Abstract
Composite adrenal medullary tumors, composed of both pheochromocytoma and ganglioneuroma, are extremely rare, as are pheochromocytomas masquerading as acute relapsing pancreatitis. We recently experienced a case of a 48-year-old male with both these phenomena. The patient complained of an acute onset of intense abdominal discomfort. At the same time, pancreatic enzymes were increased in concentration. An abdominal computed tomographic scan revealed an enlarged pancreas and a 3-cm left adrenal incidentaloma. Biochemical and 131I-MIBG scintigraphic findings were compatible with a pheochromocytoma. Yet, he had no clinical manifestations suggesting pheochromocytoma. An adrenalectomy was performed and a composite adrenal medullary tumor of pheochromocytoma and ganglioneuroma was confirmed during a pathologic examination. This case illustrates two points: 1) acute abdominal intense discomfort and hyperamylasemia may be unusual presentations of pheochromocytomas; and 2) the possibility of the pheochromocytoma, albeit rare, should be considered when a relapsing pancreatitis of uncertain etiology develops.
Keywords: Adrenal gland neoplasms, Pheochromocytoma, Ganglioneuroma, Hyperamylasemia, Pancreatitis
INTRODUCTION
On rare occasions, pheochromocytoma and ganglioneuroma develop together within a single adrenal medullary tumor1-3). It is apparent that composite tumors of that type may display symptoms referable to hormonal hypersecretion by either portion of the tumor1). Yet, pheochromocytomas commonly act in the typical manner and presented as cardiomyopathy4-6) or pancreatitis7-11) have been rarely documented. The non-specific nature of its manifestations may render prompt recognition elusive12). We recently experienced a case of a composite adrenal medullary tumor of pheochromocytoma and ganglioneuroma that was incidentally discovered and masquerading as acute relapsing pancreatitis. To our knowledge, it may be the first case in medical literature.
CASE REPORT
A 48-year-old male was referred to our endocrinologic clinic for the evaluation of an adrenal incidentaloma. Two months ago, he had been admitted to our gastroenterologic clinic at which time he complained of acute, severe intense abdominal discomfort. He claimed that the discomfort had a rapid onset reaching a maximum in 10 to 20 minutes. Further, he had band-like radiation on/to the back. He consumed small amounts of alcohol but had no significant medical and family history. Laboratory data revealed elevated serum pancreatic amylase and lipase levels (reference range shown parenthetically) of 98.8 U/L (8~53) and 603.4 U/L (0~60), respectively. A diagnosis of acute alcoholic pancreatitis was suggested. He was managed with conservative treatments including restriction of caloric intake. Ten days later, he was discharged with significant clinical improvements.
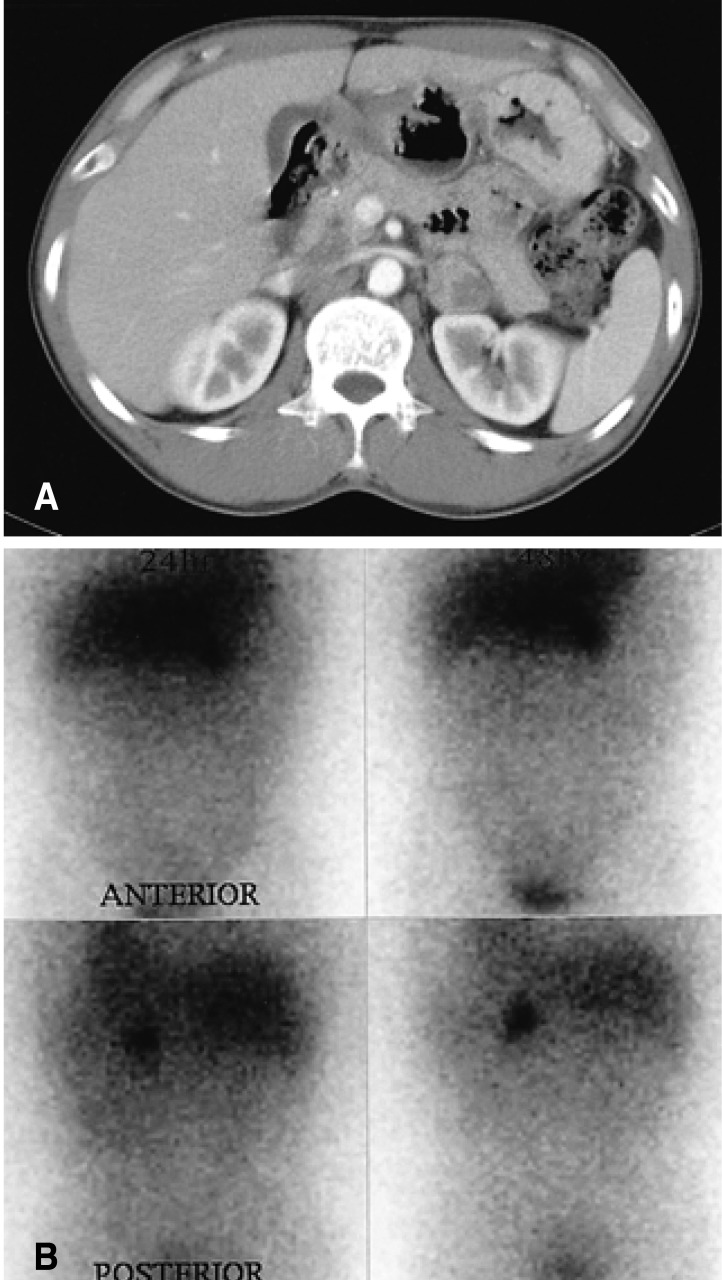
