Abstract
Purpose
The objective was to compare retinal morphology and function following intravitreal injections of bevacizumab (Avastin) or triamcinolone (Volon A) in patients with early diabetic macular edema (DME).
Patients and methods
The study was planned as a randomized, prospective, interventional clinical trial. A total of 30 diabetic patients with treatment-naïve, clinically significant macular edema were included in this study and randomized to two equal groups. One group initially received three injections of 2.5 mg bevacizumab in monthly intervals. The second group received a single injection of 8 mg triamcinolone, followed by two sham interventions. Functional and anatomic results were evaluated monthly using ETDRS vision charts and spectral-domain optical coherence tomography. According to the study protocol, retreatment after 3 months was dependent on functional and anatomic outcome in a PRN regimen.
Results
Baseline best corrected visual acuity (BCVA) was 0.30 logMAR and central retinal subfield thickness (CSRT) was 505 μm in the bevacizumab group and 0.32 logMAR and 490 μm CSRT in the triamcinolone group. After 3 months, BCVA improved to 0.23 logMAR (bevacizumab) and 358 μm CRST and 0.26 logMAR (triamcinolone) and 308 μm CSRT. After 12 months, BCVA further recovered in the bevacizumab group (0.18 logMAR) but slightly decreased in the triamcinolone group (0.36 logMAR).
Conclusion
Intravitreal bevacizumab and triamcinolone are both equally effective in reducing CSRT in early DME. After 6 months, rehabilitation of vision was comparable in both treatment arms, whereas at the final follow-up at month 12, BCVA was superior in the bevacizumab than in the triamcinolone sample. This may be related to cataract development following steroid treatment, as well as to substance-specific mechanisms within the angiogenic versus the inflammatory cascade.
Keywords: bevacizumab, triamcinolone, diabetic macular edema
Introduction
Diabetic retinopathy is considered as one of the leading causes of serious visual impairment in young- to middle-aged adults.1 To date, laser coagulation remains the mainstay for therapy of diabetic retinopathy but is, for all undoubted efficacy, associated with significant ocular side effects.2 Despite the proven effect of adequate laser therapy, recovery of visual function is rare and the demand for alternative treatment modalities is rising. Intravitreal injections of corticosteroids have been increasingly used, but treatment success is limited because of ocular side effects and severe complications. With the introduction of anti-vascular endothelial growth factor (VEGF) therapy for the treatment of multiple ocular diseases, anti-VEGF drug use also appears promising for diabetic retinopathy (DRP), as VEGF levels in vitreous and aqueous fluids relate closely to active neovascularization and macular edema.3 Both bevacizumab and triamcinolone are the most cost-effective drugs and therefore widely used in diabetic macular edema (DME). Our study evaluates and compares the effect of the two treatment strategies: intravitreal injections of 8 mg triamcinolone or 2.5 mg bevacizumab individually, as a monotherapeutic approach, in patients with early diabetic macular edema. In a prospective manner, treatment and follow-up over 12 months were managed according to a standardized protocol, using a solid real-world PRN (pro re nata) regimen. None of the patients had undergone any prior treatment for DME, which provides an optimal setting for an evaluation of functional and morphological effects and retreatment needs based on disease activity.
Patients and methods
The trial, conducted at the Department of Ophthalmology of the Medical University of Vienna, followed the tenets of the Helsinki Declaration, was registered at www.clinicaltrials.com and approved by the responsible ethics committee of the Vienna University, as well as the Austrian Agency for Health and Food Safety (AGES). Before study inclusion, the interventional study design and examinations for scientific purposes were explained to each patient in a personal interview and informed consent was obtained.
Analyses of anatomical and functional results
The best corrected visual acuity (BCVA) results are described in logMAR and central subfield retinal thickness (CSRT) measurements in μm values. Results are described as mean and 95% confidence intervals. A mixed model ANOVA was applied for comparison of treatments and time points. Time points were tested for differences to baseline by linear contrasts. Normality was tested by Lilliefors' tests. For all tests, P-values of <0.05 were considered significant.
