Abstract
Background
Amphotericin B dexoycholate is currently the standard empirical antifungal therapy for neutropenic patients with hematologic malignancies and who also have persistent fever that does not respond to antibacterial therapy. The antifungal triazoles offer a potentially safer and effective treatment alternative to Amphotericin B dexoycholate.
Methods
We assessed the efficacy and safety of intravenous itraconazole, as compared with the efficacy and safety of amphotericin B deoxycholate, as an empirical antifungal therapeutic agent in a matched case-control clinical trial from June 2004 to August 2005.
Results
Efficacy was evaluated in 96 patients (48 received itraconazole and 48 received amphotericin B deoxycholate) and all the patients who received the study drugs were evaluated for safety. The baseline demographic characteristics were well matched. The overall success rates were 47.9% for itraconazole and 43.8% for amphotericin B deoxycholate (% difference: 4.1% [95% confidence interval for the difference: -15.8 to 24]), which fulfilled the statistical criteria for the non-inferiority of itraconazole. The proportions of patients who survived for at least seven days after discontinuation of therapy or who were prematurely discontinued from the study were not significantly different between the two groups. The rates of breakthrough fungal infections and resolution of fever during neutropenia were similar in both groups. More patients who received amphotericin B deoxycholate developed nephrotoxicity, hypokalemia or infusion-related events than did those patients who received itraconazole (nephrotoxicity: 16.7% vs. 1.8%, hypokalemia: 66.7% vs. 24.6%, and infusion-related events: 41.7% vs. 3.5%, respectively).
Conclusions
Intravenous itraconazole is as effective as amphotericin B deoxycholate and it is generally better tolerated than amphotericin B deoxycholate when it is given as empirical antifungal therapy for Korean patients with persistent neutropenic fever.
Keywords: Itraconazole, Amphotericin B deoxycholate, Empirical antifungal therapy, Persistent febrile neutropenia
INTRODUCTION
The risk of invasive fungal infections (IFI) is greatly increased in patients with profound and protracted neutropenia. Invasive fungal infections are an important cause of morbidity and mortality for patients with neutropenia who have received chemotherapy for cancer. However, the early diagnosis of these infections is difficult and persistent fever may be the only symptom. A delay in treatment, while pursuing the diagnosis, may lead to increased mortality and morbidity1-4). Therefore, the practice of providing empirical antifungal therapy to neutropenic patients with persistent fever, in spite of the administration of broad spectrum antibacterial therapy, has become a standard of care1-4). Aspergillus infection is very problematic for patients with prolonged neutropenia, such as patients who are being treated for acute leukemia and also those who are undergoing hematopoietic stem cell transplantation (HSCT)1, 5). Amphotericin B deoxycholate has been considered the standard first line agent for empirical antifungal therapy, but empirical treatment with amphotericin B dexoxycholate is limited due to its acute toxic effect related to the infusion and the dose-limiting nephrotoxicity4, 6-8). Consequently, less toxic alternatives have been investigated, including lipid formulations of amphotericin B, voriconazole and caspofungin4, 9, 10). However, their high cost makes it difficult to routinely use them in most hospitals. Itraconazole is one of the broad-spectrum triazoles with improved safety and activity against the Aspergillus and Candida species11-13), and it is available as an intravenous formulation and an oral solution. Thus, itraconazole can be an alternative to amphotericin B for empirical therapy in persistently febrile neutropenic patients11).
We investigated the efficacy and safety of intravenous itraconazole, as compared with those of amphotericin B deoxycholate, as a first line empirical antifungal agent for Korean neutropenic patients with persistent fever.
MATERIALS AND METHODS
Study Design
This was a matched case control study conducted from June 2004 to August 2005 at the Catholic HSCT center. All the required institutional review boards approved the protocol.
Patient Enrollment
The patients who were eligible for the itraconazole group were at least 15 years of age, they had received chemotherapy for leukemia, lymphoma, or other hematologic malignancies or they had undergone autologous HSCT; further, they had neutropenia (an absolute neutrophil count [ANC] below 500/mm3) and persistent or recurrent fever (an axillary temperature above 38℃) despite receiving more than 3 days of systemic antibacterial therapy.
