Abstract
BACKGROUND: MicroRNA 132 (miR-132) is dysregulated in a range of human malignancies; however, its role in glioma has not been reported. The aim of this study was to profile miR-132 expression in a cohort of patients with primary glioblastoma multiforme (GBM) treated with the Stupp regimen and to correlate microRNA levels with patient outcome. METHODS: miR-132 levels relative to RNU44 were assessed by quantitative reverse transcription-polymerase chain reaction in 43 GBMs and normal brain tissue. The cohort comprised patients less than 72 years of age with Eastern Cooperative Oncology Group (ECOG) scores between 0 and 2 who had undergone 6-week concomitant radiation and temozolomide followed by adjuvant temozolomide. Survival data were available for all cases. Tumors were characterized for O6-methylguanine-DNA methyltransferase (MGMT) methylation and isocitrate dehydrogenase (IDH) 1/2 mutation status. Associations between miR-132 expression and clinical indicators were analyzed. RESULTS: Tumor miR-132 levels ranged from 0.07- to 40.4-fold increase (mean = 5.5-fold increase) relative to normal brain. High-level miR-132 (above the mean) independently predicted for a significantly shorter overall survival (P = .008). miR-132 was a stronger prognostic indicator than ECOG score (P = .012) and age at diagnosis (P = .026) but did not correlate with MGMT methylation status or extent of tumor resection. Cox regression analysis confirmed high miR-132 as the strongest predictor of outcome (P = .010) with a hazard ratio of 2.8. CONCLUSIONS: This study identified high miR-132 expression as a biomarker of poor prognosis in patients with primary GBM treated with the Stupp regimen.
Background
Glioblastoma multiforme (GBM, grade 4 astrocytoma) is the most common primary brain tumor in adults. Current standard therapy for these patients involves surgical debulking, followed by radiotherapy plus concomitant and adjuvant temozolomide (Stupp regimen) [1]. Despite aggressive therapy, prognosis is still dismal, with a 5-year survival rate of just 9% [2]. Few chemotherapeutic agents have any impact on disease outcome, and new therapies are urgently needed.
MicroRNAs (miRNAs) are small noncoding RNAs, 20 to 25 nucleotides in length that modulate gene expression in crucial cellular processes including apoptosis, differentiation, and development [3]. miRNAs regulate gene expression by direct cleavage of mRNA or by inhibiting translation through perfect or near perfect complementarity to target mRNA at the 3′ untranslated region. miRNAs are involved in virtually all biologic processes, and it is estimated that miRNAs regulate up to 60% of human gene expression [4]. miRNAs play a crucial role in human malignancy, where they have been shown to act as tumor suppressors (miR-15 and miR-16) or oncogenes (miR-155) [5,6]. Global alterations in miRNA expression have now been identified in a large number of human malignancies [7,8]. miRNA expression patterns in cancer have been shown to correlate with diagnosis, prognosis, and response to therapy [7,8]. Their utility as biomarkers is currently being evaluated in clinical trials for a range of human malignancies, including non-small cell lung carcinoma, breast cancer, leukemias and lymphomas, neuroblastoma, and ovarian and prostate cancers [8]. miRNAs have also been shown to modulate cancer cell sensitivity to chemotherapy and radiotherapy. For example, targeting of oncogenic miR-21 in glioblastoma cell lines, using an antagomir (antisense oligonucleotide), significantly increases the cytotoxic effect of taxol and 5-fluorouracil [9,10].
MicroRNA 132 (miR-132), transcribed from an intergenic region on human chromosome 17, is aberrantly expressed in lung and pancreatic cancers and in breast carcinoma tumor endothelium [11,12]. More recently, miR-132 has been shown to regulate a host of central nervous system-specific processes, including neurogenesis [13], synaptic plasticity [14], neuroendocrine-modulated inflammation [15], and differentiation of dopamine neurons [16]. It is dysregulated in several brain-related diseases, including Huntington disease [17], Parkinson disease, and schizophrenia [18]. However, a potential role for miR-132 in primary GBM has not been explored.
When evaluating the significance of a potential biomarker, it is essential to study its expression in a clinically similar group of patients to minimize potential bias from unrelated factors. In the current study, we profiled miR-132 expression in newly diagnosed patients with primary GBM who successfully completed a minimum of 18 weeks of therapy and whose standard prognostic measures were within a defined narrow range. We correlated tumor miRNA levels with these and other measures of clinical outcome to determine the potential of miR-132 levels as an independent indicator of prognosis in primary GBM.
