Significance
Nutritional bacterial endosymbionts are housed in specialized host cells and are partitioned from the host cell cytoplasm by a host-derived symbiosomal membrane. This cellular organization isolates bacterial symbionts from nutrient pools in the host cell and makes possible host control of nutrient supply to bacterial symbionts. Here, using the aphid–Buchnera nutritional endosymbiosis, we demonstrate that the most active host glutamine (precursor) transporter, Acyrthosiphon pisum glutamine transporter 1, is competitively inhibited by arginine (a Buchnera-synthesized end product). We propose a model of endosymbiosis regulation in which precursor transport is regulated by a symbiont-synthesized end product. Thus, we provide insights into the molecular mechanism of host control of bacterial endosymbiont essential nutrient biosynthesis.
Keywords: symbiosis, coevolution, amino acid/auxin permease
Abstract
Endosymbiotic associations have played a major role in evolution. However, the molecular basis for the biochemical interdependence of these associations remains poorly understood. The aphid–Buchnera endosymbiosis provides a powerful system to elucidate how these symbioses are regulated. In aphids, the supply of essential amino acids depends on an ancient nutritional symbiotic association with the gamma-proteobacterium Buchnera aphidicola. Buchnera cells are densely packed in specialized aphid bacteriocyte cells. Here we confirm that five putative amino acid transporters are highly expressed and/or highly enriched in Acyrthosiphon pisum bacteriocyte tissues. When expressed in Xenopus laevis oocytes, two bacteriocyte amino acid transporters displayed significant levels of glutamine uptake, with transporter ACYPI001018, LOC100159667 (named here as Acyrthosiphon pisum glutamine transporter 1, ApGLNT1) functioning as the most active glutamine transporter. Transporter ApGLNT1 has narrow substrate selectivity, with high glutamine and low arginine transport capacity. Notably, ApGLNT1 has high binding affinity for arginine, and arginine acts as a competitive inhibitor for glutamine transport. Using immunocytochemistry, we show that ApGLNT1 is localized predominantly to the bacteriocyte plasma membrane, a location consistent with the transport of glutamine from A. pisum hemolymph to the bacteriocyte cytoplasm. On the basis of functional transport data and localization, we propose a substrate feedback inhibition model in which the accumulation of the essential amino acid arginine in A. pisum hemolymph reduces the transport of the precursor glutamine into bacteriocytes, thereby regulating amino acid biosynthesis in the bacteriocyte. Structural similarities in the arrangement of hosts and symbionts across endosymbiotic systems suggest that substrate feedback inhibition may be mechanistically important in other endosymbioses.
Endosymbiosis is an important force in evolution that allows animals and plants to acquire novel metabolic traits and exploit nutritionally challenging environments. For example, in legume–Rhizobium symbioses, plants exchange amino acids (1, 2) and carbon photosynthate (as dicarboxylic acids, usually malate) in return for Rhizobium-provisioned fixed nitrogen (as ammonium, NH4+) (3). Nitrogen often is a limiting factor in plant growth (3), and legume–Rhizobium nitrogen fixation provides a significant proportion of all biologically accessible nitrogen (4). Nutritional symbioses are present in 10–15% of all insect species, with microbial symbionts providing essential nutrients (such as essential amino acids and vitamins) that are absent from nutritionally unbalanced diets (5–8). Importantly, endosymbionts provide novel biochemistry to host insects that allows insects to exploit otherwise inaccessible niches. In both plant and animal nutritional endosymbioses a unifying unanswered question remains: How are symbiotic partners metabolically integrated so the microbial partner meets the host demand for essential nutrients?
To advance insight into host–symbiont metabolic integration, we work with the insect–bacterial nutritional endosymbiosis of the pea aphid, Acyrthosiphon pisum and its gamma-proteobacterium Buchnera aphidicola. A. pisum feeds exclusively on plant phloem sap, which contains low concentrations of essential amino acids, the amino acids that are required but cannot be synthesized de novo by the aphid. Elegantly, the essential amino acid shortfall is compensated by the host’s collaboration with the intracellular symbiont Buchnera aphidicola, which converts abundant nonessential amino acids into essential amino acids that are supplied to the aphid [reviewed by Shigenobu and Wilson, ref. 9]. Recent in silico metabolic flux balance analysis of the A. pisum–Buchnera symbiosis demonstrates that, at least theoretically, host regulation of the supply of precursor nitrogen (amino acid) and carbon to Buchnera can impact the output of essential amino acids, thus providing a mechanism by which the host can control the production of essential amino acids (10). In fact Thomas et al. (10) propose that the symbiont’s biosynthesis of essential amino acids is exclusively host controlled, because Buchnera has lost essential amino acid biosynthesis gene regulatory elements (11). Lack of gene regulation by Buchnera is consistent with evidence from whole-genome microarray experiments that show Buchnera has a limited transcriptional response to the manipulation of dietary amino acid supply (12–15). Overall those microarray data demonstrate constitutive amino acid biosynthesis in the symbiont, precluding Buchnera from playing an active role in metabolic regulation of the symbiosis at a transcriptional level.
