;Lepr+/+;RIP-DTR and Lepr+/+;RIP-DTR mice) littermate mice that received icv leptin administration (DT-icv-leptin); Statistical analyses were done using Log-rank Test (among all groups) followed by Gehan-Breslow-Wilcoxon Test (compared each group versus DT-VGAT-icv-placebo). ***P<0.001. (numbers of mice at day 0 of DT-VGAT-icv-placebo, DT-VGAT-icvleptin and DT-icv-leptin were 5, 12 and 8, respectively). (B) Glucose levels in the blood, and body weight, and (C) food intake (D) glucagon levels in the plasma in DT-VGAT-icv-placebo, DT-VGAT-icv-leptin (non-survivors), DT-VGAT-icv-leptin (survivors), and DT-icv-leptin mice. (E) Representative distribution of cells expressing phosphorylated STAT3 (a read out of leptin-responsive neurons) in the lateral hypothalamic area (LHA), hypothalamic arcuate nucleus (ARC) and dorsomedial nucleus (DMH) of DT-VGAT-icv-placebo, DT-VGAT-icv-leptin and DT-icv-leptin mice. Anatomical location of LHA, ARC and DMH is shown in red-colored, dashed-line boxed area in top panels (Franklin and Paxinos, 2008). Statistical analyses were done using one-way ANOVA (Dunnett's Multiple Comparison Test). Values are mean ± S.E.M. (n = 3–12). Scale bar size = 100 μm. †††P<0.001, ††P<0.01, †P<0.05 versus DT-icv-leptin. See also Figure S4
Of note, leptin-responsive GABAergic neurons are localized only in ARC, lateral hypothalamic area (LHA) and dorsomedial hypothalamus (DMH) within the CNS (Vong et al., 2011). Accordingly, robust pSTAT3 staining was detected in the ARC, LHA and DMH of DT-VGAT-icv-leptin but not in DT-VGAT-icv-placebo mice (Figure 4E). The different intra-group survival outcomes and metabolic profiles displayed by DT-VGAT-icv-leptin mice could be due to i) failure to delivering the hormone into the brain, and/or ii) altered leptin sensitivity of DT-VGAT-icv-leptin non-survivors, and/or iii) DT injections ablated a significant proportion of leptin-responsive VGAT-expressing neurons in DT-VGAT-icv-leptin non-survivors. The aforementioned possibilities are unlikely because the anatomical distribution of brain pSTAT3 was comparable between DT-VGAT-icv-leptin survivors and non-survivors (Figure S4A–F).
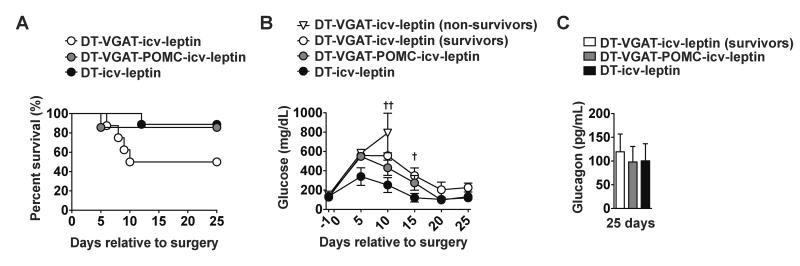
Thus, LEPRs on other non-GABAergic neuronal group(s) must play a required role. Because our results shown in Figure 2 indicated that POMCLEPRs are required, we investigated whether re-expression of LEPRs in GABAergic and also POMC neurons restores a full responsiveness to leptin administration. By following the breeding strategy described in the Experimental Procedures section, mice expressing LEPRs selectively in GABAergic and also POMC neurons (Vgat-ires-Cre;Pomc-Cre;LeprTB/TB;RIP-DTR mice) were obtained. Insulin deficiency in these mice was achieved by ip DT injections as described above and shown in Figure 1A. Re-expression of LEPRs in GABAergic and also POMC neurons had greater effects in mediating the life-saving and hyperglycemia-improving action of leptin administration compared to re-expression of LEPRs only in GABAergic neurons (Figures 5A and 5B). In fact, DT-VGAT-icv-leptin mice again displayed a partial improvement in lethality and hyperglycemia while these parameters were similarly rescued in icv-leptin-treated insulin-deficient mice expressing LEPRs concomitantly only in GABAergic and POMC neurons (DT-VGAT-POMC-icv-leptin group) compared to DT-icv-leptin controls (Figures 5A and 5B). In line with data shown in Figure 4D, DT-VGAT-icv-leptin survivors were again found to display similar levels of circulating glucagon compared to DT-icv-leptin controls (Figure 5C). Also DT-VGAT-POMC-icv-leptin mice had circulating glucagon levels undistinguishable from DT-VGAT-icv-leptin survivors and DT-icv-leptin controls (Figure 5C). At 25 days after induction of insulin deficiency insulin in the plasma was undetectable in all groups (data not shown).