Methods
Study Cohort
Following approval by the Northern Sydney Central Coast Health Human Research Ethics Committee (Protocol No. 1011-363M), patients with GBM who had consented to tissue banking and were treated between 2005 and 2010 were selected from the Sydney Neuro-Oncology Group database. All tumors were reviewed by two neuropathologists and classified as grade 4 astrocytoma (GBM), on the basis of the World Health Organization (WHO) classification criteria. A clinical cohort of GBM cases was defined according to guidelines of reporting of tumor marker studies. Cases were excluded if tumor tissue was only available from tumor recurrences or if they had a prior history of a lower-grade brain tumor. To ensure a cohesive clinical cohort, patients were excluded if age at diagnosis was greater than 72 years of age, if they had not completed treatment with 6 weeks of adjuvant temozolomide radiotherapy followed by two cycles of adjuvant temozolomide (18 weeks of therapy) or if they had an Eastern Cooperative Oncology Group (ECOG) score above 2. As part of standard practice in our unit, most patients received dexamethasone at least 24 hours before surgery. Clinical follow-up data were available for all cases, with a census date of 27 March 2013.
DNA and RNA Extraction
DNA for the determination of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation and isocitrate dehydrogenase (IDH) 1/2 mutation status and RNA for quantification of miR-132 levels were extracted from formalin-fixed paraffin-embedded tumor tissue (2 x 10 µM sections per specimen), using the RecoverAll Total Nucleic Acid Isolation Kit (Ambion/Life Technologies Australia Pty Ltd, Mulgrave, Australia), according to the manufacturer's protocol. Following extraction, nucleic acid purity was assessed (A260/A280, Nanodrop ND-1000; Thermo Scientific, Wilmington, DE), with mean A260/A280 values of 1.9 for RNA and 1.8 for DNA obtained.
Determination of MGMT Promoter Methylation Status
Bisulfite conversion of DNA (1 µg) was performed using the EpiTect Bisulfite Kit (Qiagen Pty Ltd Australia, Chadstone Centre, Australia). Pyrosequencing of bisulfite-converted DNA was performed on a PyroMark 24 (Qiagen), using the human MGMT PyroMark CpG Assay (Qiagen) and commercial controls (Epitect, Qiagen) in addition to in-house validated controls.
Determination of IDH1/2 Mutation Status
To determine the mutation status at IDH1 codon R132, which accounts for approximately 90% of IDH1 mutations in glioma [19], DNA was amplified by polymerase chain reaction (PCR), purified using the DNA Clean and Concentrator kit (Zymo Research, Irvine, CA), and commercially sequenced (Australian Genome Research Facility, Westmead, Australia) using primers spanning exon 4 [CATTTGTCTGAAAAACTTTGCTT (forward) and TCACATTATTGCCAACATGAC (reverse); amplicon size, 359 bp]. The mutation status at IDH2 codon R172 was performed as described above, except using primers spanning IDH2 exon 4 [GGTTCAAATTCTGGTTGAAAGATG (forward) and GCTAGGCGAGGAGCTCCAGT (reverse); amplicon size, 289 bp].
Relative Quantification of miR-132
cDNA was synthesized from 10 ng of total RNA from each tumor or a commercial pool of normal brain RNA (Ambion FirstChoice Human Brain Reference Total RNA, a pool of 23 subjects; Life Technologies) using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems/Life Technologies). Relative quantification using TaqMan assays [quantitative reverse transcription-PCR (qRT-PCR); Applied Biosystems] was performed on an AB7900HT real-time PCR instrument (Applied Biosystems). Small nucleolar RNA C/D box 44 (SNORD44, also referred to as RNU44) was chosen as the endogenous normalizer for miR-132 expression because of its low variance between normal brain tissue and tumor irrespective of grade (data not shown) [20]. All assays were performed in triplicate. All procedures were performed according to the manufacturer's instructions.
Statistical Analysis
We examined correlations between miR-132 expression and indicators of clinical outcome using linear regression analysis. Clinical biomarkers were analyzed by Kaplan-Meier survival analysis (using a log-rank test) and by multivariate analysis using a Cox proportional hazards regression model. The prognostic strength of miR-132 was further validated using k-fold validation test, which assessed the susceptibility of the data set to outliers. Statistical analyses were performed using SPSS software version 21 (SPSS Australasia Pty Ltd, Chatswood, Australia). In all cases, a P value of ≤ .05 was regarded as significant.
Results
Characterization of a Clinical Cohort of Patients with GBM
A cohort of 43 patients with primary GBM uniformly treated with concurrent and adjuvant temozolomide therapy was selected for inclusion in this study. The median age at diagnosis was 59 years, and median overall survival was 15 months (Table 1). Our cohort included only patients with a favorable preoperative ECOG score of 0 (53%), 1 (42%), or 2 (5%; Table 2) and who had completed the initial 18 weeks of the Stupp regimen. This provided a homogenous group in terms of diagnosis, treatment, and overall performance suitable for the evaluation of novel prognostic biomarkers. Gross total resection was performed in 91% of cases (Table 2).