Patients
Each of the 30 study patients (mean age: 59±11 years, 12 male, 18 female) enrolled presented with clinical significant macular edema because of systemic diabetes mellitus diagnosed for >3 months. Four patients had a history of cataract surgery (three patients in the triamcinolone and one patient in the bevacizumab group). Previous macular laser photocoagulation or intravitreal injection therapy, active proliferative diabetic retinopathy (PDRP) with necessity of panretinal laser treatment, or panretinal laser treatment within the past 6 months were defined as exclusion criteria. Patients were required to have a baseline CSRT of at least 300 μm and BCVA of 20/25 to 20/400 Snellen equivalent in the study eye.
Regimen and follow-up
The study was designed as prospective, randomized, double-masked, comparative interventional case series. Patients were randomly assigned to one of two treatment arms: 15 eyes received three injections of 2.5 mg bevacizumab, and 15 eyes received one initial injection of 8 mg triamcinolone, and 2 sham injections after 4 and 8 weeks, respectively. After 12 weeks (±1 week) and application of either three injections of 2.5 mg bevacizumab (Avastin, Roche Pharma AG, Vienna, Austria) or one injection of 8 mg triamcinolone (Volon A, Dermapharm GmbH, Vienna, Austria) and two consecutive sham injections, PRN retreatment criteria were defined as follows: if SD-OCT showed any evidence of intraretinal or subretinal fluid or a 100 μm increase in central subfield retinal thickness from the thinnest measurement from any prior scheduled study visit, or a decrease in BCVA >5 letters compared with the score from the previous scheduled study visit. If one of these conditions was met, patients received further injections of bevacizumab or injections of triamcinolone every 3 months with sham injections within the intervals between therapeutic interventions, dependent on which treatment arm they were originally assigned to.
At each monthly visit, the following predefined examinations were obtained: BCVA testing using ETDRS charts at a 2-m distance (logMAR), slit-lamp examination including intraocular pressure (IOP) measurement (Goldmann Applanation Tonometry, Haag-Streit GmbH, Wedel, Germany), fundus biomicroscopy and retinal morphology scans (CSRT, 512 × 126 Cirrus OCT, Carl Zeiss Meditec, Jena, Germany). Fluorescein angiography (Heidelberg Engineering, Heidelberg, Germany) was performed at baseline and in 3-month intervals. Glycated hemoglobin (HbA1c) and creatinine levels were obtained monthly.
Injection technique
In all subjects, intraocular injections were performed under sterile conditions in the surgery unit following standardized procedures.4 A volume of 0.1 ml containing either 8 mg triamcinolone (Volon A, Dermapharm GmbH) or 2.5 mg bevacizumab (Avastin, Roche Pharma AG) was injected at 3.5 mm distance from the limbus through the inferotemporal pars plana. Patients in the triamcinolone group were injected the steroid only during the initial treatment, and two subsequent injections were mimicked: the same preinjection procedure was performed, and then the physician exerted light pressure on the conjunctiva using an empty syringe without a needle.