However, seven days after he was discharged, the same symptoms and signs re-developed at which time he also described palpitation, facial flushing and resting hand tremor in the previous five months that were developed slightly, briefly and occasionally. On admission, the patient's body temperature was 36.6℃ while blood pressure was 110/70 mmHg. The pulse rate was 72 beats/minute and the respiratory rate was 19/minute. His height and body weight were 158 cm and 52 kg, respectively. The physical examination was unremarkable except for direct tenderness in the epigastric area. Laboratory data revealed normal blood cell counts, plasma glucose and serum electrolyte levels. The results of liver and renal function tests were also within the normal ranges. However, serum pancreatic amylase and lipase levels were elevated to 81.4 U/L (8~53) and 100.9 U/L (0~60), respectively. The serum calcium level was 2.22 mmol/L (2.15~2.50) and triglyceride level was 0.66 mmol/L (<2.83). The serum CPK-MB (creatinine phosphokinase-MB isoform) level was 1.8 µg/L (0.7~3.8) and the troponin-I level was 0.3 µg/L (0~0.5). The findings of a chest roentgenogram, electrocardiogram, and 24-hour holter monitor showed nothing signigficant. Echocardiographic findings were normal except for mild concentric left ventricular hypertrophy. An abdominal computed tomographic scan showed a mildly enlarged pancreatic change without abnormal fluid collection and necrosis. In addition, he showed a left adrenal mass, 3×3 cm in diameter, which was heterogeneously low attenuated and enhanced after intravenous contrast administration (Figure 1A).
Figure 1.
(A) Abdominal computed tomographic scan shows a mildly enlarged pancreatic change and a left adrenal mass, 33 cm in diameter, which was heterogeneously low attenuated and enhanced after intravenous contrast administration. (B) 131I-MIBG scintigraphic findings shows marked uptake in a single location corresponding to the left adrenal mass.
About one month after the second discharge, we performed medical tests for discriminating the functional status of the adrenal incidentaloma. The urinary metanephrine level was elevated to 24.7 µmol/24h (0.5~8.1) and the urinary VMA (vanillylmandelic acid) level was normal at 27.5 µmol/24h (7~33). The results of plasma catecholamines, baseline plasma ACTH and cortisol, urinary-free cortisol, uplight plasma renin activity and aldosterone and serum DHEA-S (dehydroepiandrosterone sulfate) levels were also within normal ranges. The results of 5-HIAA (5-hydroxyindoleacetic acid) and thyroid function tests were also normal. 131I-MIBG (Metaiodobenzyl guanidine) scintigraphy was performed for the confirmation of the provisional diagnosis and localization of additional extra-adrenal tumors. 131I-MIBG scintigraphic findings showed a marked uptake in a single location corresponding to the left adrenal mass (Figure 1B). Ultrasonographic findings of the neck were unremarkable and serum intact PTH and calcitonin levels were 14.39 pg/mL (15~65) and 1.4 ng/mL (0~10), respectively. Therefore, we could exclude pheochromocytoma from familial association of multiple endocrine neoplasia type II. An -adrenoceptor blocker (doxazocin mesylate 4mg qd for 11 days) was prescribed preoperatively to prevent an operative adrenal crisis. Then, the left adrenal gland was surgically removed trans-abdominally through a subcostal incision.