Table 1.
Basic Characteristics of the GBM Cohort (n = 43).
| Variable | Range | Median (SD) |
| Overall survival | 6–50 months | 15.0 months (10.7) |
| Age at diagnosis | 27–71 years | 59.0 years (9.8) |
Table 2.
Clinicopathologic Features of the GBM Cohort and Association with Clinical Outcome.
| Variable | Percentage of Cohort | Overall Survival* Median (95% CI) | Significance |
| Age at Diagnosis | 0.026† | ||
| <50 years | 23% | 15 months (5.7–24.3) | |
| 50–65 years | 61% | 17 months (12.0–22.0) | |
| >65 years | 16% | 12 months (5.6–18.4) | |
| Gender | 0.472 | ||
| Male | 77% | 17 months (11.4–22.6) | |
| Female | 23% | 13 months (6.9–19.1) | |
| ECOG score | 0.012† | ||
| 0 | 53% | 19 months (15.5–22.5) | |
| 1 | 42% | 13 months (12.2–13.7) | |
| 2 | 5% | 10 months | |
| Extent of Gross Resection | 0.295 | ||
| Partial | 9% | 22 months (10.2–33.8) | |
| Total | 91% | 15 months (12.3–17.7) | |
| MGMT Methylation | 0.432 | ||
| Unmethylated | 76% | 13 months (10.1–15.9) | |
| Methylated | 24% | 16 months (7.7–24.3) | |
| Status | |||
| Alive | 5% | ||
| Deceased | 95% |
Kaplan-Meier survival analysis results.
Statistically significant.
In this cohort, age at diagnosis (<50, 50–65, and >65 years) was significantly correlated with patient outcome (P = .026), as was ECOG score (P = .012; Table 2). MGMT status and extent of resection were not prognostic. Using DNA extracted from fixed tumor tissue specimens, we determined either IDH1 or 1DH2 mutation status for 91% of cases. No tumors contained a mutation for IDH1 or 1DH2 or had elements of a lower-grade lesion on histopathology, consistent with primary GBM. In three patients (12%), we identified a synonymous variant at IDH1 G105 [single nucleotide polymorphism database identifier (dbSNP ID), rs11554137; catalogue of somatic mutations in cancer identifier (COSMIC ID), COSM253316]. This has been previously reported as a somatic variant in hematopoietic and lymphoid tissue but not in glioma (COSMIC: http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/).
High Expression of miR-132 in GBM Independently Predicts for a Worse Survival Outcome
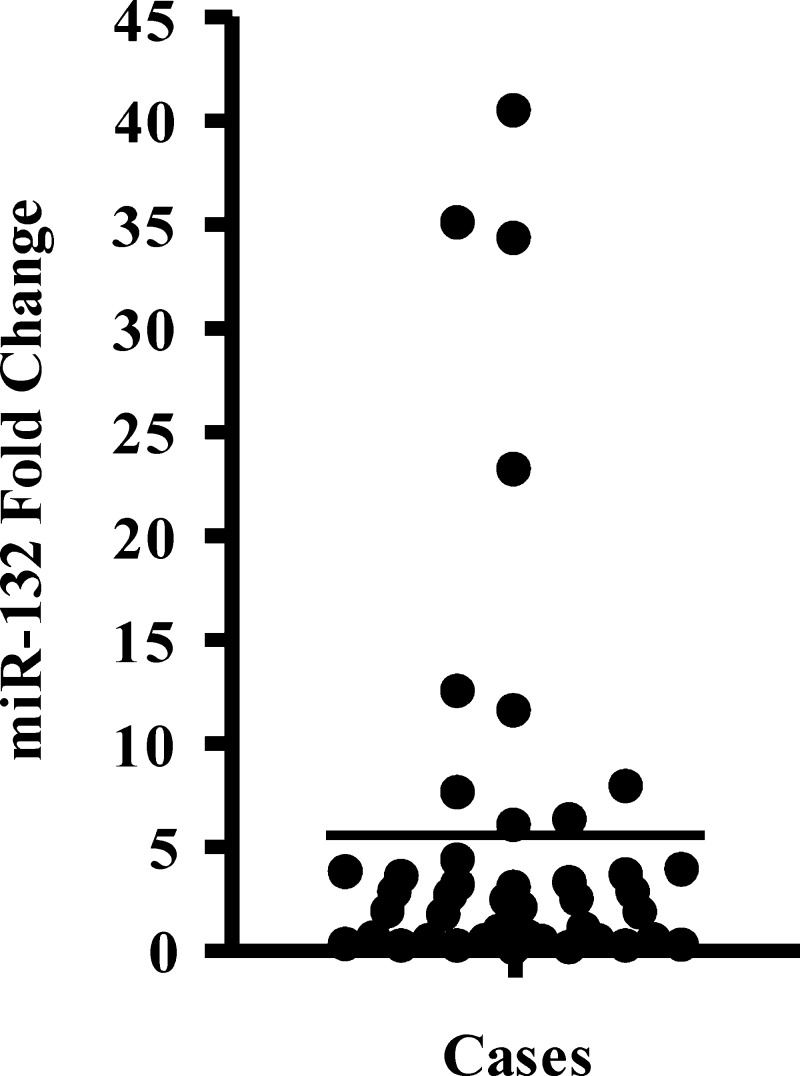
We quantified the expression of miR-132 and the endogenous reference RNU44 by qRT-PCR in tumor RNA from our cohort of patients with GBM and the pooled normal brain RNA. miR-132 (normalized to RNU44) was found to be increased in the GBM cohort, with expression ranging from 0.07- to 40.4-fold increase relative to normal brain RNA and a mean increase of 5.5-fold (Figure 1).