Results
Correlation of BCVA and CSRT
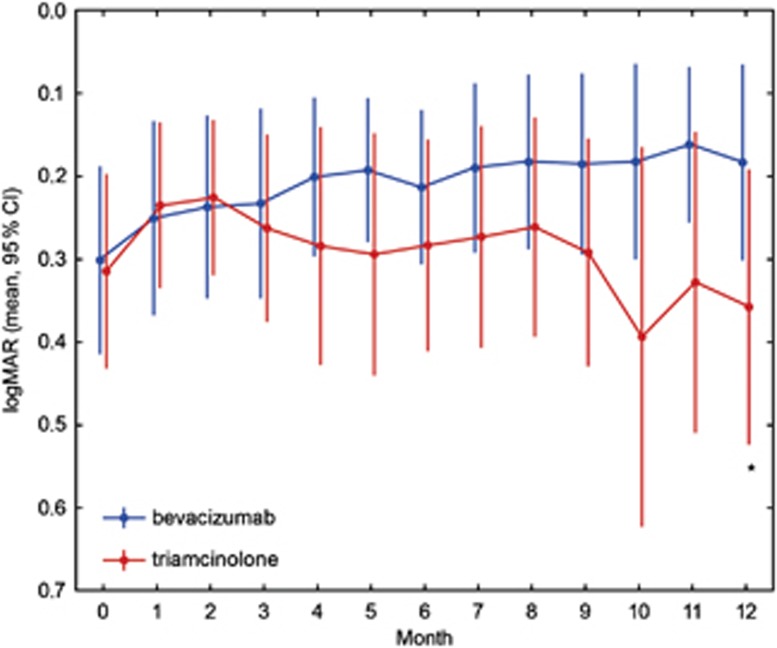
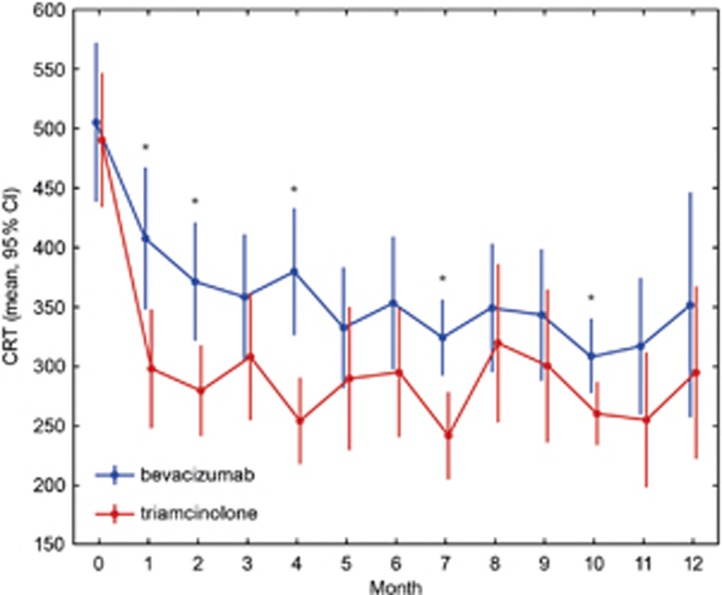
Baseline characteristics of both groups were similar, with a visual acuity (VA) of 0.30 logMAR for the bevacizumab and 0.32 logMAR for the triamcinolone group and a CSRT of 505 and 490 μm, respectively. At 3 months, after three consecutive injections of bevacizumab or one injection of triamcinolone and two sham injections, the results were as follows: VA was 0.23 logMAR in the bevacizumab and 0.26 logMAR for the triamcinolone sample (difference baseline/3 months: P>0.1); CSRT was 358 μm for bevacizumab and 308 μm for triamcinolone group (difference baseline/3 months: P<0.01). At 6 months, VA remained stable in both groups with no statistical difference between groups (bevacizumab: 0.22 logMAR; triamcinolone: 0.28 logMAR, P>0.05), together with a relatively constant CSRT of 353 μm in the bevacizumab and 295 μm in the triamcinolone group (intergroup comparison: P=0.07). The improvement in VA as compared with baseline did not reach statistical significance at this point in either group (P>0.05); however, retinal thickness showed significant thinning (P<0.01) compared with initial presentation. After a follow-up period of 12months, VA improved to 0.18 logMAR and CSRT remained at a value of 351 μm in the bevacizumab cohort. Repeated administration of triamcinolone induced a slight decrease in BCVA to 0.36 logMAR, despite considerable recovery of retinal anatomy toward 296 μm CSRT. Compared with baseline, the reduction in retinal thickness was statistically significant in both groups (P<0.01), but resolution of edema was enhanced with triamcinolone treatment. In contrast, improvement in BCVA was significantly superior in the bevacizumab group as compared with the triamcinolone group (P<0.05). Table 1 summarizes the mean value for BCVA and CSFT at each monthly interval indicating 95% confidence intervals. In Figures 1 and 2, the change in BCVA and CSFT is shown. Although retinal function shows a trend for superior outcomes for each single subsequent interval until month 12 in favor of bevacizumab, morphological retinal thinning is continuously more intensive with triamcinolone therapy.