Figure 5. LEPRs in GABAergic and POMC neurons are sufficient to fully mediate the anti-diabetic action of leptin in the context of insulin deficiency.
(A) Kaplan-Meier survival analyses were performed on DT-treated mice expressing LEPRs selectively in both GABAergic and POMC neurons (Vgat-ires-Cre;Pomc-Cre;LeprTB/TB;RIP-DTR mice), GABAergic neurons (Vgat-ires-Cre;LeprTB/TB;RIP-DTR mice) and LEPR-intact control (composed of Vgat-ires-Cre; Pomc-Cre;Lepr+/+;RIP-DTR, Vgat-ires-Cre;Lepr+/+;RIP-DTR, Pomc-Cre;Lepr+/+;RIP-DTR and Lepr+/+;RIP-DTR mice) mice that received icv leptin (25 ng/hour) administration (DT-VGAT-POMC-icv-leptin, DT-VGAT-icv-leptin and DT-icv-leptin); Statistical analyses were done using by Log-rank Test (numbers of mice at day 0 of DT-VGAT-icv-leptin, DT-VGAT-POMC-icv-leptin and DT-icv-leptin were 8, 7 and 9, respectively). (B) Glucose levels in the blood of DT-VGAT-icv-leptin (non-survivors), DT-VGAT-icv-leptin (survivors), VGAT-POMC-icv-leptin and DT-icv-leptin and (C) glucagon levels in the plasma in DT-VGAT-icv-leptin (survivors), DT-VGAT-POMC-icv-leptin and DT-icv-leptin at 25 days. Statistical analyses were done using one-way ANOVA (Dunnett's Multiple Comparison Test). Values are mean ± S.E.M. (n = 3–9). ††P<0.05, †P<0.05 versus DT-icv-leptin.
Altogether, our results demonstrate that concomitant expression of LEPRs only in GABAergic and POMC neurons is sufficient to fully mediate the life-saving and anti-diabetic effects of leptin administration in the context of insulin deficiency and that the vast majority of these actions are via LEPRs in GABAergic neurons.
Improved liver, soleus, and iBAT metabolism underlie the actions of leptin in insulin deficiency
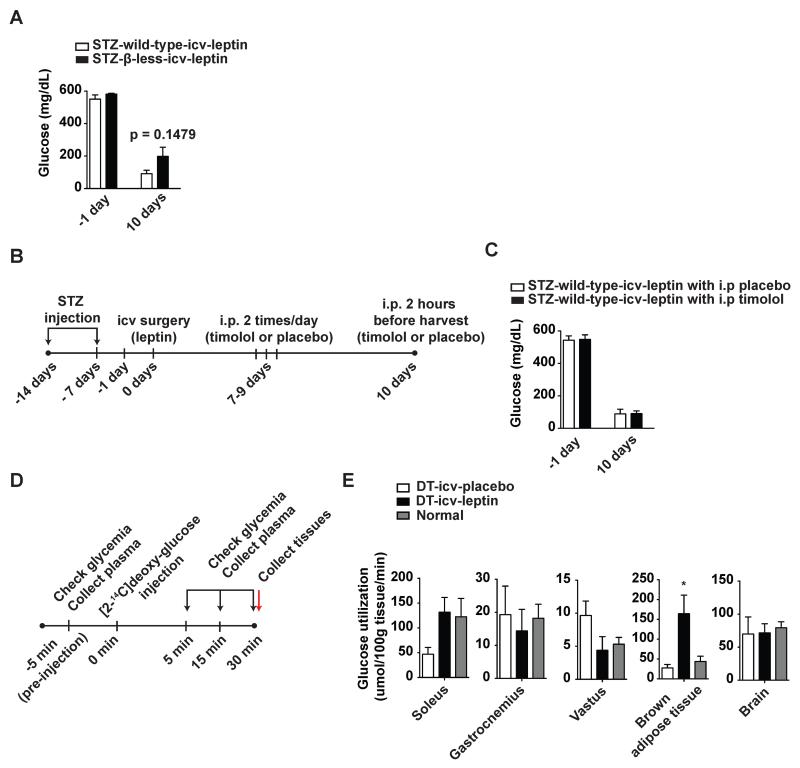
To gather further insights on the mechanisms underpinning the leptin-dependent pathway enabling life without insulin we interrogated the role of β-adrenergic receptors. These receptors have been previously indicated as important components of hypothalamic-dependent pathway governing glucose metabolism (Haque et al., 1999; Minokoshi et al., 1999). Thus, to directly test whether β-adrenergic receptors are downstream effectors of the neurocircuitry engaged by leptin, we assessed the metabolic outcomes of icv leptin administration in mice devoid of the three known β-adrenergic receptors (β-less mice) (Bachman et al., 2002). As shown in Figure 6A, icv leptin administration rescued hyperglycemia to similar extents in STZ-treated wild-type (STZ-wild-type-icv-leptin) and β-less (STZ-β-less-icv-leptin) mice. These genetic results were further corroborated by pharmacological data. Indeed, administration of the β-adrenergic receptor blocker timolol did not alter the anti-diabetic actions of icv leptin administration in STZ-treated wild-type mice (Figure 6B and 6C). Collectively, these data indicate that β-adrenergic receptors are dispensable for the anti-diabetic action of leptin in the context of insulin deficiency.