Figure 1.
miR-132 expression in the GBM cohort (n = 43). Individual qRT-PCR results for miR-132 (normalized to RNU44) were plotted as fold change relative to the normal brain control. Mean of miR-132 expression of the GBM cohort (5.5-fold expression) is indicated by the line.
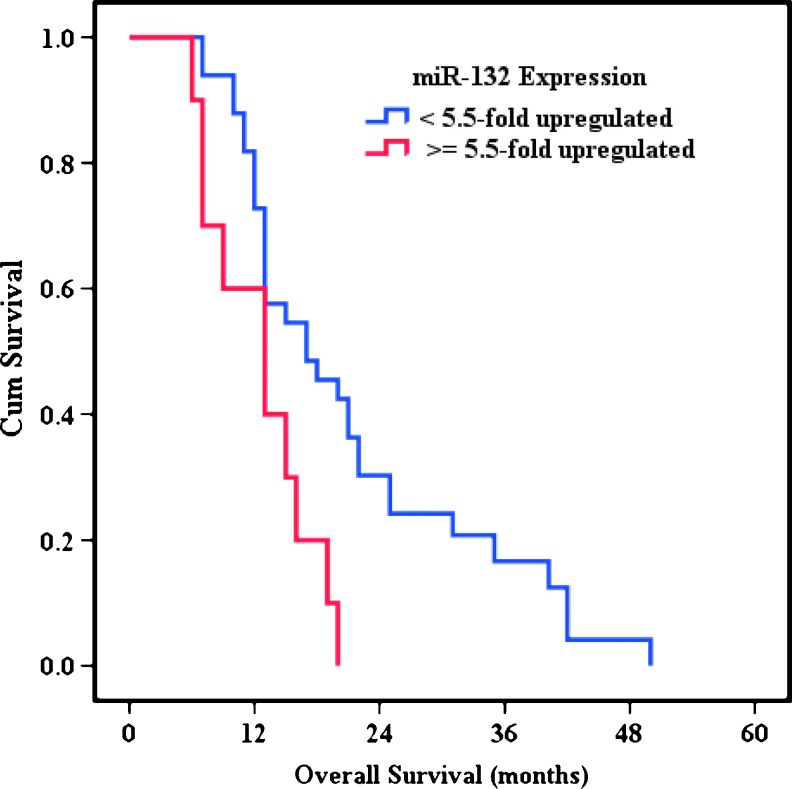
Patients were then dichotomized into two subgroups, using the mean overexpression of miR-132 in the cohort (n = 43 cases) as the threshold. High miR-132 expression (≥5.5-fold up-regulation) was prognostic for a significantly worse survival outcome, with a median overall survival of 13 months [95% confidence interval (CI) = 6.9–19.1, n = 10] versus 17 months (95% CI = 9.1–24.9, n = 33) in the low-expression subgroup (P = .008; Figure 2). A k-fold validation test was performed to examine the possibility that our result was influenced by outliers. This analysis showed that median overall survival months in the high-miR-132 subgroup was 13 months (95% CI = 7.0–16.0) versus 17.5 months (95% CI = 13.0–22.0) in the low-miR-132 subgroup, confirming that miR-132 expression is a robust prognostic marker.
Figure 2.
Kaplan-Meier survival analysis according to miR-132 level. Patients with high miR-132 levels (≥5.5-fold up-regulation; n = 10) had a significantly poorer outcome than those with low miR-132 levels (<5.5-fold up-regulation; n = 33; P = .008).
Linear regression analyses confirmed that miR-132 expression was not related to other markers of clinical outcome, including ECOG score, age at diagnosis, MGMT methylation status, or extent of resection (Table W1). We also performed Cox regression analysis to compare prognostic miR-132 with age at diagnosis and ECOG score, also shown to be prognostic in this cohort. This analysis showed that miR-132 was the strongest prognostic indicator for outcome (P = .01; Table 3). The analysis also revealed that the hazard ratio for patients with a higher miR-132 level was 2.8.