If symbiont’ biosynthesis of essential amino acids is exclusively host controlled, by what mechanism is it controlled? One potential way that A. pisum could regulate symbiont’s biosynthesis of essential amino acids is by regulating the supply of precursor amino acids to Buchnera (10). Central to the A. pisum–Buchnera symbiosis is the exchange of amino acids between symbiotic partners, with Buchnera receiving nonessential amino acids from host aphids and returning essential amino acids to the host (9, 16, 17). Buchnera populations are densely packed and housed in large, specialized bacteriocyte cells located in the aphid hemolymph. Within each bacteriocyte, Buchnera are partitioned from the cytoplasm by a symbiosomal membrane of host origin (18, 19) that is formed by endocytosis of the host cell membrane (20). The symbiosomal membrane sits at the host–symbiont interface, making the symbiont in effect extracellular or outside the host cytoplasm. Very little is known about the origin, structure, and transport properties of the symbiosomal membrane, although it is likely to be of significant importance for nutritional symbiosis function. Transport of amino acids from aphid hemolymph across the bacteriocyte membrane, symbiosomal membrane, and Buchnera inner and outer cell membranes is fundamental to symbiotic function (Fig. S1). Previously we established that the A. pisum genome contains 40 putative nutrient amino acid transporters (AATs) that belong to the amino acid polyamine organocation (APC) superfamily (21, 22). On the basis of bacteriocyte gene-expression analyses (16, 21, 23, 24) and a quantitative proteome analysis (25), we hypothesize that five transporters have an important functional role in bacteriocytes.
The most likely amino acid target for host control of symbiotic function in the A. pisum–Buchnera symbiosis is glutamine. Buchnera cannot synthesize seven nonessential amino acids (asparagine, aspartate, glutamate, glutamine, proline, serine, and tyrosine) (9, 11). In the case of aspartate, glutamate, glutamine, serine, and tyrosine, A. pisum compensates for Buchnera’s biosynthetic deficiency by synthesizing these amino acids in bacteriocytes (16). Biosynthesis of these five nonessential amino acids requires a single amino acid precursor: glutamine. Glutamine is the dominant hemolymph amino acid in aphids and is available for transport into bacteriocytes (26). Experimentally isolated A. pisum bacteriocytes transport 14C-glutamine via a high-capacity glutamine transporter of unknown identity (26). Furthermore, Buchnera require glutamine as a substrate for the biosynthesis of the essential amino acids arginine, histidine, and tryptophan (9, 16), necessitating the transport of glutamine across the A. pisum symbiosomal membrane and the Buchnera inner and outer membranes. In sum, glutamine, the primary amino acid imported into bacteriocytes, is critical for the biosynthesis of nonessential and essential amino acids. Currently, the molecular identity of the A. pisum bacteriocyte glutamine AATs is unknown.
The current study had two objectives: (i) to identify bacteriocyte glutamine transporter(s), and (ii) to characterize glutamine transport function. To meet these objectives, we expressed A. pisum bacteriocyte AATs in African clawed frog (Xenopus laevis) oocytes and screened A. pisum bacteriocyte transporters for glutamine uptake. Based on functional characterization and immunolocalization of the most active bacteriocyte glutamine transporter, we propose a model for the regulation of bacteriocyte amino acid biosynthesis. In our proposed model, the accumulation of the essential amino acid arginine in A. pisum hemolymph reduces transport of the precursor nonessential amino acid glutamine in to bacteriocytes, thereby regulating bacteriocyte biosynthesis of nonessential and essential amino acids.
Results
Analysis of the Relative Expression of A. pisum Bacteriocyte AATs.