Patients were excluded if they had been treated with other investigational drugs, if they were receiving medications known to interact with itraconazole, or if they had history of invasive fungal infections during the previous neutropenic period. We excluded patients with liver dysfunction (an aminotransferase level 5 times or more than the upper limit of normal, a bilirubin or alkaline phosphatase level more than 3 times the upper limit of normal) or renal dysfunction (a serum creatinine level more than 2.5 times the upper limit of normal). Pregnant women and children under 15 years of age were also excluded. The patients who were receiving antifungal prophylaxis with oral itraconazole were excluded as well. The patients were stratified according to the risk of underlying disease. High-risk patients were those who had undergone autologous HSCT or who had relapsed or refractory leukemia.
The eligibility and exclusion criteria for the amphotericin B deoxycholate group were the same as for the itraconazole group. The patients in the amphotericin B deoxycholate group were randomly selected from those who received amphotericin B deoxycholate during the study period, and they were matched for age, gender, risk status, the underlying diagnoses and the treatment modalities of the underlying disease.
Administration of Study Medication
Intravenous itraconazole, 200 mg (Sporanox IV, Janssen Pharmaceutica, Beerse, Belgium) was administered by infusion as a 40% hydroxypropyl-β-cyclodextrin solution in water every 12 hours for the first 48 hours, and this was followed by 200 mg daily from day 3 until defervescence and recovery from neutropenia (ANC > 500/mm3 for at least 3 consecutive days). Amphotericin B deoxycholate (Fungizone, Bristol-Myers Squibb, Princeton, New Jersey, USA) was infused intravenously over 4 hours at a daily dose of 0.5 to 1.5 mg/kg of body weight until defervescence and recovery from neutropenia (ANC > 500/mm3 for at least 3 consecutive days). Infusion-related adverse events were treated with hydrocortisone, antihistamines or antipyretics based on the decision of the clinicians.
Analysis
Efficacy was assessed for the patients who received itraconazole for 3 or more days. The safety and tolerability were assessed for all patients who had received at least one dose of itraconazole.
Efficacy assessments and definition
The primary efficacy endpoint was a favorable overall response, as determined by a five-component endpoint that has been used in previous studies of empirical antifungal therapy4, 9, 10, 14). Treatment was considered successful if all five of the following criteria were met: 1) successful treatment of any baseline fungal infection, 2) the absence of any breakthrough fungal infection during therapy or within 7 days after the completion of therapy, 3) survival for at least 7 days after discontinuation of therapy, 4) resolution of fever (defined as a temperature below 37℃ for at least 2 days) during neutropenia, and 5) no premature discontinuation of the study therapy because of drug-related toxicity or lack of efficacy. Secondary efficacy assessments were made for each component of the primary endpoint. The survival rates of both groups also were assessed.
Invasive fungal infection was defined according to the EORTC/MSG criteria15). Baseline fungal infections were defined as those that developed within the first 24 hours after entry into the study. Breakthrough fungal infections were defined as those that occurred during therapy or within 7 days after completion of therapy4, 9, 10).
Safety assessments
Clinical adverse events were monitored prospectively for the patients who received itraconazole during the study. The patients who were selected for the control group were reviewed for adverse events. Nephrotoxicity and hepatotoxity were defined as an increment of one or more grades above the baseline grade level, based on the Common Toxicity Criteria (CTC) version 2.0., which was published by the National Cancer Institute.
Statistical analysis
The primary analysis was designed to demonstrate whether the outcome of the intravenous itraconazole group was not inferior to that of the amphotericin B deoxycholate group. The efficacy of itraconazole was considered clinically non-inferior to that of amphotericin B deoxycholate if the difference in response rates to the treatment was not greater than 25%. Assuming a 60% response rate in both groups, a sample size of 96 patients (48 patients per group) was required to achieve a study power of 80% at a two-sided significance level of 5%. Thus, a 95 percent confidence interval for the difference in response rates, within 25 percentage points in either direction, was required for intravenous itraconazole to be considered not inferior to amphotericin B deoxycholate. All the statistical tests were interpreted at the 5% significance level. A two-sided 95% confidence interval was calculated where appropriate. The chi-square test or Fisher's exact test was used to compare differences in the proportions. Student's t-test was used for the continuous variables. Kaplan-Meier estimates of survival time were determined and the differences in survival times between the two groups were assessed with a log-rank test at the 5% significance level.