Table 1. Overview of visual acuity (BCVA; logMAR) and central subfield thickness (CSFT; μm) results of the triamcinolone and the bevacizumab groups at each quartile over 12 months.
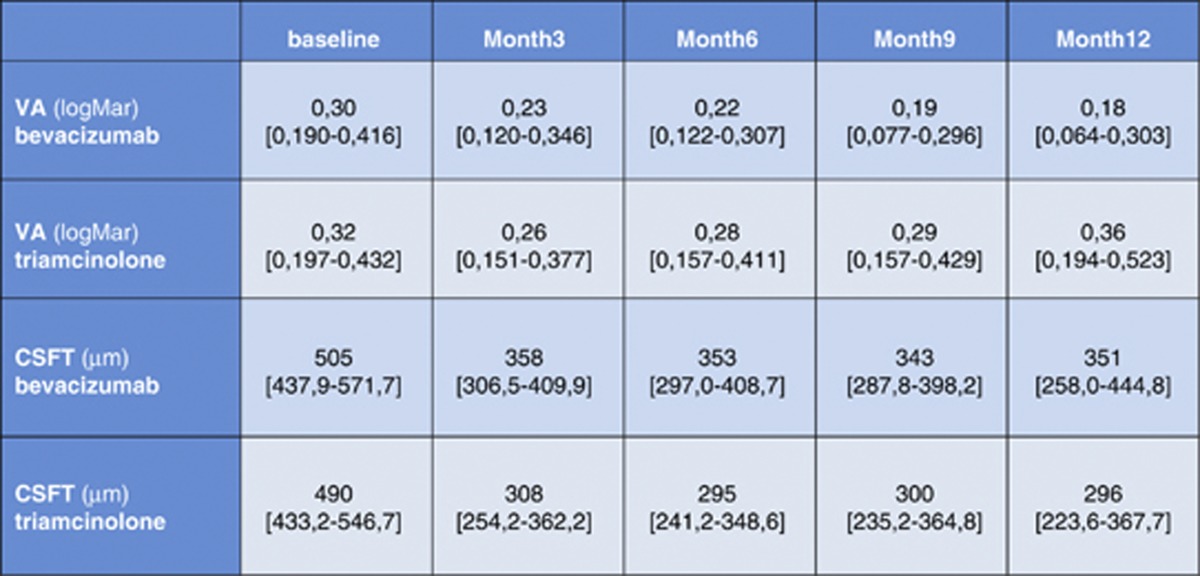
Figure 1.
Change in BCVA of both groups over 12 months in logMAR values. Blue symbols represent the bevacizumab, and red symbols represent the triamcinolone values. Asterisks indicate significant differences between groups. After 12 months, the functional results of the bevacizumab-treated cohort were significantly superior compared with the triamcinolone group.
Figure 2.
SD-OCT measurements of retinal thickness (CRT) over 1 year. The X axis represents values (μm) as mean and 95% confidence intervals. Bevacizumab (blue line) as well as triamcinolone (red line) induced a significant reduction of retinal thickness because of resolution of macular edema. Asterisks indicate significant differences between groups.