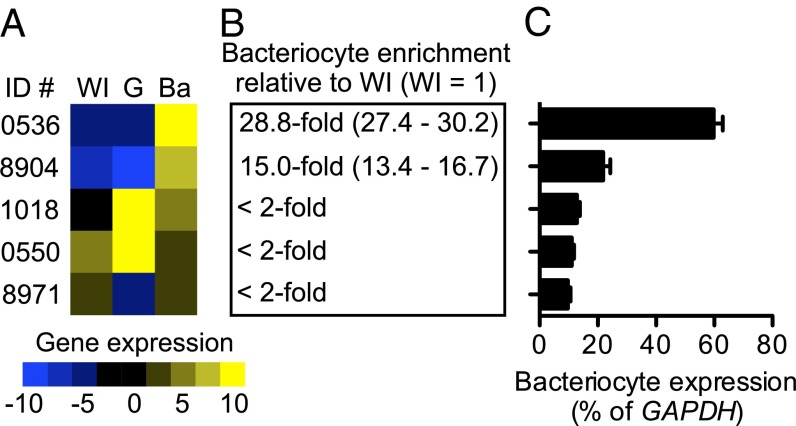
Previously, in A. pisum we identified 40 APC superfamily AATs, with 18 transporters belonging to APC transporter family [transporter classification number (T.C #) 2.A.3] and 22 belonging to the amino acid/auxin permease transporter family (T.C # 2.A.18) (21). Here we reanalyze existing expression data for 17 candidate A. pisum bacteriocyte AATs and show that, based on gene expression, only five transporters—ACYPI000536 (LOC100159138), ACYPI000550 (LOC100159152), ACYPI001018 (LOC100159667), ACYPI008904 (LOC100168178), and ACYPI008971 (LOC100168251)—are expressed in bacteriocytes at levels >10% of GAPDH expression (Fig. 1). We predict that these five transporters are functionally important at the aphid–Buchnera symbiotic interface. Transporters ACYPI000536 and ACYPI008904 are enriched in bacteriocytes by 28.8-fold and 15.0-fold, respectively, compared with whole-insect expression levels (Fig. 1 A and B) and also are highly expressed in bacteriocytes as compared with other ATTs in the A. pisum APC superfamily (Fig. 1C) (21). The remaining transporters ACYPI000550, ACYPI001018, and ACYPI008971 are not enriched in bacteriocytes relative to whole-insect expression levels (Fig. 1 A and B) but are highly expressed compared with other ATTs in the A. pisum APC superfamily (21) (Fig. 1C). Notably, these patterns of AAT expression are consistent across three genetically discrete A. pisum lines (Fig. S2).
Fig. 1.
Quantitative gene-expression analysis of A. pisum (lineage LSR1) bacteriocyte AATs. (A) AAT gene-expression levels were obtained using real-time qPCR in whole adult females (WI) and in gut (G), and bacteriocyte (Ba) tissues. Gene expression was normalized to GAPDH and compiled into a heat map, as described in Materials and Methods, showing high expression (z-score = 10; yellow) to low expression (z-score = −10; blue) (n = 3). (B) Transporters enriched more than twofold in bacteriocyte tissue relative to whole insect (WI) are indicated with confidence limits (35) in parentheses (n = 3). (C) Levels of AAT expression in bacteriocyte tissue shown as a percentage of GAPDH and ranked from high (0536) to low (8971) bacteriocyte expression. n = 3. Error bars represent SEM. Transporter sequences are available from the National Center for Biotechnology Information by appending ACYPI00 to each of the transporter identifier numbers.
A. pisum Bacteriocyte AATs Transport Glutamine.
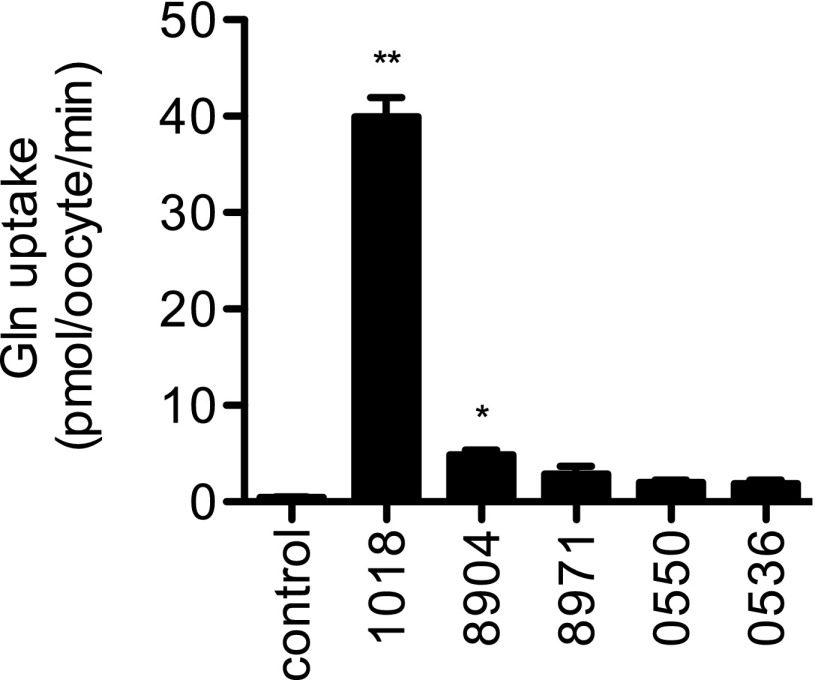
Two of the five AATs we predict to be functionally important at the A. pisum–Buchnera symbiotic interface transport glutamine. When expressed in Xenopus oocytes, these two transporters, ACYPI001018 and ACYPI008904, have significant levels of glutamine transport compared with water-injected controls (P < 0.01, one-way AVOVA followed by Dunnett’s posttest) (Fig. 2). Under our standard assay conditions (as described in Materials and Methods), transporter ACYPI001018 (LOC100159667) is the dominant glutamine transporter, transporting 7.6-fold more glutamine than ACYPI008904, and hereafter is referred to as “A. pisum glutamine transporter 1” (ApGLNT1).