Figure 6. Leptin administration induces glucose uptake in soleus and iBAT of insulin-deficient mice.
(A) Glucose level in the blood of insulin-deficient β-less mice (that do not express any types of β-adrenergic receptors) and their wild-type controls that received icv leptin (25 ng/hour) administration (STZ-β-less-icv-leptin and STZ-wild-type-icv-leptin groups, respectively). Insulin deficiency was induced by administration of STZ as shown in Figure 2. (B) Experimental design and (C) glucose level in the blood of STZ-treated FBV/NJ mice that received icv administration of leptin (25 ng/hour) followed by administrations of β-adrenergic-receptor blocker timolol (1 mg/kg BW) or placebo (0.9% saline solution). Mice were injected ip twice a day from day 7 up to day 9 and one time 2 hours before sacrifice. (D) Experimental design of assessment of basal glucose utilization. The experiment was carried out 10 days after icv administration as described in Figure 1A. (E) Glucose utilization levels in soleus muscle, gastrocnemius muscle, vastus muscle, interscapular brown adipose tissue and brain of DT-icv-leptin, DT-icv-placebo and normal mice. Values are mean ± S.E.M. (n = 4–6). Statistical analyses were done using unpaired t-test or one-way ANOVA (Tukey's Multiple Comparison Test) *P<0.05 versus DT-icv-placebo mice. See also Figure S5 and Table S1 and S2.
We and others have reported that the improved hyperglycemia engendered by leptin administration to insulin-deficient rodents is not the result of increased glucose excretion (Fujikawa et al., 2010; Wang et al., 2010; Yu et al., 2008). To determine the fate of glucose in insulin-deficient mice undergoing leptin administration, we used glucose tracing analyses (Figure 6D). Our results indicate that icv leptin administration does not affect glucose uptake in brain as well as gastrocnemius and vastus skeletal muscle while it significantly enhances glucose uptake in soleus muscle and iBAT of DT-treated RIP-DTR mice (Figure 6E). To further elucidate the peripheral effects of icv leptin administration in insulin-deficient mice, hepatic gene expression and metabolites levels in STZ-Leprflox/flox-icv-leptin and STZ-Leprflox/flox-icv-placebo mice (same animals as shown in Figure 2B) were assessed by transcriptomics and metabolomics assays, respectively. The transcriptomics analysis revealed that more than 800 genes were significantly (p<0.001) down- or up-regulated by icv leptin administration (Figure S5A). The metabolomics analysis indicated that 23 metabolites were significantly (p<0.01) altered by icv leptin administration (Figure S5B and Table S1). Of note, pathway analysis indicated that several metabolic pathways including those relevant to glucose metabolism were significantly altered (p<0.001) by icv leptin administration (Figure S5C and Table S2). We particularly focused on glucose and glycogen metabolism because lower circulating glucagon levels brought on by icv leptin administration may affect those pathways. In line with our results that hepatic glycogen levels were not altered by icv leptin administration (Figure 1F) (Fujikawa et al., 2010), we found many genes and metabolites relevant to this pathway that were not significantly altered by icv leptin administration (Figure S5D and Table S2). On the other hand, hepatic glucose content and the expression of glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase 1 (both of which encode for enzymes of the glycolytic pathway) were reduced by icv leptin administration (Figure S5D). These results indicate that although not normalized, the liver of icvleptin-treated insulin-deficient mice displayed improved metabolomics and transcriptomics profiles compared to icv-placebo-treated insulin-deficient mice. Collectively, our results suggest that increased glucose uptake and utilization by soleus and iBAT and improved hepatic metabolism are components of the mechanism underlying the anti-diabetic action of leptin in the context of insulin deficiency.