RESULTS
Patient Population
A total of 57 patients received at least one dose of itraconazole and so they were included for the safety analysis. Among the 57 patients in the itraconazole group, 48 were included in the efficacy evaluation. Nine patients were excluded from the efficacy evaluation because all of them received less than 3 days of intravenous itraconazole due to various reasons; 4 of them stopped receiving itraconazole because they had been treated with it before, 2 of them because they had been receiving oral itraconazole as antifungal prophylaxis, 2 of them because they did not meet the definition of persistent neutropenic fever and one patient refused to receive itraconazole. Among the patients who received amphotericin B deoxycholate during the study period and who met the inclusion and exclusion criteria, 48 were randomly selected for the efficacy and safety analysis. The study groups were similar with respect to age, gender, the underlying hematologic diseases, the risk status and treatment modalities for the underlying diseases. Most of the patients received oral fluconazole (100 mg/day), which was used for antifungal prophylaxis (91.7% [n=44] in the itraconazole group and 95.8% [n=46] in the amphotericin B deoxycholate group). Antibacterial therapy, including the use of aminoglycosides and modification of the initial antibiotic therapy, was also similar between the two treatment groups. The SAPS II score, at the time of entry to the study, was similar in the two groups. The median duration of neutropenia was 17 days in the itraconazole group and 15 days in the amphotericin B deoxycholate group; the rate of failure to recover from neutropenia was 8.3% in the former group and 10.3% in the latter group. The median duration of administration of the study drugs was 10 days and 13.5 days for the itraconazole group and the amphotericin B deoxycholate group, respectively. The mean daily dose of amphotericin B was 1.02±0.4 mg/kg. Three patients in the itraconazole group (one with candidemia, one with probable invasive pulmonary aspergillosis and one with probable Aspergillus sinusitis) and two patients in the amphotericin B group (one with probable invasive pulmonary aspergillosis and one with probable Aspergillus sinusitis) had baseline fungal infections within the first 24 hours after entry into the study. The demographics and clinical characteristics are summarized in Table 1.
Table 1.
Demographics and clinical characteristics of patients receiving itraconazole or amphotericin B.
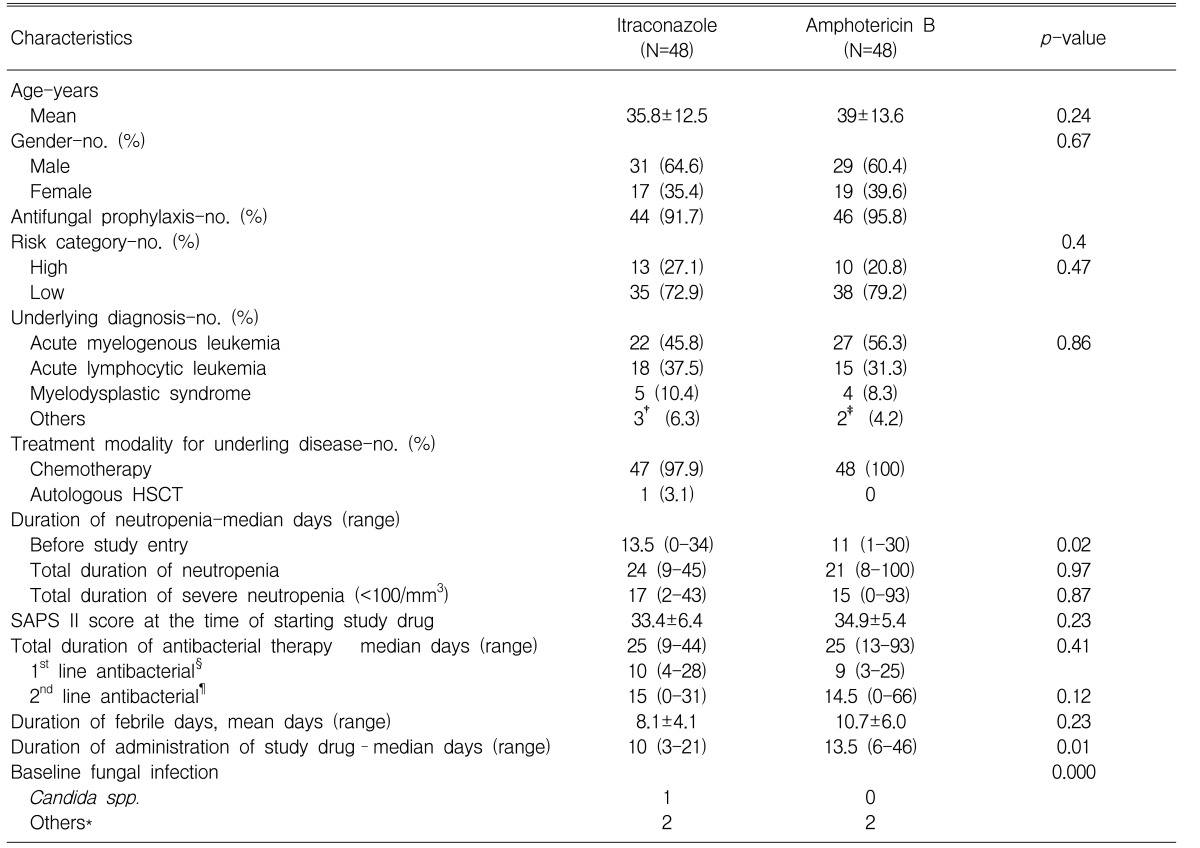
†included 1 case of chronic myelogenous leukemia, 1 case of acute biphenotype leukemia and 1 case of lymphoma.