Fig. 2.
Glutamine transport activity of A. pisum bacteriocyte AATs. The most highly expressed and/or enriched A. pisum bacteriocyte transporters (ACYPI001018, ACYPI008904, ACYPI008971, ACYPI000550, and ACYPI000536) were functionally expressed in X. laevis oocytes and were screened for their ability to transport 1 mM glutamine. All transport data are shown as raw uptake into either water-injected oocytes (control) or transporter-injected oocytes (1018, 8904, 8971, 0550, and 0536). For each transporter, n = 24–35. Error bars represent SEM. Bars marked with asterisks are significantly different from control. *P < 0.01; **P < 0.001; one-way ANOVA followed by Dunnett’s posttest.
ApGLNT1 Is a Glutamine Transporter Competitively Inhibited by Arginine.
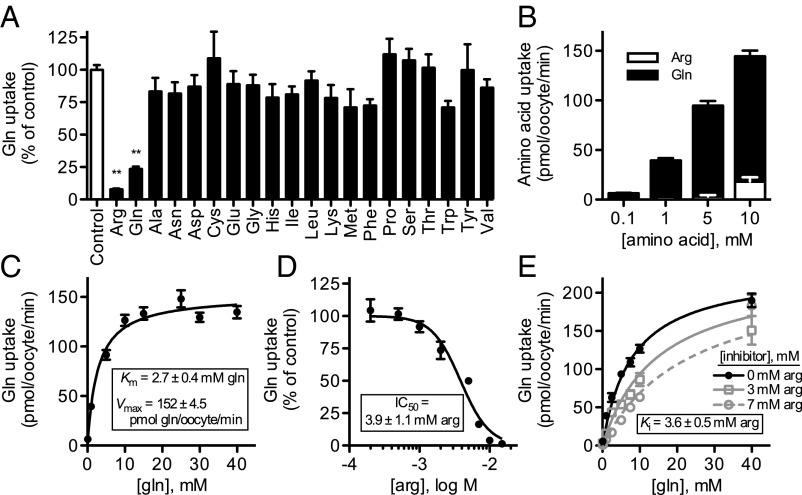
ApGLNT1 has very narrow substrate selectivity; amino acid competition (inhibition) with a 10-fold molar excess of competing amino acid (with the exception of tyrosine that, because of low solubility, was used at a 2.5-fold molar excess; Fig. 3A) demonstrates that only arginine significantly inhibits glutamine transport (P < 0.001, one-way ANOVA followed by Dunnett’s posttest). These competition assays show that ApGLNT1 has higher binding affinity for arginine than for glutamine (Fig. 3A). To determine if arginine is recognized as a substrate and transported by ApGLNT1, we performed transport assays with 14C-arginine in parallel with standard 14C-glutamine transport assays as a positive control (Fig. 3B). At low arginine concentrations (0.1 mM, 1 mM, and 5 mM), we could not detect arginine transport. Measurable arginine transport was detected at higher concentrations (10 mM arginine) but only at low levels relative to glutamine transport: 10 mM arginine transport was ∼12% of 10 mM glutamine transport. Amino acid competition experiments (Fig. 3A) and analyses of arginine transport (Fig. 3B) demonstrate that ApGLNT1 binds arginine efficiently but transports arginine poorly. In contrast, the transport rate of ApGLNT1 can be saturated with increasing concentrations of glutamine, yielding estimated Km and Vmax values of 2.7 ± 0.4 mM glutamine and 152.0 ± 4.5 pmol glutamine per oocyte/min, respectively (Fig. 3C).
Fig. 3.
Functional characterization of A. pisum AAT ApGLNT1. Transport function and substrate selectivity of ApGLNT1 were investigated by functional expression in X. laevis oocytes. (A) Inhibition of 14C-glutamine transport (1 mM initial extracellular concentration) in competition assays containing 10 mM unlabeled amino acid (as indicated, with the exception of tyrosine, which was used at 2.5 mM because of low solubility). Transport is displayed as percentage of no competing amino acid (control). (B) Transport of 14C-glutamine and 14C-arginine, at indicated concentrations. (C) Kinetics of glutamine transport by ApGLNT1. Glutamine transport was fitted to Michaelis–Menten equations, and Km and Vmax were determined by nonlinear regression using Prism 5.0c software. (D) Inhibition of glutamine transport (1 mM initial extracellular concentration) by arginine competition at the indicated concentrations. Glutamine transport is displayed as a percentage of control glutamine transport experiments with no competing arginine. The arginine inhibition curve was fitted to a sigmoid concentration–inhibition equation with variable slope using Prism 5.0c software. (E) Kinetics of glutamine transport by ApGLNT1 in the presence of inhibitor arginine. Glutamine transport with and without inhibitor arginine (at indicated concentrations) was fitted to competitive inhibition equations, and Ki was determined by nonlinear regression using Prism 5.0c software. All transport was corrected for background transport into control (water-injected oocytes). Each value is the mean ± SEM; n = 10–12 in A–D; n = 7 in E. Bars marked with asterisks are significantly different from control. **P < 0.001; one-way ANOVA followed by Dunnett’s posttest.