Table 3.
Multivariate Analysis of Prognostic Markers Using a Cox Proportional Hazards Regression Model.
| Prognostic Marker | Regression Coefficient | SE | P Value | Hazard Ratio | 95% CI for Hazard Ratio | |
| Lower | Upper | |||||
| miR-132 mean | 1.044 | 0.407 | .010† | 2.842 | 1.281 | 6.305 |
| Age at diagnosis | 0.340 | 0.292 | .245 | 1.405 | 0.792 | 2.491 |
| ECOG score | 0.670 | 0.312 | .032† | 1.953 | 1.059 | 3.601 |
Performed using 1 df.
Statistically significant.
Discussion
The ability of miRNAs to guide clinical decision making is becoming evident, with more than 100 ongoing trials utilizing miRNAs as biomarkers [8]. miRNA modulation is also being pursued as a novel therapeutic intervention in patients with cancer, with the unique ability to fine-tune the expression of multiple targets/pathways simultaneously, which is of particular relevance to GBM, given its molecular heterogeneity [21]. In this study, we identified miR-132 to be upregulated in a subset of primary GBM tumors and prognostic for a poor outcome in these patients (P = .008). The prognostic value of miR-132 was confirmed by k-fold leave-one-out cross-validation. Furthermore, miR-132 was a stronger indicator for outcome than the established factors age at diagnosis and ECOG score. The prognostic ability of miR-132 was also independent of MGMT methylation status, another established marker of outcome, predictive of sensitivity to alkylating chemotherapy [22] and radiotherapy [23].
miR-132 has been reported to modulate a range of processes within the endothelial, neuronal, and immune compartments (Table 4) [24] with targets involved in neuronal morphogenesis, inflammation and chromatin remodeling, and angiogenesis [12,14–16,18,24–35]. In glioma cells, knockdown of p300, a validated target of miR-132, has been shown to promote invasion [36]. Overexpression of miR-132 increases the excitability of cortical neurons in response to glutamate [34], which is released at excitotoxic levels by glioma cells [37]. This in turn is thought to contribute to the death of peritumoral neurons, facilitating tumor expansion, tumor necrosis, local inflammation, and glioma-related seizures [37]. In support of our findings, a previous study has also shown that levels of miR-132 are high in GBM tumors (n = 12 tumors). However, this study compared levels to oligodendroglial tumors (n = 14 tumors) rather than normal tissue and did not classify GBM tumors on the basis of IDH mutation status [38].
Table 4.
Reports of miR-132 Expression and Function.
| Pathway | Finding | Profiling Method | Validated mRNA Target | References |
| Angiogenesis | • miR-132 expression in HUVEC cells is induced by VEGF and bFGF | qRT-PCR | p250RasGAP | [12] |
| • miR-132 is highly expressed in the endothelium of human breast carcinoma (n = 12 tumors) and hemangiomas (n = 68 tissues) but not in normal endothelium | LNA probes (in situ hybridization) | |||
| • Transfection of HUVEC cells with miR-132 increases their proliferative and tube-forming capacity | ||||
| • Treatment of HUVEC cells with anti-miR-132 significantly decreases VEGF-induced phosphorylation of MEK-1 | ||||
| • Targeted delivery of anti-miR-132 to tumor endothelium (through a liposome carrier and αvβ3 integrin-targeting peptide) suppresses angiogenesis and tumor burden in an orthotopic xenograft model of human breast cancer | ||||
| Immune function/inflammation | • miR-132 is highly upregulated in lymphatic endothelial cells following infection with Kaposi sarcoma-associated herpesvirus and after infection of monocytes with herpes simplex virus and human cytomegalovirus | miRNA microarray and qRT-PCR | p300 | [43] |
| • miR-132 is upregulated in primary human macrophages treated with LPS | Spotted microarray (customized, in-house miRNA microarray) and qRT-PCR | AChE | [15] | |
| • Overexpression of miR-132 through lentiviral infections downregulates acetylcholine activity in primary bone marrow-derived macrophages | ||||
| • miR-132 is one of three miRNAs upregulated in NK cells following prolonged treatment with IL-12 and negatively regulates the IL-12 signaling pathway, modulating NK cell responsiveness to further IL-12 stimulation | qRT-PCR | STAT4 | [28] | |