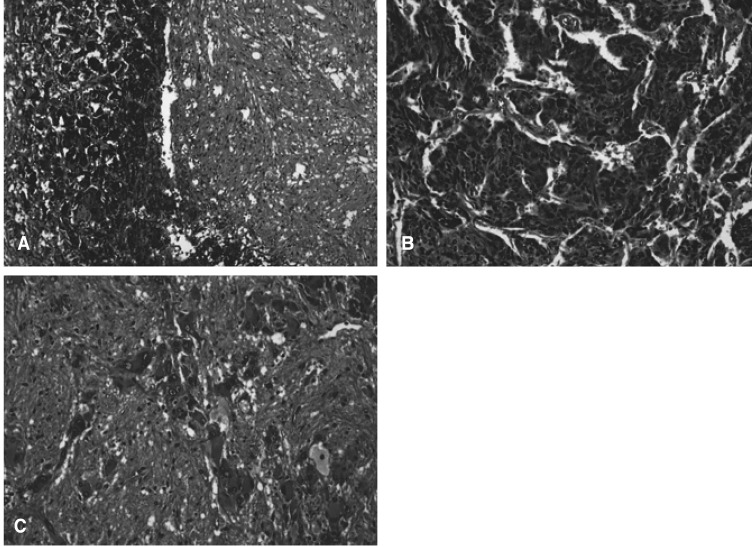
On pathologic examination, a composite adrenal medullary tumor of pheochromocytoma and ganglioneuroma was confirmed. On gross examination, a well-defined solid tumor measuring 2.8×2.5×2.5 cm was found within the adrenal gland and cut surface of the tumor was yellowish brown. The microscopic appearance of the tumor showed an abrupt transition of two different patterns of typical pheochromocytoma and a large area of ganglioneuroma (Figure 2A). In the pheochromocytoma area, organoid nests, called "Zellballen" pattern, of polygonal cells surrounded by fibrovascular stroma were observed. Each cell had granular basophilic or amphophilic cytoplasm and intracytoplasmic hyaline globules, positively stained by PAS (periodic acid-Schiff's reagent). Its round or oval nuclei had a prominent nucleoli which contained inclusion-like structures and showed moderate pleomorphism with hyperchromasia (Figure 2B). At the ganglioneuroma area, uncapsulated clusters of fully matured ganglion cells surrounded by fascicles of spindle (Schwann-like) cells were shown (Figure 2C). On immunohistochemical staining, it was weakly positive for NSE (neuron-specific enolase), chromogranin and synaptophysin, negative for HMB45 and CK in the pheochromocytoma component, and positive for S-100 protein in the ganglioneuroma component. The patient tolerated the operation well and had an smooth recovery. He was discharged on the 7th post-operative day. After surgery, there were no episodes of intense abdominal discomfort. Further, palpitation, facial flushing and hand tremor were resolved. Now, the patient has remained well and free of any symptoms for more than eight months.
Figure 2.
(A) Light micrograph of the tumor showed abrupt transition of two different patterns of typical pheochromocytoma and large area of ganglioneuroma (H&E stain, ×40). (B) Light micrograph of the pheochromocytoma component. Organoid nest called "Zellballen" pattern, granular basophilic or amphophilic cytoplasm, intracytoplasmic hyaline globules, and round or oval nuclei with prominent nucleoli are noted (H&E, ×400). (C) Light micrograph of the ganglioneuroma component. Uncapsulated clusters of fully matured ganglion cells surrounded by fascicles of spindle (Schwann-like) cells are noted (H&E stain, ×100).