We further investigated the ability of arginine to compete with glutamine for ApGLNT1 uptake by performing additional amino acid competition (inhibition) assays in the presence of increasing concentrations of unlabeled arginine (Fig. 3D). These assays demonstrate that arginine inhibits glutamine uptake with an estimated IC50 of 3.9 ± 1.1 mM arginine. Furthermore, assays of glutamine transport kinetics in the presence of 3 mM and 7 mM arginine demonstrate an increase in glutamine Km, with retention of a Vmax indistinguishable from that estimated in the absence of arginine (one-way ANOVA, F = 2.102, P = 0.151). This increase in Km with no change in Vmax indicates that arginine competitively inhibits glutamine transport with an estimated Ki of 3.6 ± 0.5 mM arginine (Fig. 3E).
ApGLNT1 Localizes to the A. pisum Bacteriocyte Plasma Membrane.
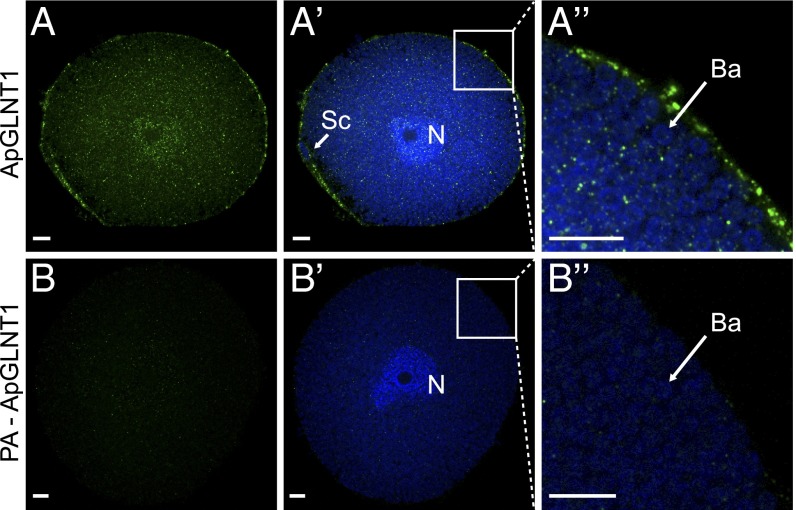
Aphid bacteriocytes isolated from young adult females appear as large, irregular spherical cells (diameter 133.9 ± 4.3 μm, mean ± SEM; n = 10), with a polyploid nucleus and densely packed with coccoid B. aphidicola symbionts (diameter 2.7 ± 0.1 μm, mean ± SEM; n = 10) (Fig. 4), as previously described (18, 19). The immunolocalization of ApGLNT1 protein in A. pisum bacteriocytes using rabbit monospecific antibodies to ApGLNT1 reveals punctate staining in the bacteriocyte cytoplasmic spaces that are not occupied by Buchnera symbionts and extensive staining of the bacteriocyte plasma membrane and sheath cell (Fig. 4 A–A′′). The observed localization of ApGLNT1 on the plasma membrane is consistent with the transport of glutamine from A. pisum hemolymph to the bacteriocyte cytosol (26). Similar staining patterns were not observed in control localizations that were performed with either peptide-preadsorbed primary ApGLNT1 antibodies (Fig. 4 B–B′′) or secondary-only antibodies (Fig. S3), confirming the specificity of the ApGLNT1 immunolocalization. Importantly, these localization patterns are consistent across three independent experiments (Fig. S3).
Fig. 4.
Immunolocalization of ApGLNT1 in isolated A. pisum bacteriocyte cells. (A) Localization pattern of anti-ApGLNT1 antibody staining (green) in isolated A. pisum bacteriocyte cells. (A′) Merge of the anti-ApGLNT1 image and DAPI-stained nuclear DNA (blue). (A′′) Magnified region of bacteriocyte cell (as indicated) showing a merge of anti-ApGLNT1 localization (green) and DAPI-stained nuclear DNA (blue). (B–B′′) Comparable control experiments were performed with isolated A. pisum bacteriocytes with peptide preadsorbed (PA) anti-ApGLNT1 antibody. The secondary antibody is Alexa-Fluor 568 donkey anti-rabbit IgG (H+L). For all images a single representative confocal plane is shown for three replicated localization experiments (Fig. S3). (Scale bars: 10 μm.) Ba, Buchnera aphidicola cell; N, bacteriocyte nucleus; Sc, sheath cell.