| • miR-132 is significantly upregulated in IgE-activated human and mouse mast cells | miRNA microarray and qRT-PCR | HB-EGF | [29] | |
| • miR-132 is one of five miRNAs significantly upregulated in peripheral blood mononuclear cells isolated from patients with rheumatoid arthritis (n = 16) compared to controls (n = 4) | qRT-PCR | [35] | ||
| • miR-132 is induced in primary human adipose-derived stem cells following serum deprivation. Overexpression of miR-132 in this cell line promotes an increase in proinflammatory chemokines IL-8 and MCP-1 and promotes NF-κB activation (through an increase in acetylated p65) | qRT-PCR | SIRT1 | [31] | |
| Glial/neuronal signaling | •Overexpression of miR-132 in embryonic stem cells reduces the differentiation of dopamine neurons | qRT-PCR | [16] | |
| • miR-132 is significantly upregulated in cultured cortical neuronal cells in response to BDNF treatment | qRT-PCR | NR2B, NR2A, GluR1* | [14] | |
| • miR-132 is significantly upregulated in cultured astroglial cells in response to bFGF | qRT-PCR | [44] | ||
| • miR-132 expression is significantly upregulated in LβT2 pituitary gonadotrope cells, following treatment with GnRH | qRT-PCR | [45] | ||
| • miR-132 expression is rapidly induced by synaptic activity in hippocampal neurons. miR-132 expression promotes dendrite growth and branching | qRT-PCR | p250RhoGAP | [27] | |
| • miR-132 is required for dendrite maturation in newborn neurons of the adult hippocampus | qRT-PCR | [13] | ||
| Neurologic disease | •miR-132 expression is significantly downregulated in the prefrontal cortex of schizophrenia subjects (n = 35; n = 16) compared to normal health controls (n = 34; n = 15), in two independent cohorts | miRNA microarray and qRT-PCR | DNMT3A, GATA2, DPYSL3 | [18] |
| • miR-132 is one of four miRNAs identified as important regulators of τ exon 10 splicing and is downregulated in sporadic progressive supranuclear palsy dementia cases (n = 8) compared to nondimentia controls (n = 8) | qRT-PCR | PTBP2 | [30] | |
| • miR-132 identified as a regulator of seizure-induced neuronal death. Depletion of hippocampal miR-132 levels using LNA-modified anti-miR-132 oligonucleotides (antagomirs) protected mice against seizure damage, in a mouse model of epileptic tolerance | TaqMan Low Density Array (Applied Biosystem, Foster City, CA) | [46] | ||
| • miR-132 is one of nine miRNAs downregulated in two transgenic mouse models of Huntington disease | miRNA microarray and qRT-PCR | [17] | ||
| Carcinogenesis | • miR-132 is significantly upregulated in PDAC (n = 11), compared to adjacent benign (n = 6) and normal (n = 4) tissue | qRT-PCR | Rb1 | [26] |
| • miR-132 is overexpressed in lung tumor tissue (n = 123) and pancreatic tumor tissue (n = 39) compared to normal control tissue (n = 123 and 12, respectively) | miRNA microarray (customized) | [11] | ||
| • miR-132 was part of a five-miRNA panel distinguishing responders and nonresponders to ifosfamide therapy (n = 27 high-grade osteosarcoma cases), with miR-132 reduced in responders | TaqMan Low Density Array | [47] |
HUVEC indicates human umbilical vein endothelial cell; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; LNA, locked nucleic acid; MEK-1, MAPK/ERK kinase 1; LPS, lipopolysaccharide; AChE, acetylcholinesterase; NK, natural killer; STAT4, signal transducer and activator of transcription 4; IL-12, interleukin-12; HB-EGF, heparin-binding EGF-like growth factor; IL-8, interleukin-8; MCP-1, monocyte chemoattractant protein; NF-κB, nuclear factor-κ-light-chain enhancer of activated B cells; SIRT1, sirtuin 1; BDNF, brain-derived neurotrophic factor; NR2B, glutamate receptor, ionotrophic, N-methyl D-aspartate 2B; NR2A, glutamate receptor, ionotropic, N-methyl D-aspartate 2A, GluR1, glutamate receptor, ionotropic, AMPA 1; GnRH, gonadotropin-releasing hormone; DNMT3A, DNA (cytosine-5-)-methyltransferase 3 α; GATA2, GATA-binding protein 2; DPYSL3, dihydropyrimidinase-like 3; PTBP2, polypyrimidine tract-binding protein 2; PDAC, pancreatic ductal adenocarcinoma; Rb1, retinoblastoma 1.
With the exception of references [11], [15], and [18], microarrays were Agilent technology (Santa Clara, CA).
Indirect targets.
