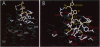Abstract
To identify determinants that form nonapeptide hormone binding domains of the white sucker Catostomus commersoni [Arg8]vasotocin receptor, chimeric constructs encoding parts of the vasotocin receptor and parts of the isotocin receptor have been analyzed by [(3,5-3H)Tyr2, Arg8]vasotocin binding to membranes of human embryonic kidney cells previously transfected with the different cDNA constructs and by functional expression studies in Xenopus laevis oocytes injected with mutant cRNAs. The results indicate that the N terminus and a region spanning the second extracellular loop and its flanking transmembrane segments, which contains a number of amino acid residues that are conserved throughout the nonapeptide receptor family, contribute to the affinity of the receptor for its ligand. Nonapeptide selectivity, however, is mainly defined by transmembrane region VI and the third extracellular loop. These results are complemented by a molecular model of the vasotocin receptor obtained by aligning its sequence with those of other G-protein coupled receptors as well as that of bacteriorhodopsin. The model indicates that amino acid residues of transmembrane regions II-VII that are located close to the extracellular surface also contribute to the binding of vasotocin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acher R. Molecular evolution of biologically active polypeptides. Proc R Soc Lond B Biol Sci. 1980 Oct 29;210(1178):21–43. doi: 10.1098/rspb.1980.0116. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. Structure and function of receptors coupled to G proteins. Curr Opin Cell Biol. 1994 Apr;6(2):180–190. doi: 10.1016/0955-0674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M., Seibold A., Gilbert S., Ishido M., Barberis C., Antaramian A., Brabet P., Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992 May 28;357(6376):333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- Chini B., Mouillac B., Ala Y., Balestre M. N., Trumpp-Kallmeyer S., Hoflack J., Elands J., Hibert M., Manning M., Jard S. Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J. 1995 May 15;14(10):2176–2182. doi: 10.1002/j.1460-2075.1995.tb07211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R. Expanding horizons for receptors coupled to G proteins: diversity and disease. Curr Opin Cell Biol. 1994 Apr;6(2):191–197. doi: 10.1016/0955-0674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Huang R. R., Strader C. D. Localization of agonist and antagonist binding domains of the human neurokinin-1 receptor. J Biol Chem. 1992 Dec 25;267(36):25664–25667. [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Snider R. M., Lowe J. A., 3rd, Nakanishi S., Schwartz T. W. Different binding epitopes on the NK1 receptor for substance P and non-peptide antagonist. Nature. 1993 Mar 25;362(6418):345–348. doi: 10.1038/362345a0. [DOI] [PubMed] [Google Scholar]
- Gorbulev V., Büchner H., Akhundova A., Fahrenholz F. Molecular cloning and functional characterization of V2 [8-lysine] vasopressin and oxytocin receptors from a pig kidney cell line. Eur J Biochem. 1993 Jul 1;215(1):1–7. doi: 10.1111/j.1432-1033.1993.tb18000.x. [DOI] [PubMed] [Google Scholar]
- Hausmann H., Meyerhof W., Zwiers H., Lederis K., Richter D. Teleost isotocin receptor: structure, functional expression, mRNA distribution and phylogeny. FEBS Lett. 1995 Aug 21;370(3):227–230. doi: 10.1016/0014-5793(95)00832-t. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby V. J., Chow M. S., Smith D. D. Conformational and structural considerations in oxytocin-receptor binding and biological activity. Annu Rev Pharmacol Toxicol. 1990;30:501–534. doi: 10.1146/annurev.pa.30.040190.002441. [DOI] [PubMed] [Google Scholar]
- Kaupmann K., Bruns C., Raulf F., Weber H. P., Mattes H., Lübbert H. Two amino acids, located in transmembrane domains VI and VII, determine the selectivity of the peptide agonist SMS 201-995 for the SSTR2 somatostatin receptor. EMBO J. 1995 Feb 15;14(4):727–735. doi: 10.1002/j.1460-2075.1995.tb07051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Tanizawa O., Mori K., Brownstein M. J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992 Apr 9;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- Kojro E., Eich P., Gimpl G., Fahrenholz F. Direct identification of an extracellular agonist binding site in the renal V2 vasopressin receptor. Biochemistry. 1993 Dec 14;32(49):13537–13544. doi: 10.1021/bi00212a020. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992 May 28;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- Mahlmann S., Meyerhof W., Hausmann H., Heierhorst J., Schönrock C., Zwiers H., Lederis K., Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1342–1345. doi: 10.1073/pnas.91.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M., Sawyer W. H. Design, synthesis and some uses of receptor-specific agonists and antagonists of vasopressin and oxytocin. J Recept Res. 1993;13(1-4):195–214. doi: 10.3109/10799899309073655. [DOI] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Mouillac B., Chini B., Balestre M. N., Elands J., Trumpp-Kallmeyer S., Hoflack J., Hibert M., Jard S., Barberis C. The binding site of neuropeptide vasopressin V1a receptor. Evidence for a major localization within transmembrane regions. J Biol Chem. 1995 Oct 27;270(43):25771–25777. doi: 10.1074/jbc.270.43.25771. [DOI] [PubMed] [Google Scholar]
- Probst W. C., Snyder L. A., Schuster D. I., Brosius J., Sealfon S. C. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992 Jan-Feb;11(1):1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Regoli D., Drapeau G., Dion S., D'Orléans-Juste P. Receptors for substance P and related neurokinins. Pharmacology. 1989;38(1):1–15. doi: 10.1159/000138512. [DOI] [PubMed] [Google Scholar]
- Schöneberg T., Liu J., Wess J. Plasma membrane localization and functional rescue of truncated forms of a G protein-coupled receptor. J Biol Chem. 1995 Jul 28;270(30):18000–18006. doi: 10.1074/jbc.270.30.18000. [DOI] [PubMed] [Google Scholar]
- Sharif M., Hanley M. R. Peptide receptors. Stepping up the pressure. Nature. 1992 May 28;357(6376):279–280. doi: 10.1038/357279a0. [DOI] [PubMed] [Google Scholar]
- Skach W. R., Shi L. B., Calayag M. C., Frigeri A., Lingappa V. R., Verkman A. S. Biogenesis and transmembrane topology of the CHIP28 water channel at the endoplasmic reticulum. J Cell Biol. 1994 May;125(4):803–815. doi: 10.1083/jcb.125.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T., Saito M., Mochizuki S., Watanabe Y., Hashimoto S., Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem. 1994 Oct 28;269(43):27088–27092. [PubMed] [Google Scholar]
- Teeter M. M., Froimowitz M., Stec B., DuRand C. J. Homology modeling of the dopamine D2 receptor and its testing by docking of agonists and tricyclic antagonists. J Med Chem. 1994 Sep 2;37(18):2874–2888. doi: 10.1021/jm00044a008. [DOI] [PubMed] [Google Scholar]
- Thibonnier M., Auzan C., Madhun Z., Wilkins P., Berti-Mattera L., Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J Biol Chem. 1994 Feb 4;269(5):3304–3310. [PubMed] [Google Scholar]
- Ufer E., Postina R., Gorbulev V., Fahrenholz F. An extracellular residue determines the agonist specificity of V2 vasopressin receptors. FEBS Lett. 1995 Mar 27;362(1):19–23. doi: 10.1016/0014-5793(95)00150-8. [DOI] [PubMed] [Google Scholar]