‡included 1 case of chronic myelogenous leukemia and 1 case of acute biphenotype leukemia.
§1st line antibacterial agents were ceftazidime or cefepime with an aminoglycoside.
¶2nd line antibacterial agents were carbapenem and glycopeptide.
*included probable invasive fungal infections. Refer to the text for more detailed data.
HSCT: hematopoietic stem cell transplantation, SAPS II score: new simplified acute physiology score
Efficacy
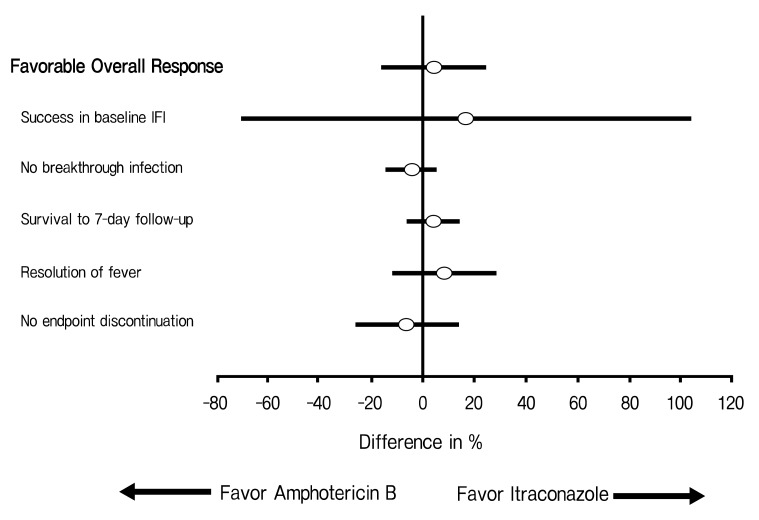
In the analysis of the primary endpoint, 47.9% of the patients who received intravenous itraconazole and 43.8% of the patients who received amphotericin B deoxycholate had a favorable overall response (difference, +4.1 [95% CI, -15.8 to +24.0]) (Table 2); thus, intravenous itraconazole fulfilled the statistical criteria for non-inferiority to amphotericin B deoxycholate. The rates of each component of the primary end point are demonstrated in Table 2 and Figure 1. The rate of a successful outcome among those patients with baseline fungal infections and the rate of occurrence of breakthrough fungal infections were not statistically different between both groups. The breakthrough fungal infections in this study were either proven or probable IFI, when not including the possible IFIs. However, possible IFIs that progressed in spite of the administration of a study drug occurred in 16 patients (33.3%) in the amphotericin B deoxycholate group (33.3%) and in 12 patients (25%) in the itraconazole group. The proportion of patients who survived for at least seven days after therapy and the rates of resolution of fever during the neutropenic period were not different between the two groups. Premature discontinuation of the study drug, either due to toxicity or the lack of efficacy, occurred at similar proportions for both groups. However, more patients in the amphotericin B deoxycholate group discontinued the study drug because of toxicity, and this is in contrast to the itraconazole group in which more patients discontinued the study drug because of the lack of efficacy.
Table 2.