Discussion
The significance of our data is threefold. First, our gene-expression analysis of A. pisum bacteriocyte AATs (Fig. 1 and Fig. S2) supports accumulating evidence from A. pisum bacteriocyte transcriptomes (16, 23) and a quantitative bacteriocyte proteome analysis (25) that two transporters, ACYPI000536 and ACYPI008904, are the most highly expressed and enriched AATs in A. pisum bacteriocytes relative to whole-insect levels. In addition, our data demonstrate that three AATs, ACYPI000550, ACYPI001018, and ACYPI008971, are highly expressed in A. pisum bacteriocytes (Fig. 1 and Fig. S2). Second, by finding that two bacteriocyte-expressed AATs (ACYPI001018 and ACYPI008904) transport glutamine (Fig. 2) we extend the work of Sasaki and Ishikawa (26), who demonstrated that isolated A. pisum bacteriocytes have high-capacity 14C-glutamine transport activity. Third, based on detailed functional analysis of ApGLNT1 (Fig. 3) and localization of ApGLNT1 to the plasma membrane of bacteriocyte cells (Fig. 4 and Fig. S3), we propose a model of substrate feedback inhibition in which ApGLNT1 regulates precursor nonessential amino acid transport into bacteriocytes; we hypothesize that this inhibition functions to regulate the bacteriocyte’s biosynthesis of nonessential and essential amino acids (Fig. 5).
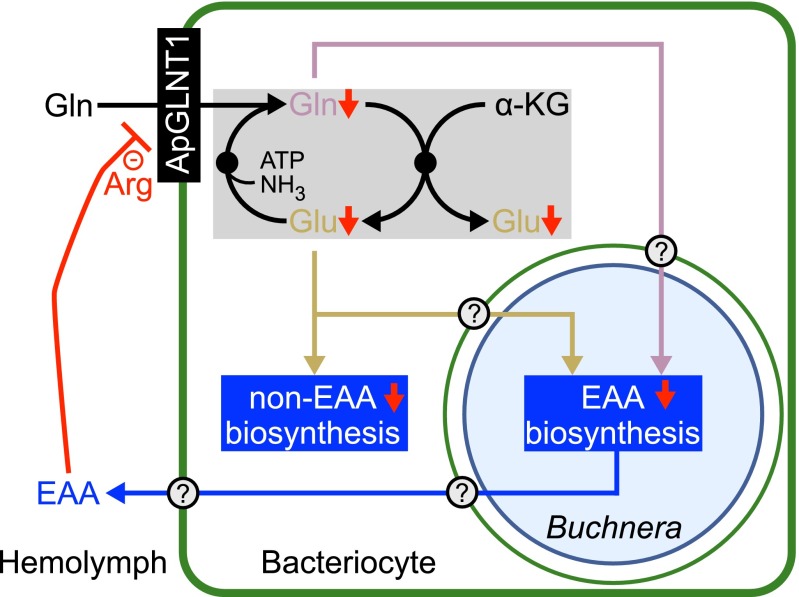
Fig. 5.
A proposed model for the regulation of glutamine transport at the A. pisum–Buchnera symbiotic interface. The nonessential amino acid (non-EAA) glutamine (Gln) is transported from A. pisum hemolymph across the A. pisum bacteriocyte membrane by transporter ApGLNT1 (ACYPI001018). Glutamine and glutamate (Glu) are readily interconverted in the bacteriocyte cytoplasm via the GOGAT cycle (shaded gray), which is up-regulated in bacteriocytes relative to whole-insect expression levels (16). Glutamine and glutamate are both required precursors and amino donors for shared A. pisum–Buchnera essential amino acid (EAA) biosynthesis pathways. In our proposed model, based on spatial localization and functional characterization of ApGLNT1, accumulating levels of the essential amino acid arginine in A. pisum hemolymph act as a negative regulator of glutamine transport (−), resulting in shut down of amino acid biosynthesis (red arrows). The multiple membranes of the symbiosome include the A. pisum bacteriocyte membrane (thick green line), the A. pisum symbiosomal membrane (thin circular green line), and the inner and outer membranes of Buchnera (thin circular blue line). Uncharacterized transporters are indicated by a question mark.
Glutamine and glutamate are the only amino acid precursors and amino donors required for bacteriocyte biosynthesis of amino acids (9, 10, 16). Early studies with isolated bacteriocytes demonstrated that glutamine, but not glutamate, is transported across the bacteriocyte cell membrane (26). More recently, and consistent with these early observations, metabolic reconstruction of the biosynthesis of amino acids in the bacteriocyte revealed that transport of a single amino acid, glutamine, is required for collaborative A. pisum and Buchnera biosynthesis of 19 protein-coding amino acids (9, 16). From glutamine, aphids synthesize glutamate in bacteriocyte cells via the GOGAT cycle (16). Thus, glutamine transport from hemolymph, where it is the most abundant free amino acid (26), into bacteriocytes, coupled with glutamate synthesis via the GOGAT cycle, provides the amino acid precursors and amino donors required for the biosynthesis of 10 essential amino acids (arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine) and nine nonessential amino acids (alanine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine, and tyrosine) (Fig. S4) (9, 16, 26). The biosynthesis pathway for the 20th amino acid, asparagine, is not highly expressed and/or enriched in bacteriocytes, likely because asparagine is found at high concentrations in the phloem-sap diet (27) and hemolymph of A. pisum (26).