The relevance of miR-132 in glioma is further highlighted by research linking miR-132 levels to current treatment as well as potential targeted therapeutic approaches for this malignancy. Both dexamethasone and extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitors (UO126 and PD98059) have been shown to attenuate miR-132 expression mediated by brain-derived neurotrophic factor (BDNF) in cortical neurons [14]. Dexamethasone is an anti-inflammatory and immunosuppressive glucocorticoid that is used routinely in the treatment of patients with GBM [39]; however, its mechanism of action is not fully understood. Given this relationship between miR-132 and dexamethasone, we cannot rule out the possibility that miR-132 expression was modulated by dexamethasone dose. However, as patients with miR-132 elevation as well as those without would have received dexamethasone before the sample being taken, this should not have influenced the study outcome. miR-132 may also be involved in a positive feedback mechanism with ERK activation. This is of interest as blockade of ERK signaling using mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitors is currently being trialed in glioma [40]. miR-based interventions may also sensitize patients to therapy. In an orthotopic mouse model of GBM, established using patient-derived prominin-1 (CD133) positive cancer stem cells, intracranial delivery of polyethyleneimine encapsulated miR-145 (a tumor-suppressor miRNA) synergized with radiotherapy and temozolomide treatment [41].
Conclusions
In conclusion, we identified that high expression of miR-132 was associated with poor prognosis for primary GBM treated with the Stupp regimen and independent of age of diagnosis, ECOG score, and MGMT methylation. These results in combination with the demonstrated mechanistic links between miR-132 and glioma treatment warrant future evaluation of this biomarker in patients with GBM treated with the Stupp regimen as well as those receiving radiation alone or those with secondary GBM. Therapeutic strategies aimed at modulating miRNA expression hold strong potential and represent just under half of US patents relating to miRNAs [21,42]. New treatments for GBM are urgently needed, and combining miR-132-targeted therapy with temozolomide-based therapy may improve the outcome of these patients.
Supplementary Material
Acknowledgments
The authors thank Sally Fielding for collecting clinical outcome data used in this study, Maggie Lee for technical assistance, and Jillian Patterson for assistance with statistical analyses.
Footnotes
This work was supported by the Sydney Neuro-Oncology Group. The authors declare that they have no competing interests.
This article refers to supplementary material, which is designated by Table W1 and is available online at www.transonc.com.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 4.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 8.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 9.Ren Y, Zhou X, Mei M, Yuan XB, Han L, Wang GX, Jia ZF, Xu P, Pu PY, Kang CS. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer. 2010;10:27. doi: 10.1186/1471-2407-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Y, Kang CS, Yuan XB, Zhou X, Xu P, Han L, Wang GX, Jia Z, Zhong Y, Yu S, et al. Co-delivery of as-miR-21 and 5-FU by poly(amidoamine) dendrimer attenuates human glioma cell growth in vitro. J Biomater Sci Polym Ed. 2010;21:303–314. doi: 10.1163/156856209X415828. [DOI] [PubMed] [Google Scholar]
- 11.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, et al. MicroRNA132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. MicroRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, Kunugi H, Hashido K. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010;165:1301–1311. doi: 10.1016/j.neuroscience.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 15.Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Li T, Wang Y, Tang Y, Cui H, Zhang X, Chen D, Shen N, Le W. miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J Cell Sci. 2012;125:1673–1682. doi: 10.1242/jcs.086421. [DOI] [PubMed] [Google Scholar]
- 17.Lee ST, Chu K, Im WS, Yoon HJ, Im JY, Park JE, Park KH, Jung KH, Lee SK, Kim M, et al. Altered microRNA regulation in Huntington's disease models. Exp Neurol. 2011;227:172–179. doi: 10.1016/j.expneurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Miller BH, Zeier Z, Xi L, Lanz TA, Deng S, Strathmann J, Willoughby D, Kenny PJ, Elsworth JD, Lawrence MS, et al. MicroRNA-132 dysregulation in schizophrenia has implications for both neurodevelopment and adult brain function. Proc Natl Acad Sci USA. 2012;109:3125–3130. doi: 10.1073/pnas.1113793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan Y, Mizoguchi M, Yoshimoto K, Hata N, Shono T, Suzuki SO, Araki Y, Kuga D, Nakamizo A, Amano T, et al. MiRNA-196 is upregulated in glioblastoma but not in anaplastic astrocytoma and has prognostic significance. Clin Cancer Res. 2010;16:4289–4297. doi: 10.1158/1078-0432.CCR-10-0207. [DOI] [PubMed] [Google Scholar]