Outcomes of empirical antifungal therapy
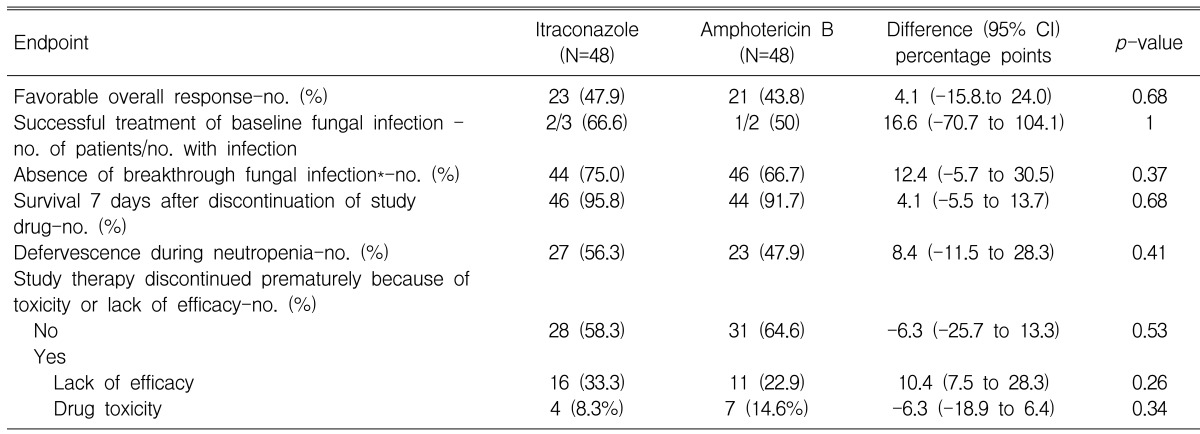
*Breakthrough fungal infections were one case of disseminated aspergillosis (brain and lung), one case of cerebral aspergillosis and two cases of invasive pulmonary aspergillosis (IPA) in the itraconazole group, and two cases of IPA in the amphotericin B deoxycholate group.
Figure 1.
Differences between the treatment groups for the rate of overall response and the components of the primary endpoint.
Safety and Toxicity
Overall, the patients tolerated itraconazole better than amphotericin B. Fewer itraconazole recipients had drug-related adverse events and had to withdraw from treatment because of toxicity, although this was not statistically significant. Infusionrelated adverse events, nephrotoxicity and hypokalemia occurred much more frequently and with greater severity in those patients who received amphotericin B deoxycholate than in those patients who received itraconazole. There was no significant difference between the two groups for the occurrence of hepatotoxicity. Nearly 41% of the patients who received amphotericin B deoxycholate experienced infusion-related events, in contrast to only 3.5% of the patients who received itraconazole. The most common infusion-related events in the amphotericin B deoxycholate group were fever and chills. Eight patients in the amphotericin B deoxycholate group had nephrotoxicity, and three of them had nephrotoxicity of more than two grades above the baseline. In contrast, only one patient in the itraconazole group experienced nephrotoxicity, which was one grade above the baseline. The safety and toxicity results are summarized in Table 3.
Table 3.
Safety and toxicity of empirical treatment with itraconazole and amphotericin B deoxycholate
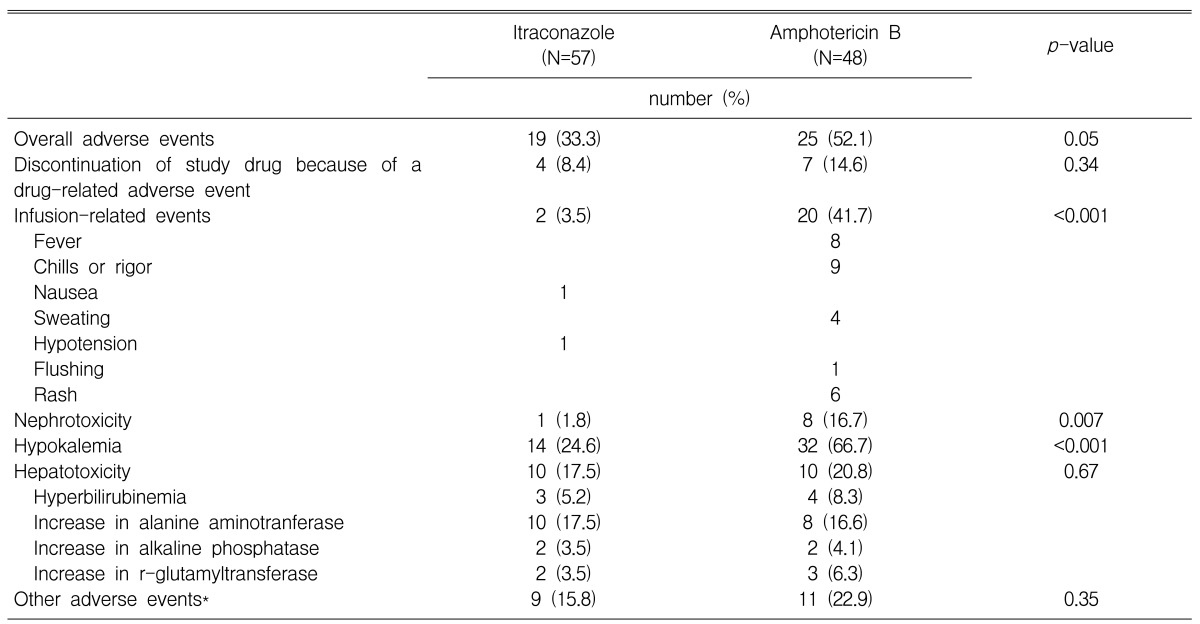
*included 2 cases of drug fever and 7 cases of skin rash in the itraconazole group; and 7 cases of drug fever and 4 cases of skin rash in the amphotericin B deoxycholate group.