Morphological response to treatment
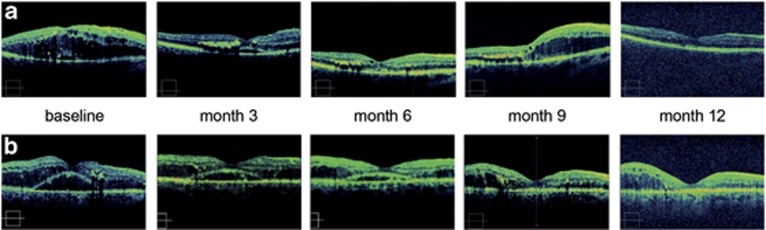
Triamcinolone as well as bevacizumab induced beneficial effects on the disintegrated retinal morphology due to DME. Both agents effectively induced thinning of the retina and dissolution of central cysts and intra- and subretinal fluid (Figures 3a and b). This effect seemed to be more pronounced in the triamcinolone sample where significant improvement occurred efficiently and homogenously with a predominant effect on the foveal region following one initial injection (Figure 3a). With bevacizumab the onset of fluid dissolution appeared to be more decelerated, usually necessitating repeated injections to booster this effect (Figure 3b). In both cohorts, within the treatment-free intervals, recurrent edema primarily developed parafoveally—this response pattern was also more distinct in the steroid-treated eyes (Figures 3a and b). Following retreatment, decrease in edema was also faster and more intensive with triamcinolone, as CRT in this cohort was regularly below bevacizumab values.
Figure 3.
(a) Retinal morphology under intravitreal treatment with triamcinolone over 1 year. In this patient, triamcinolone induced an obvious resolution of macular edema with sub- and intraretinal components. Accumulation of hard exudates is clearly visible in the outer nuclear layer. Recurrent edema typically developed in the parafoveal area and was successfully retreated with a further injection. (b) OCT images documenting the retinal response to anti-VEGF treatment with bevacizumab over 1 year. Consecutive injections induced a distinct regression of macular edema with decrease of intra- and subretinal fluid. Continuous treatment was necessary to maintain a stable effect. An exemplary case is shown at baseline with intra- and subretinal fluid. Typically subretinal fluid has subsided completely at month 12, but intraretinal cysts within the outer nuclear layer remain and are responsible for an increased CSRT at the last visit.
Influence of diabetes-related parameters
Individual values for HbA1c (in mmol/mol Hb) and renal function (creatinine in mg/dl) were tested monthly to evaluate similar physical conditions between both groups in general and during follow-up. The duration of diabetes had no influence on any parameter or treatment response.
Number of treatments
In the bevacizumab sample, patients received a mean of 9.0 out of 12 and in the steroid sample 2.7 out of 4 possible injections.
Adverse events
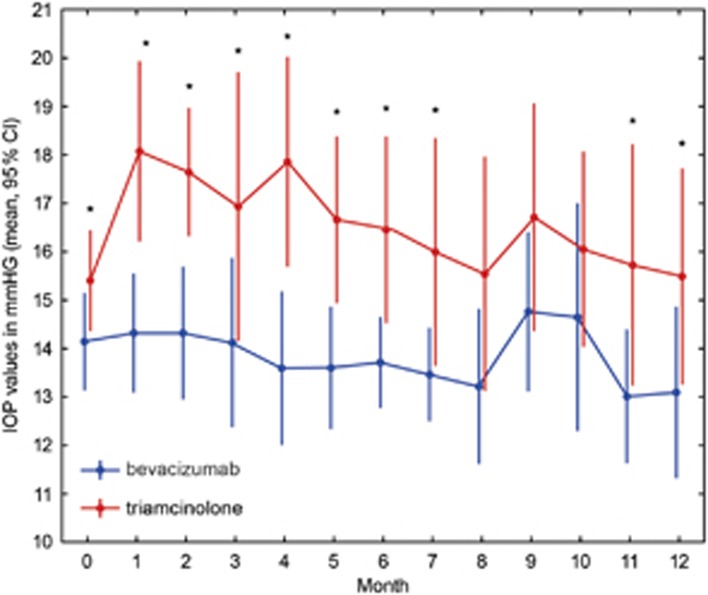
Within the observational period of 12 months, IOP remained on a constant level (baseline/month 12: P=0.3 bevacizumab group; P=0.9 triamcinolone group) in both groups (Figure 4). No adverse systemic or ocular events occurred in either group. No inflammatory response or endophthalmitis was observed in any eye at any time. Cataract development was present in both groups, but obviously more distinct in the steroid sample. None of the patients required cataract surgery within the study period, but eight probands of the triamcinolone sample underwent surgery within 1 year after study expiration.