Localization of ApGLNT1 to the plasma membrane of bacteriocytes (Fig. 4 and Fig. S3) is consistent with the transport of glutamine from A. pisum hemolymph into bacteriocyte cells (26). On the basis of our glutamine transport assays (Fig. 2), we argue that ApGLNT1 is the dominant transporter of glutamine into A. pisum bacteriocytes. We hypothesize that the low levels of glutamine transport by ACYPI008904 and the absence of any significant glutamine transport by ACYPI008971, ACYPI000550, and ACYPI000536 are caused by these transporters not recognizing glutamine as a preferred substrate, and we anticipate that these four transporters function at the bacteriocyte membrane and/or symbiosomal membrane to transport other amino acids. However, although ApGLNT1 is the most efficient bacteriocyte glutamine transporter when expressed in Xenopus oocytes, we cannot exclude the possibility that ACYPI000536, ACYPI000550, ACYPI008904, and ACYPI008971 play important roles in glutamine transport at the A. pisum–Buchnera symbiotic interface. The low glutamine-transport activity of these four bacteriocyte transporters in Xenopus oocytes under standard assay conditions may not accurately reflect their ability to transport glutamine in vivo. Current work is underway to localize these four transporters to the bacteriocyte and/or the symbiosomal membrane (Fig. 5) and to investigate further their transport properties and substrate selectivity.
Exactly how is amino acid biosynthesis in the bacteriocyte regulated? Here we present biochemical evidence consistent with previous in silico metabolic reconstructions by Thomas et al. (10) that the host-regulated supply of metabolic precursors impacts the biosynthetic output of bacteriocytes. ApGLNT1 transports glutamine from A. pisum hemolymph into bacteriocytes (Fig. 4). Transport of glutamine by ApGLNT1 is competitively inhibited by an end-product metabolite, arginine (Fig. 3). Putting these data together, we present a model of substrate feedback inhibition in which accumulating levels of the essential amino acid arginine in A. pisum hemolymph inhibit the transport of the precursor glutamine into bacteriocytes (Fig. 5). In turn, the reduced glutamine transport into bacteriocytes results in reduced biosynthesis of nonessential and essential amino acids (Fig. 5 and Fig. S4). In aphids, because of missing genes in the urea cycle (28), arginine is not synthesized de novo and therefore is an essential amino acid. Our regulatory model proposes that arginine assimilation reduces the arginine concentration in hemolymph to a level at which glutamine transport into bacteriocytes no longer is inhibited, thereby restarting the biosynthesis of amino acids in the bacteriocyte. The regulation of precursor glutamine transport into bacteriocytes by an end-product metabolite arginine is sufficient to regulate the biosynthesis of amino acids in the bacteriocyte in response to host demand.
The mechanism of substrate feedback inhibition is more commonly associated with regulation of enzymes in linear multistep metabolic pathways, in which the first reaction step is inhibited by accumulation of reaction product from the final step in the pathway (29, 30). Recently, competitive substrate inhibition has been demonstrated for the high-capacity Escherichia coli xylose transporter XylE, which has high binding affinity for glucose but does not transport glucose (31). Mechanistically, competitive inhibition of xylose transport by glucose may play an important role in E. coli catabolite repression, a mechanism that allows E. coli to use glucose preferentially before xylose (32). Here we report that competitive substrate feedback inhibition can operate at the level of a metabolite substrate transporter at a symbiotic interface.
Compartmentalization of host and Buchnera amino acid biosynthesis enzymes from A. pisum hemolymph facilitates an AATs functioning as a key nutrient regulator at the symbiotic interface. Remarkably, the compartmentalization observed at the aphid–Buchnera symbiotic interface has a striking similarity to the legume–Rhizobium symbiotic interface (33). Differentiated Rhizobium symbionts (called “Rhizobium bacteroids”) are housed in specialized plant cells, and symbionts are partitioned from the cytoplasm by a peribacteroid membrane, which is equivalent to the A. pisum symbiosomal membrane. Upon the establishment of a functional symbiotic association, free-living Rhizobium bacteroids surrender their ability to synthesize the branched-chain amino acids (isoleucine, leucine, and valine) and become dependent on the host plant for supply of these nutrients, a phenomenon that is called “symbiotic auxotrophy” (2). Significantly, the supply of branched-chain amino acids to Rhizobium bacteroids provides a mechanism by which legume host plants regulate the development and persistence of Rhizobium bacteroids (2, 34). Likewise, as a result of extensive genome erosion, Buchnera cannot synthesize seven nonessential amino acids (asparagine, aspartate, glutamate, glutamine, proline, serine, and tyrosine) (11) and therefore also can be classified as symbiotic auxotrophs. In the aphid host the biosynthetic pathways for five of the seven nonessential amino acids are up-regulated in bacteriocytes to compensate for Buchnera gene loss (16). Thus, transport of all seven nonessential amino acids across the bacteriocyte symbiosomal membrane to Buchnera symbionts by as yet uncharacterized transporters is predicted to occur. Likewise, bacteriocyte-synthesized essential amino acids and potentially nonessential amino acids are returned to the A. pisum hemolymph via uncharacterized transporters (Fig. 5). Our future work will focus on these uncharacterized AATs with the aim of testing metabolic models that necessitate the flux of metabolites between A. pisum and Buchnera.