Mortality
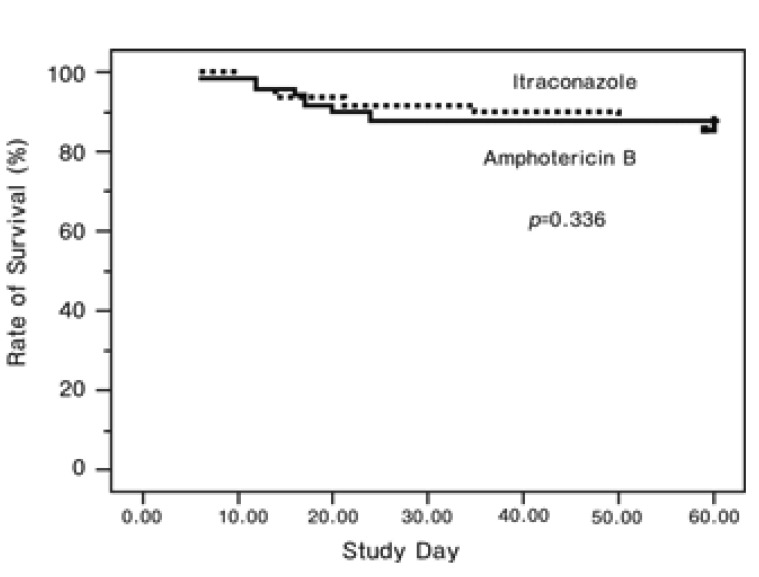
There was no significant difference in overall mortality between the itraconazole and amphotericin B deoxycholate groups. The Kaplan-Meier analysis demonstrated that the survival rates after 60 days follow-up after entry into the study were similar between the two groups (Figure 2). The causes of death in the itraconazole group were two cases of pneumonia (possible IFI), one case of pneumonia and brain abscess (probable IFI), and one case of septic shock. The causes of death in the amphotericin B deoxycholate group were six cases of pneumonia (possible IFI) and one case of typhlitis and septic shock.
Figure 2.
The Kaplan-Meier curves demonstrate the rate of survival after therapy according to the treatment group.
DISCUSSION
This study demonstrated that the efficacy of intravenous itraconazole was similar to that of amphotericin B deoxycholate as a first-line empirical antifungal therapy for patients with persistent fever and neutropenia after chemotherapy or autologous HSCT for treating hematologic diseases. The rate of a favorable overall response was 47.9% for empirical therapy with itraconazole compared with 43.8% for empirical therapy with amphotericin B deoxycholate. The number of breakthrough infections and the frequency of premature discontinuation of the study drug because of a lack of efficacy were not statistically different between the itraconazole group and the amphotericin B deoxycholate group. Four patients treated with itraconazole and two patients treated with amphotericin B deoxycholate had breakthrough fungal infections, and all of these were considered as either probable or proven IFI. However, in the clinical setting, the possible IFI that progressed in spite of administration of a study drug, but that was not included as breakthrough infections in this study, occurred more frequently in the amphotericin B deoxycholate group (n=16, 33.3%) than in the itraconazole group (n=12, 25%). Therefore, in terms of preventing IFI, both drugs appeared to have a similar effect. Although rates of defervescence were similar in both groups, four itraconazole recipients were changed to an alternative systemic antifungal therapy because of persistent fever, and their responses were considered as a lack of efficacy. In contrast, none of the patients treated with amphotericin B deoxycholate were changed to an alternative antifungal therapy because of persistent fever. Since this was not a blind trial, the more frequent changes of therapy in the itraconazole group may have been due to the lack of experience by the physicians with a new approach to empirical therapy. This could explain why the duration of administration of the study drug was significantly longer in the amphotericin B deoxycholate group than in the itraconazole group. The rates of successful treatment of baseline fungal infections were not statistically different between the two groups. However, the number of baseline fungal infections was too small to compare the efficacy. It should be taken into account that one case with Candida inconspicua blood stream infection did not respond to itraconazole; moreover there is an increasing incidence of infections due to non-albicans Candida spp. that are resistant to triazole, which could limit the use of itraconazole as an empirical antifungal agent in patients with persistent febrile neutropenia16). The rates for resolution of fever during neutropenic periods were similar between the two groups. There was no significant difference in the survival rates over seven days after discontinuation of the study drugs between the two groups, as well as the 60-day survival rates after entry into the trial.