Discussion
In this study we unraveled a neurocircuitry whereby leptin engages hypothalamic GABALEPRs and POMCLEPRs to permit survival and maintain euglycemia in the context of complete insulin deficiency. This conclusion was drawn mainly because concomitant re-expression of LEPRs in GABAergic and POMC neurons is sufficient to mediate virtually all of the life-saving and anti-diabetic actions of leptin administration in insulin-deficient mice (Figure 5). Our results also clarified the contribution that each one of the two aforementioned neuronal populations exerts on this pathway. As shown in Figure 2, the effects of icv leptin administration on survival, food intake and circulating glucagon level do not require POMCLEPRs. Although our results indicate that POMCLEPRs are required for mediating the effect on hyperglycemia their contribution to this action is marginal. In fact, data shown in Figure 2 indicate that the ability of icv leptin administration to suppress hyperglycemia in the STZ- and DT-induced models of insulin deficiency is only slightly diminished in mutants lacking POMCLEPRs. Also, these receptors alone are not sufficient for mediating any of the effects of icv leptin administration in the context of insulin deficiency (Figure 3). On the other hand, our data strongly suggest that LEPRs in GABAergic neurons mediate a large component of the hormonal effects on survival and glucose metabolism in the context of complete insulin deficiency (Figure 4). Thus, the beneficial effects of leptin administration in insulin-depleted mice are largely mediated by direct action of the hormone leptin on GABALEPRs while only a minimal component of these effects is mediated by POMCLEPRs.
Unger and colleagues have provided experimental support to the idea that leptin improves diabetes of insulin-deficient rodents in part via diminishing circulating glucagon content and action (Lee et al., 2012; Wang et al., 2010; Yu et al., 2008). Our results are in line with this notion. Indeed, in our leptin-treated insulin-deficient mouse models displaying ameliorated hyperglycemia circulating glucagon level and action were also improved (Figures 1F, 2D, 2G, 4D, and 5C). Combined with data shown in Figures S1A–D indicating that LEPR-B is not expressed by pancreatic α-cells, our results suggest that the hyperglucagonemia-lowering effect of leptin is not via direct action of the hormone on pancreatic α-cells but rather via indirect action through hypothalamic GABAergic neurons. Further studies will be needed to unravel all components of this GABAergic-neuron-pancreatic α-cell axis.
Although GABAergic neurons are widely distributed throughout the CNS, the sub-group of GABAergic neurons also able to express LEPRs is restricted to in the ARC, DMH, and LHA (Figure 4E); of note, these results are in line with previously reported data (Vong et al., 2011). Therefore, the direct action of leptin on one or several of the aforementioned three sites must be important for the life-saving and anti-diabetic action of the hormone in the context of insulin deficiency (Figure 4E). Among the plausible candidates are agouti related protein (AgRP)-expressing neurons as they are i) located in the ARC, ii) GABAergic, and iii) known to express LEPRs (Vianna and Coppari, 2011). Thus, to directly test whether AgRP neurons are indeed the crucial neurons in this neurocircuitry, assessing the outcomes of leptin administration in insulin-deficient mice expressing LEPRs only in AgRP neurons appears as the ideal experimental approach. Such mutants can theoretically be generated by breeding the LeprTB allele to Agrp-ires-Cre transgene (Tong et al., 2008). Nevertheless, our attempts to achieve this goal failed owing to the nearly 100% frequency of detectable Cre-recombined LeprTB allele in sites in which AgRP neurons are not present in Agrp-ires-Cre;LeprTB/TB mice. Tackling the relevance of LEPRs in DMH and/or LHA is currently a technically very difficult task mainly because of the lack of DMH- and/or LHA-specific-Cre mouse lines. Thus, future studies aimed at sorting out the contribution that LEPRs expressed by GABAergic neurons within the ARC, DMH, and LHA on the life-saving and anti-diabetic action of leptin in the context of insulin deficiency are warranted.