DISCUSSION
Although pheochromocytoma may occur at multiple sites and in association with a number of other tumors, the presence of both pheochromocytoma and ganglioneuroma within a single tumor is extremely rare. Only a few cases have been described previously1-3). While pheochromocytoma is a tumor that originated from the adrenal medullary chromaffin cells, ganglioneuroma represents a tumor from autonomic ganglion cells or their precursors. Embriologically, both chromaffin and ganglion cells are derived from neural crest cells and migrate to somatic areas13). It is apparent that a composite tumor of pheochromocytoma and ganglioneuroma may display symptoms referable to hormonal hypersecretion by either portion of the tumor1), and Moore et al.2) described hormonal hypersecretion in approximately three-fourths of the reported cases. Clinically active pheochromocytoma may produce the classic symptoms of headache, palpitation, and excessive perspiration in 50% of the cases. In addition, hypertension, either sustained or paroxysmal, is the cardinal feature of pheochromocytoma14). In our case, while the 24-hour urinary metanephrine level was elevated to 24.7 mol/24h (reference range, 0.5~8.1), no definite manifestations referable to catecholamine hypersecretion were identified prior to diagnosis. In other words, the patient had no hypertension or any classic symptoms of pheochromocytoma. He only complained of palpitation, facial flushing and hand tremor which developed only slightly, briefly and occasionally. Further, there were no episodes during the hospitalized days. Reviews have placed frequency of hypertension at 72.4%, with that of sustained hypertension at only 47.9% in pheochromocytomas12), and Moore et al.2) noted that only four of the 13 patients had associated hypertension in composite adrenal medullary tumors. The reason for a lack of endocrine abnormalities and symptoms of pheochromocytoma component in some composite adrenal tumors has not been shown conclusively. One possible hypothesis is the autoregulation of the pheochromocytoma cells by the ganglion cells in the ganglioneuroma component15).
Pheochromocytomas commonly do not behave in the classic manner, which may render prompt recognition elusive12). The signs and symptoms are often absent and can be unusually presented as catecholamine-induced cardiomyopathy4-6) or hyperamylasemia7-11). In review of the literature, the amylase was almost exclusively of the S-type in the pheochromocytoma- associated hyperamylasemia. Moreover, the pancreas itself was morphologically normal on image or surgical locations and lipase was within the normal range. In general, hyperamylasemia was probably caused by tumor production or ischemic injury to amylase containing tissue. In pheochromocytoma, the source of hyperamylasemia was thought to be pulmonary endotherial cells under ischemic damage caused by a potent vasoconstrictive action of circulating catecholamines16). According to the theory of Perrier el al.9), functioning pheochromocytoma causes a catecholamine-induced cardiomyopathy, which contributes to the failure of the left ventricle, followed by pulmonary edema and release of amylase from hypoxic lung tissue. However, in our case, there was no clinical evidence of cardiomyopathy or myocardial ischemia based on electrocardiographic, echocardiographic findings and cardiac biochemical marker, and the clinical pictures of the patient suggested acute pancreatitis. The provisional diagnosis seemed reasonable because of the acute onset of abdominal intense discomfort, elevated serum pancreatic enzyme levels and a mildly enlarged pancreas on image.
We did not perform an analysis of amylase isoenzyme by the electrophoretic method, but pancreatic amylase, not total amylase, was assayed by a enzymatic photometric test, which is based on the method of monoclonal antibodies to inhibit the salivary enzyme and which distinguish pancreatic and salivary amylase sufficiently17, 18). Therefore, it could be suggested that the pancreatitis was caused by the pheochromocytoma in our case. The mechanism of this was considered to be ischemic damage of the pancreas itself by vasoconstrictive action of circulating catecholamines16). The second suggested mechanism was dysfunction of the sphincter of Oddi caused by adrenergic action of circulating catecholamines. This possibility was low because the sphincter of Oddi was relaxed by adrenergic stimulation in general19). However, paradoxical contraction by down regulation of adrenoceptors was as probable as circulatory collapse or hypotension seen in pheochromocytoma.
In conclusion, pheochromocytoma can be present in a variety ways without hypertension and classic symptoms. Therefore, it is vital to recognize the rare presentations of pheochromocytoma to avoid an unsuspected lethal course. We recently experienced a case of 48-year-old male with incidentally discovered a composite adrenal medullary tumor of pheochromocytoma and ganglioneuroma that was manifested as catecholamine-induced relapsing pancreatitis without hypertension. Herein, we described the case with the review of the literature.