Previous studies have demonstrated that amphotericin B deoxycholate was associated with several dose-limiting toxicities. Infusion-related toxicity, including chills or rigor, fever and cardiovascular events, were the most common limitations for the empirical use of amphotericin B dexoycholate. Nephrotoxicity had been reported in previous studies to occur in an average of 27% of cases (range: 1% to 55%) and hypokalemia occurred in approximately 18% (range: 4% to 42%)3, 4, 17-19). The development of serious metabolic toxicities prevented effective dosing and necessitated treatment withdrawal altogether. Such toxic events have been reported less frequently, but they were not eliminated, even when lipid-based formulations of amphotericin B deoxycholate were used4, 9, 18, 19). In this study, itraconazole was superior to amphotericin B deoxycholate with respect to safety and tolerability. In particular, the number of patients who experienced infusion-related toxicity and nephrotoxicity was considerably less in the itraconazole group than in the amphotericin B deoxycholate group. Hepatotoxicity, which is known to be the most common toxicity caused by itraconazole20), occurred in similar proportions of both groups.
The limitation of this study was that it was not a randomized or blinded clinical trial, and it included a relatively small number of patients. There might have been a possible selection bias in choosing patients for the control group, even though their clinical characteristics were matched to those of both study groups. Consequently, evaluating the treatment responses and adverse events might have been biased. This trial did not include the pharmacokinetic data of intravenous itraconazole for the patients with neutropenia. Plasma itraconazole concentrations greater than 250 ng/mL are known to be active against most itraconazole-susceptible fungi21). Although an intravenous infusion of itraconazole circumvents the problems of bioavailability and poor compliance, it cannot influence the cytochrome P450-determined rates of metabolism22). Due to the inter-patient variations of metabolism, it was difficult to determine whether or not an inappropriate blood level of itraconazole would lead to treatment failure. As a result, we are planning to evaluate the pharmacokinetic data of intravenous itraconazole in neutropenic patients. The first and most important shortcoming of itraconazole, as well as for most azoles, remains the potential for hazardous interactions with other drugs, especially in those patients who are receiving chemotherapy (e.g. vincristine and cyclophosphamide) and transplant recipients (e.g. cyclosporine A and tacrolimus)22-24). Second, the spectrum of antifungal drugs remains suboptimal, especially when considering the growing diversity of offending species such as Fusarium spp., Scedosporium spp. and the Zygomycetes.
In conclusion, this study demonstrated that intravenous itraconazole is an appropriate alternative to amphotericin B deoxycholate for empirical antifungal therapy in neutropenic Korean patients with persistent fever, despite the administration of broad-spectrum antibacterial therapy. In terms of toxicity and safety, intravenous itraconazole has potential advantages over amphotericin B deoxycholate. However, drug interactions should be carefully considered before the use of intravenous itraconazole, and it's appropriate to change to a different agent in the empirical setting for patients receiving prophylactic oral itraconazole.