It is of interest that while POMCLEPRs are sufficient to mediate the effects of leptin on glucose metabolism in rodents able to secrete insulin (Berglund et al., 2012) they are not sufficient to mediate these glucoregulatory effects of the hormone in rodents devoid of insulin (Figure 3). These results demonstrate that insulin is necessary for POMC neurons to trigger the cascade of events that bring about reduced hyperglycemia induced by leptin. Along these lines, in rodents able to produce insulin hypothalamic-mediated control of glucose metabolism (as for example in skeletal muscle and iBAT) relies on the activation of sympathetic nervous system (SNS) neurons (these cells secrete catecholamines whose targets are adrenergic receptors) (Minokoshi et al., 1999; Ramadori et al., 2011). However, our genetic and pharmacological data shown in Figures 6A and 6C indicate that β-adrenergic receptors are dispensable for hypothalamic-dependent glucoregulatory actions of leptin in rodents unable to produce insulin. It is important to note that our results cannot rule out the possibility that other types of adrenergic receptors (e.g.: α-adrenergic receptors) and/or receptors whose ligands secreted by SNS neurons are not catecholamines are important components of the aforementioned mechanism. Nevertheless, we suggest that alternative non-SNS pathways are engaged by leptin to increase glucose uptake in skeletal muscle and iBAT in the context of insulin deficiency (Figures 6E). These pathways may include parasympathetic nervous system neurons and/or humoral factor(s) and future studies will be needed to directly test this hypothesis. Collectively, our data and previously published results mentioned above indicate that different pathways can be engaged by hypothalamic neurons in order to keep glucose metabolism in check in the presence vs. absence of insulin.
From a therapeutic viewpoint, our results could pave the way for developing the urgently needed improved therapies for the millions of people affected by insulin deficiency (e.g.: T1DM and some T2DM patients) (Butler et al., 2007; Coppari and Bjorbaek, 2012; Talchai et al., 2012). In fact, the sole therapeutic available to these patients is insulin administration that, as discussed in the Introduction section, bears several limitations and can even underlie some of the serious morbidities seen in these patients (Larsen et al., 2002; Orchard et al., 2003). Although these pre-clinical results indicate that leptin therapy may represent an ideal alternative, its applicability in insulin-deficient humans seems improbable. Indeed, several clinical trials failed to show effectiveness of leptin administration in patients who are not severely hypoleptinemic or totally lacking leptin; yet, the vast majority of T2DM and insulin-treated T1DM subjects are not known to be severely hypoleptinemic or leptin deficient (Coppari and Bjorbaek, 2012). Therefore, targeting the cellular and molecular components underlying the effects of leptin in insulin deficiency may prove efficacious in the clinical arena. Here, we identified crucial components of this pathway; hence we believe that our results bear important clinical significance. In fact, harnessing hypothalamic GABAergic/POMC neurocircuitry and/or its downstream effector components (e.g.: brown adipocytes) may permit survival and greatly improve hyperglycemia in people suffering from diseases characterized by insulin deficiency.
Experimental Procedures
Animal
All mice were housed with standard chow diet and water available ad libitum in a light- and temperature-controlled environment. We used male mice. Care of mice was within the Institutional Animal Care and Use Committee (IACUC) guidelines, and all the procedures were approved by the University of Texas Southwestern Medical Center IACUC.
Induction of insulin deficiency by diphtheria toxin or streptozotocin injection
We used 3–4 months of old male RIP-DTR mice whose body weight was approximately 30 g. Diphtheria toxin (Sigma, MO, USA) was dissolved in sterile 0.9% NaCl and intraperitoneally (ip) administered into RIP-DTR mice to ablate pancreatic β-cells (0.5 μg/kg B.W.; on day 0, 3, 5). Streptozotocin (Sigma) was dissolved in cold-sterile 0.9% NaCl and immediately ip administered (150 mg/kg B.W.; two times at one week interval) into mice (Fujikawa et al., 2010).
icv leptin administration
Leptin (Peprotech, NJ, USA; 25 ng/hour/0.11 μL) was chronically intracerebroventricularly (icv) administered by the osmotic pump (Alzet, CA, USA) as previously described (Fujikawa et al., 2010). A sterile phosphate-buffered saline (pH = 7.4, Invitrogen, CA, USA) solution was administered into the control group as placebo treatment.
Assessment of energy substrates and hormones levels
Daily glycemia, plasma and pancreatic insulin were measured as previously described (Fujikawa et al., 2010). Plasma glucagon was measured as previously described (Berglund et al., 2012).