- 21.Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 2012;18:282–289. doi: 10.1016/j.drudis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 23.Rivera AL, Pelloski CE, Gilbert MR, Colman H, De La Cruz C, Sulman EP, Bekele BN, Aldape KD. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116–121. doi: 10.1093/neuonc/nop020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanet A, Tacheny A, Arnould T, Renard P. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 2012;40:4742–4753. doi: 10.1093/nar/gks151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez-Saavedra M, Antoun G, Yanagiya A, Oliva-Hernandez R, Cornejo-Palma D, Perez-Iratxeta C, Sonenberg N, Cheng HY. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum Mol Genet. 2011;20:731–751. doi: 10.1093/hmg/ddq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JK, Henry JC, Jiang J, Esau C, Gusev Y, Lerner MR, Postier RG, Brackett DJ, Schmittgen TD. miR-132 and miR-212 are increased in pancreatic cancer and target the retinoblastoma tumor suppressor. Biochem Biophys Res Commun. 2011;406:518–523. doi: 10.1016/j.bbrc.2011.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, et al. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA. 2008;105:9093–9098. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Lei Y, Zhang H, Hou L, Zhang M, Dayton AI. Micro-RNA regulation of STAT4 protein expression: rapid and sensitive modulation of IL-12 signaling in human natural killer cells. Blood. 2011;118:6793–6802. doi: 10.1182/blood-2011-05-356162. [DOI] [PubMed] [Google Scholar]
- 29.Molnár V, Érsek B, Wiener Z, Tömböl Z, Szabó PM, Igaz P, Falus A. MicroRNA-132 targets HB-EGF upon IgE-mediated activation in murine and human mast cells. Cell Mol Life Sci. 2012;69:793–808. doi: 10.1007/s00018-011-0786-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PY, Delay C, Girard J, Papon MA, Planel E, Sergeant N, Buée L, Hébert SS. MicroRNA-132 loss is associated with tau exon 10 inclusion in progressive supranuclear palsy. Hum Mol Genet. 2011;20:4016–4024. doi: 10.1093/hmg/ddr330. [DOI] [PubMed] [Google Scholar]
- 31.Strum JC, Johnson JH, Ward J, Xie H, Feild J, Hester A, Alford A, Waters KM. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat Genet. 2010;42:1101–1108. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- 33.Carrillo ED, Escobar Y, González G, Hernández A, Galindo JM, García MC, Sáanchez JA. Posttranscriptional regulation of the β2-subunit of cardiac L-type Ca2+ channels by microRNAs during long-term exposure to isoproterenol in rats. J Cardiovasc Pharmacol. 2011;58:470–478. doi: 10.1097/FJC.0b013e31822a789b. [DOI] [PubMed] [Google Scholar]
- 34.Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, et al. MicroRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54:813–829. doi: 10.1016/j.neuron.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panicker SP, Raychaudhuri B, Sharma P, Tipps R, Mazumdar T, Mal AK, Palomo JM, Vogelbaum MA, Haque SJ. p300- and Myc-mediated regulation of glioblastoma multiforme cell differentiation. Oncotarget. 2010;1:289–303. doi: 10.18632/oncotarget.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Groot J, Sontheimer H. Glutamate and the biology of gliomas. Glia. 2011;59:1181–1189. doi: 10.1002/glia.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lages E, Guttin A, El Atifi M, Ramus C, Ipas H, Dupré I, Rolland D, Salon C, Godfraind C, deFraipont F, et al. MicroRNA and target protein patterns reveal physiopathological features of glioma subtypes. PLoS One. 2011;6:e20600. doi: 10.1371/journal.pone.0020600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 40.Little AS, Smith PD, Cook SJ. Mechanisms of acquired resistance to ERK1/2 pathway inhibitors. Oncogene. 2013;32:1207–1215. doi: 10.1038/onc.2012.160. [DOI] [PubMed] [Google Scholar]
- 41.Yang YP, Chien Y, Chiou GY, Cherng JY, Wang ML, Lo WL, Chang YL, Huang PI, Chen YW, Shih YH, et al. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials. 2012;33:1462–1476. doi: 10.1016/j.biomaterials.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 42.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 43.Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RS, Gotch F, Boshoff C. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat Cell Biol. 2010;12:513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- 44.Numakawa T, Yamamoto N, Chiba S, Richards M, Ooshima Y, Kishi S, Hashido K, Adachi N, Kunugi H. Growth factors stimulate expression of neuronal and glial miR-132. Neurosci Lett. 2011;505:242–247. doi: 10.1016/j.neulet.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Godoy J, Nishimura M, Webster NJ. Gonadotropin-releasing hormone induces miR-132 and miR-212 to regulate cellular morphology and migration in immortalized LβT2 pituitary gonadotrope cells. Mol Endocrinol. 2011;25:810–820. doi: 10.1210/me.2010-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimenez-Mateos EM, Bray I, Sanz-Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T, Saugstad JA, Simon RP, et al. miRNA expression profile after status epilepticus and hippocampal neuroprotection by targeting miR-132. Am J Pathol. 2011;179:2519–2532. doi: 10.1016/j.ajpath.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gougelet A, Pissaloux D, Besse A, Perez J, Duc A, Dutour A, Blay JY, Alberti L. Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int J Cancer. 2010;129:680–690. doi: 10.1002/ijc.25715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.