References
- 1.Wingard JR. Empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis. 2004;39(Suppl 1):S38–S43. doi: 10.1086/383052. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA. Empirical antifungal therapy: new options, new tradeoffs. N Engl J Med. 2002;346:278–280. doi: 10.1056/NEJM200201243460410. [DOI] [PubMed] [Google Scholar]
- 3.Klastersky J. Empirical antifungal therapy. Int J Antimicrob Agents. 2004;23:105–112. doi: 10.1016/j.ijantimicag.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Holcenberg JS. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia: National Institute for Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999;340:764–771. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 5.Yoo JH, Choi JH, Lee DG, Choi SM, Shin WS, Kim CC. Analysis of invasive fungal infection after hematopoietic stem cell transplantation or chemotherapy in patients with hematologic diseases. Infect Chemother. 2004;36:40–45. [Google Scholar]
- 6.Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am J Med. 1982;72:101–111. doi: 10.1016/0002-9343(82)90594-0. [DOI] [PubMed] [Google Scholar]
- 7.The EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. Am J Med. 1989;86:668–672. doi: 10.1016/0002-9343(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 8.Karp JE, Merz WG, Charache P. Response to empiric amphotericin B during antileukemic therapy: induced granulocytopenia. Rev Infect Dis. 1991;13:592–599. doi: 10.1093/clinids/13.4.592. [DOI] [PubMed] [Google Scholar]
- 9.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, Yanovich S, Stiff P, Greenberg R, Donowitz G, Schuster M, Reboli A, Wingard J, Arndt C, Reinhardt J, Hadley S, Finberg R, Laverdiere M, Perfect J, Garber G, Fioritoni G, Anaissie E, Lee J. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225–234. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 10.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, Cornely OA, Bourque MR, Lupinacci RJ, Sable CA, dePauw BE. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 11.Boogaerts M, Winston DJ, Bow EJ, Garber G, Reboli AC, Schwarer AP, Novitzky N, Boehme A, Chwetzoff E, De Beule K. Intravenous and oral itraconazole versus intravenous amphotericin B deoxycholate as empirical antifungal therapy for persistent fever in neutropenic patients with cancer who are receiving broad-spectrum antibacterial therapy: a randomized, controlled trial. Ann Intern Med. 2001;135:412–422. doi: 10.7326/0003-4819-135-6-200109180-00010. [DOI] [PubMed] [Google Scholar]
- 12.Manavathu EK, Cutright JL, Chandrasekar PH. Organism-dependent fungicidal activities of azoles. Antimicrob Agents Chemother. 1998;42:3018–3021. doi: 10.1128/aac.42.11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barchiesi F, Colombo AL, McGough DA, Fothergill AW, Rinaldi MG. In vitro activity of itraconazole against fluconazole-susceptible and -resistant Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:1530–1533. doi: 10.1128/aac.38.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett JE, Powers J, Walsh T, Viscoli C, de Pauw B, Dismukes W, Galgiani J, Glauser M, Herbrecht R, Kauffman C, Lee J, Pappas P, Rex J, Verweij P. Forum report: issues in clinical trials of empirical antifungal therapy in treating febrile neutropenic patients. Clin Infect Dis. 2003;36(Suppl 3):S117–S122. doi: 10.1086/367839. [DOI] [PubMed] [Google Scholar]
- 15.Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F, Denning DW, Donnelly JP, Edwards JE, Erjavec Z, Fiere D, Lortholary O, Maertens J, Meis JF, Patterson TF, Ritter J, Selleslag D, Shah PD, Stevens DA, Walsh TJ. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller MA, Diekemal DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. Am J Med. 2000;108:282–289. doi: 10.1016/s0002-9343(99)00457-x. [DOI] [PubMed] [Google Scholar]
- 18.White MH, Bowden RA, Sandler ES, Graham ML, Noskin GA, Wingard JR, Goldman M, van Burik JA, McCabe A, Lin JS, Gurwith M, Miller CB. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia. Clin Infect Dis. 1998;27:296–302. doi: 10.1086/514672. [DOI] [PubMed] [Google Scholar]
- 19.Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia: L Amph/ABLC Collaborative Study Group. Clin Infect Dis. 2000;31:1155–1163. doi: 10.1086/317451. [DOI] [PubMed] [Google Scholar]
- 20.Tucker RM, Haq Y, Denning DW, Stevens DA. Adverse events associated with itraconazole in 189 patients on chronic therapy. J Antimicrob Chemother. 1990;26:561–566. doi: 10.1093/jac/26.4.561. [DOI] [PubMed] [Google Scholar]
- 21.Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, Lancaster M, Odds FC, Rinaldi MG, Walsh TJ, Barry AL. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on antifungal susceptibility testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 22.Prentice AG, Glasmacher A. Making sense of itraconazole pharmacokinetics. J Antimicrob Chemother. 2005;56(Suppl 1):i17–i22. doi: 10.1093/jac/dki220. [DOI] [PubMed] [Google Scholar]
- 23.Maertens J, Boogaerts M. The place for itraconazole in treatment. J Antimicrob Chemother. 2005;56(Suppl 1):i33–i38. doi: 10.1093/jac/dki222. [DOI] [PubMed] [Google Scholar]
- 24.Marr KA, Leisenring W, Crippa F, Slattery JT, Corey L, Boeckh M, McDonald GB. Cyclophosphamide metabolism is affected by azole antifungals. Blood. 2004;103:1557–1559. doi: 10.1182/blood-2003-07-2512. [DOI] [PubMed] [Google Scholar]