Figure 4.
Results of intraocular pressure (IOP) measurements in mm Hg (mean and 95% CI) of both groups over 12 months. Blue symbols represent values of the bevacizumab, and red symbols represent values of the triamcinolone sample. Asterisks indicate significant differences between groups. Already at baseline, mean IOP values were slightly elevated in the triamcinolone sample. However, IOP remained constant in both groups over the entire study period.
Discussion
A characteristic complication and major reason for vision impairment at an early stage of diabetic retinopathy is macular edema (DME).5 As inflammatory mechanisms seem to play a major role in the development of DME,6, 7 direct intravitreal delivery of therapeutic agents is about to slowly replace laser therapy, long considered as first-line treatment for DME.8 This knowledge initiated multiple studies demonstrating the benefit of anti-inflammatory and anti-VEGF substances in the treatment of DME. However, almost all prospective trials used ranibizumab as therapeutic agent with various regimens,9, 10, 11 and only few studies report direct comparison between bevacizumab and triamcinolone (mainly presenting short-time results following one single injection7, 12, 13). To reach maximal benefit and reduce intravitreal and retinal VEGF to a minimum level, we chose a dose of 2.5 mg bevacizumab, which proved to be safe in recent studies.14 For steroid treatment, we injected a dosage of 8 mg every 3 months, which might prolong the beneficial outcome without major retinal toxicity.15 Both drugs clearly induced a rapid and highly significant effect on the restoration of retinal morphology, and gained similar anatomical results after 12 months. Because of our flexible PRN treatment regimen, macular edema intermittently recurred and necessitated regular retreatment in both arms to stabilize the beneficial effect. The amount of required retreatments in each study arm is similar to the treatment frequency in other trials, using lower dosages of medication, and implies that a higher dosage is not significantly associated with superior or prolonged outcome. With the lower retreatment frequency, more fluctuations were noted with triamcinolone, possibly indicating a need to shorten the fixed 3-month retreatment interval, as even steroid implants showed cessation of response earlier than anticipated pharmacologically.16 In our trial, rehabilitation of function was comparable in both treatment arms up to month 4. This short time outcome is in good accordance with the results of other studies,7, 12, 13 when a single triamcinolone or bevacizumab injection was temporarily effective in improving function, but limited by vision loss due to recurrent edema a few weeks after intervention. After 4 months, VA slowly, but continuously, improved in the bevacizumab group, whereas in the triamcinolone sample, VA decreased toward a baseline level (Figure 1). These findings may be related to the diversity of mechanisms of both treatment modalities, but a contributing factor is obviously the higher rate of cataract formation in the triamcinolone group, particularly at the end of the study. Within 1 year after study completion, 8 out of 15 steroid-treated eyes were assigned to cataract surgery, followed by a considerable improvement of visual acuity (mean 0.4 Sn preoperatively to 0.6 Sn postoperatively). These retrospectively analyzed data emphasize the obvious impact of steroid-related lens opacification. Therefore, to provide optimal benefit and safety, accurate patient selection is essential and pretreatment aspects should be taken into account. Triamcinolone is not an adequate first-line therapy, especially for young phakic patients, and the visual outcome with steroids is certainly inferior in the ‘real world' for an extended time period, unless cataract surgery is offered earlier than in patients without steroid impact. Even if cataract surgery is not estimated as a major problem today, the increased risk of postoperative CME or endophthalmitis, especially in diabetic patients, should be considered. On the other hand, it is proven that bevacizumab, even if delivered in minimal vitreal concentrations, downregulates VEGF plasma levels17 and may provoke cardiovascular events,18, 19 as diabetic patients are innately exposed to increased cardiovascular risk factors. The therapeutic mechanisms of the drugs and the retinal structures are comparable, but not identical. The direct comparison in a standardized setting proves that both independent pathways—anti-angiogenic and anti-inflammatory—contributed to a solid therapeutic effect.