References
- 1.Kragel PJ, Johnston CA. Pheochromocytoma-ganglioneuroma of the adrenal. Arch Pathol Lab Med. 1985;109:470–472. [PubMed] [Google Scholar]
- 2.Moore PJ, Biggs PJ. Compound adrenal medullary tumor. South Med J. 1995;88:475–478. doi: 10.1097/00007611-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Okumi M, Matsuoka Y, Tsukikawa M, Fujimoto N, Sagawa S, Itoh K. A compound tumor in the adrenal medulla-pheochromocytoma combined with ganglioneuroma: a case report. Hinyokika Kiyo. 2000;46:887–890. [PubMed] [Google Scholar]
- 4.Gilsanz FJ, Luengo C, Conejero P, Peral P, Avello F. Cardiomyopathy and phaeochromocytoma. Anaesthesia. 1983;38:888–891. doi: 10.1111/j.1365-2044.1983.tb12258.x. [DOI] [PubMed] [Google Scholar]
- 5.Attar MN, Moulik PK, Salem GD, Rose EL, Khaleeli AA. Phaeochromocytoma presenting as dilated cardiomyopathy. Int J Clin Pract. 2003;57:547–548. [PubMed] [Google Scholar]
- 6.Mootha VK, Feldman J, Mannting F, Winters GL, Johnson W. Pheochromocytoma-induced cardiomyopathy. Circulation. 2000;102:E11–E13. doi: 10.1161/01.cir.102.1.e11. [DOI] [PubMed] [Google Scholar]
- 7.Kwon JH, Chang KY, Moon SJ, Hong SI, Shin WS, Jeon HK, Seung KB. A case of pheochromocytoma with acute pancreatitis and catecholamine-induced cardiomyopathy. Korean J Med. 2004;67:S767–S770. [Google Scholar]
- 8.Kim SY, Kim JH, Kim CH, Nam SW, Kim YJ, Kim JI, Park SH, Han JY, Kim JG, Jeong GW, Sun HS. A case of pheochromocytoma with hyperamylasemia. Korean J Gastroenterol. 2003;42:172–175. [PubMed] [Google Scholar]
- 9.Perrier NA, van Heerden JA, Wilson DJ, Warner MA. Malignant pheochromocytoma masquerading as acute pancreatitis: a rare but potentially lethal occurrence. Mayo Clin Proc. 1994;69:366–370. doi: 10.1016/s0025-6196(12)62222-8. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ, Miller RF, Webb AR, Russell RC. Phaeochromocytoma presenting as acute hyperamylasaemia and multiple organ failure. Can J Anaesth. 1994;41:244–247. doi: 10.1007/BF03009839. [DOI] [PubMed] [Google Scholar]
- 11.Munk Z, Tolis G, Jones W, Fallen E, McLean P. Pheochromocytoma presenting with pulmonary edema and hyperamylasemia. Can Med Assoc J. 1977;116:357–359. [PMC free article] [PubMed] [Google Scholar]
- 12.Werbel SS, Ober KP. Pheochromocytoma: update on diagnosis, localization, and management. Med Clin North Am. 1995;79:131–153. doi: 10.1016/s0025-7125(16)30088-8. [DOI] [PubMed] [Google Scholar]
- 13.Weston JA. The migration and differentiation of neural crest cells. Adv Morphog. 1970;8:41–114. doi: 10.1016/b978-0-12-028608-9.50006-5. [DOI] [PubMed] [Google Scholar]
- 14.Manger WM, Gifford RW., Jr . Clinical and experimental pheochromocytoma. 2nd ed. New York: Blackwell Scientific Publication Ltd.; 1996. p. 570. [Google Scholar]
- 15.Aiba M, Hirayama A, Ito Y, Fujimoto Y, Nakagami Y, Demura H, Shizume K. A compound adrenal medullary tumor (pheochromocytoma and ganglioneuroma) and a cortical adenoma in the ipsilateral adrenal gland: a case report with enzyme histochemical and immunohistochemical studies. Am J Surg Pathol. 1988;12:559–566. doi: 10.1097/00000478-198807000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Jam I, Shoham M, Wolf RO, Mishkin S. Elevated serum amylase activity in the absence of clinical pancreatic or salivary gland disease: possible role of acute hypoxemia. Am J Gastroenterol. 1978;70:480–488. [PubMed] [Google Scholar]
- 17.Tietz NW, Burlina A, Gerhardt W, Junge W, Malfertheiner P, Murai T, Otte M, Stein W, Gerber M, Klein G. Multicenter evaluation of a specific pancreatic isoamylase assay based on a double monoclonal-antibody technique. Clin Chem. 1988;34:2096–2102. [PubMed] [Google Scholar]
- 18.Junge W, Troge B, Klein G, Poppe W, Gerber M. Evaluation of a new assay for pancreatic amylase: performance characteristics and estimation of reference intervals. Clin Biochem. 1989;22:109–114. doi: 10.1016/s0009-9120(89)80007-4. [DOI] [PubMed] [Google Scholar]
- 19.Dahlstrand C, Dahlstrom A, Ahlman H. Adrenergic and VIP-ergic relaxatory mechanisms of the feline extrahepatic biliary tree. J Auton Nerv Syst. 1989;26:97–106. doi: 10.1016/0165-1838(89)90157-4. [DOI] [PubMed] [Google Scholar]