Assessment of mRNA and protein contents
mRNA levels and protein contents in the hypothalamus, pancreas and liver were determined by the previous described methods with slight modifications (Fujikawa et al., 2010).
Immunohistochemistry
Phosphorylation of STAT3 in the brain was determined as previously described (Scott et al., 2009). Pancreatic glucagon and insulin distribution was determined as previously described (Fujikawa et al., 2010)
Assessment of basal tissue-specific glucose uptake
Mice were fasted for 1.5 h and then ip injected with 150 uCi of [2-14C]deoxy-glucose. Samples to measure blood glucose and plasma 14C specific activity were taken at t = −5 (pre-injection), 5, 15, and 30 min. Mice were then euthanized immediately after the final sample and tissues were quickly dissected and frozen in liquid nitrogen. Methods and calculations to determine tissue-specific glucose uptake (Rg) are previously described (Fueger et al., 2003; Kraegen et al., 1985).
Statistical Analysis
With the exception of microarray and metabolomics results, all data sets were analyzed for statistical significance using PRISM (GraphPad, CA) as indicated in each of the Figure legends. Error bars in all figures represent S.E.M
Microarray data
The microarray date was deposited to The National Center for Biotechnology (accession number is GSE48598).
Supplementary Material
Acknowledgements
We thank Drs. Mirco Gallie, Kim Ki Woo for providing technical advices, Massimo Attanasio, Claes Wollheim, Christian Bjørbæk and Paolo Sassone-Corsi for critical reading of the manuscript, and Joyce Repa for providing some of quantitative real time PCR primers. This work was supported by Juvenile Diabetes Research Foundation (Post-doctoral fellowship 3-2011-405 to T.F.), American Heart Association (Post-doctoral fellowship to G.R. and Scientist Development Grant to R.C.), National Science Foundation (NSFIIS-0513376 to P.B.) and by National Institutes of Health Grants (FDK092083A to E.D.B.; F32 DK078478 to L.V; R01 DK089044, R01 DK075632, P30 DK046200 and p30DK057521 to B.B.L.; RL1 DK081185 and R37 DK053301 to J.K.E.; LM010235 and NLM T15LM07443 to P.B.; R01 DK080836 and R01 DK091680 to R.C.). This work has also received the unrestricted support of the Louis-Jeantet Foundation (to R.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr., Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J, Jr., Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. The Journal of clinical investigation. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nature clinical practice Endocrinology & metabolism. 2007;3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- Choi YH, Fujikawa T, Lee J, Reuter A, Kim KW. Revisiting the Ventral Medial Nucleus of the Hypothalamus: The Roles of SF-1 Neurons in Energy Homeostasis. Frontiers in neuroscience. 2013;7:71. doi: 10.3389/fnins.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr., et al. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia. 2009;52:35–37. doi: 10.1007/s00125-008-1205-7. [DOI] [PubMed] [Google Scholar]
- Denroche HC, Levi J, Wideman RD, Sequeira RM, Huynh FK, Covey SD, Kieffer TJ. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60:1414–1423. doi: 10.2337/db10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos GU. The Mouse Brain in Stereotaxic Coordinates. Academic Press; 2008. [Google Scholar]
- Fueger PT, Heikkinen S, Bracy DP, Malabanan CM, Pencek RR, Laakso M, Wasserman DH. Hexokinase II partial knockout impairs exercise-stimulated glucose uptake in oxidative muscles of mice. American journal of physiology Endocrinology and metabolism. 2003;285:E958–963. doi: 10.1152/ajpendo.00190.2003. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107:17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48:1706–1712. doi: 10.2337/diabetes.48.9.1706. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of clinical investigation. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjorbaek C. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol. 1985;248:E353–362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- Larsen J, Brekke M, Sandvik L, Arnesen H, Hanssen KF, Dahl-Jorgensen K. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–2641. doi: 10.2337/diabetes.51.8.2637. [DOI] [PubMed] [Google Scholar]
- Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, Unger RH. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14972–14976. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell metabolism. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. The Journal of clinical investigation. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna CR, Coppari R. A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology. 2011;152:11–18. doi: 10.1210/en.2010-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr., Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Chen L, Clark GO, Lee Y, Stevens RD, Ilkayeva OR, Wenner BR, Bain JR, Charron MJ, Newgard CB, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci U S A. 2010;107:4813–4819. doi: 10.1073/pnas.0909422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.