The authors declare no conflict of interest.
References
- Rogers SL, Tikellis G, Cheung N, Tapp R, Shaw J, Zimmet PZ, et al. Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008;31 (4:761–763. doi: 10.2337/dc07-1622. [DOI] [PubMed] [Google Scholar]
- Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1995;113 (9:1144–1155. [PubMed] [Google Scholar]
- Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140 (2:256–261. doi: 10.1016/j.ajo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jaissle GB, Szurman P, Bartz-Schmidt KU. [Recommendation for the implementation of intravitreal injections—statement of the German Retina Society, the German Society of Ophthalmology (DOG) and the German Professional Association of Ophthalmologists (BVA)] Klin Monbl Augenheilkd. 2005;222 (5:390–395. doi: 10.1055/s-2005-858231. [DOI] [PubMed] [Google Scholar]
- Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96 (10:1501–1510. doi: 10.1016/s0161-6420(89)32699-6. [DOI] [PubMed] [Google Scholar]
- Patel JI, Tombran-Tink J, Hykin PG, Gregor ZJ, Cree IA. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006;82 (5:798–806. doi: 10.1016/j.exer.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Shimura M, Nakazawa T, Yasuda K, Shiono T, Iida T, Sakamoto T, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145 (5:854–861. doi: 10.1016/j.ajo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report no. 3. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27 (4:254–264. doi: 10.1097/00004397-198702740-00005. [DOI] [PubMed] [Google Scholar]
- Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33 (11:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118 (4:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119 (4:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Paccola L, Costa RA, Folgosa MS, Barbosa JC, Scott IU, Jorge R. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study) Br J Ophthalmol. 2008;92 (1:76–80. doi: 10.1136/bjo.2007.129122. [DOI] [PubMed] [Google Scholar]
- Shahin MM, El-Lakkany RS. A prospective, randomized comparison of intravitreal triamcinolone acetonide versus intravitreal bevacizumab (avastin) in diffuse diabetic macular edema. Middle East Afr J Ophthalmol. 2010;17 (3:250–253. doi: 10.4103/0974-9233.65496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Arevalo JF, Berrocal MH, Maia M, Roca JA, Morales-Cantón V, et al. Comparison of two doses of intravitreal bevacizumab as primary treatment for macular edema secondary to branch retinal vein occlusions: results of the Pan American Collaborative Retina Study Group at 24 months. Retina. 2009;29 (10:1396–1403. doi: 10.1097/IAE.0b013e3181bcef53. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno JM, Montero JA, Bayon A, Rueda J, Vidal M. Retinal toxicity of intravitreal triamcinolone acetonide at high doses in the rabbit. Exp Eye Res. 2007;84 (2:342–348. doi: 10.1016/j.exer.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Chan A, Leung LS, Blumenkranz MS. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant (Ozurdex) for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Clin Ophthalmol. 2011;5:1043–1049. doi: 10.2147/OPTH.S13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Ogata N, Matsuoka M, Wada M, Takahashi K, Nishimura T. Plasma levels of vascular endothelial growth factor and pigment epithelium-derived factor before and after intravitreal injection of bevacizumab. Br J Ophthalmol. 2010;94 (9:1215–1218. doi: 10.1136/bjo.2008.156810. [DOI] [PubMed] [Google Scholar]
- Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128 (10:1273–1279. doi: 10.1001/archophthalmol.2010.223. [DOI] [PubMed] [Google Scholar]
- Sacu S, Pemp B, Weigert G, Matt G, Garhofer G, Pruente C, et al. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Visual Sci. 2011;52 (6:3046–3050. doi: 10.1167/iovs.10-5842. [DOI] [PubMed] [Google Scholar]