In summary, our work identifies ApGLNT1 as the dominant glutamine transporter at the bacteriocyte plasma membrane, and we demonstrate that ApGLNT1 has very narrow substrate selectivity, with the activity of glutamine transport competitively inhibited by the essential amino acid arginine. In this study we focused on detailed functional characterization of an A. pisum bacteriocyte AAT in experimentally amenable Xenopus oocytes; importantly, our work makes significant progress by providing a model for regulation of a nutritional endosymbiosis. This result is consistent with the idea that the biosynthesis of amino acid in the bacteriocyte can be regulated by controlling the transport of the precursor into bacteriocytes (10). It remains to be seen whether symbiotic regulation by substrate feedback inhibition of a transporter is mechanistically important in other highly compartmentalized, nutritionally obligate endosymbioses.
Materials and Methods
Real-Time Quantitative PCR.
Real-time quantitative PCR (qPCR) was used to compare AAT gene expression in different tissues using 2−ΔΔCT methodology (35). Reaction conditions and primer sequences were described previously by Price et al. (21). AAT gene expression was normalized to GAPDH (ACYPI009769, LOC100169122) as previously described (21). For data presentation in heat maps, the ΔCT for each transporter in each tissue was transformed into z-scores × 10 where the z-score of a raw expression value x is z = (x − μ)/σ, where μ and σ are the mean and SD, respectively, of ΔCT values for all transporters across all tissues.
Cloning Full-Length A. pisum Bacteriocyte AAT Genes.
A. pisum bacteriocyte transporter coding sequences (CDS) for ACYPI000536 (LOC100159138), ACYPI000550 (LOC100159152), ACYPI001018 (LOC100159667), ACYPI008904 (LOC100168178), and ACYPI008971 (LOC100168251) were amplified from A. pisum bacteriocyte cDNA using Phusion High Fidelity DNA Polymerase (Finnzymes). All PCR primers contained a 5′ optimized Kozak initiation sequence for efficient translation (36) and a 5′ NotI site and a 3′ BamHI site (primer sequences are listed in Table S1). Amplification reactions were performed on a Mastercycler ep thermal cycler (Eppendorf) using an initial hold of 98 °C for 30 s followed by 30 cycles of 98 °C for 10 s, 60 °C for 30 s, and 72 °C for 90 s, and after cycling a hold at 72 °C for 5 min. Amplified coding sequences were digested with NotI and BamHI and cloned into the respective sites of pcDNA3.1 (Invitrogen). All expression constructs were fully sequenced using Sanger sequencing in standard ABI Big Dye terminator v3.1 reactions. Reaction products were analyzed on 3130xl genetic analyzer (ABI), and sequence data were assembled into contiguous sequences using Sequencher (version 4.9).
Expression of A. pisum AATs in Xenopus Oocytes.
Oocytes were surgically removed from adult female X. laevis frogs (Nasco). The care and use of X. laevis frogs in this study were approved by the University of Miami Animal Research Committee and met the guidelines of the National Institutes of Health. Follicle cells were removed with Collagenase B (Boehringer Mannhem) for 2 h at room temperature. Capped cRNA encoding each transporter was generated using T7 mMESSAGE mMACHINE kits (Ambion) and was polyadenylated using the poly(A) tailing kit (Ambion). For expression of each transporter, 23–46 ng of cRNA was injected into stage V–VI oocytes. Control oocytes were injected with an equivalent volume of water. Oocytes were incubated at 16 °C in Barth’s saline [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.3 mM CaNO3, 0.41 mM CaCl2, 0.82 mM MgSO4, 15 mM Hepes (pH 7.6) and 2.5 µg/mL ciprofloxacin] for 1–3 d before functional uptake assays.
Functional Analysis of A. pisum AATs in Xenopus Oocytes.
Xenopus oocyte standard transport assays were based on methods previously described by Yao et al. (37). Briefly 10–12 oocytes injected with cRNA were washed twice and allowed to equilibrate for 15 min at room temperature in transport buffer [25 mM citrate buffer (pH 6.0), 100 mM NaCl, 2 mM KCl, 1 mM MgCl2]. To initiate uptake assays, oocytes were placed on a rotatory shaker at room temperature, and at time 0 the transport buffer was replaced with 200 µL transport buffer containing defined amino acids and either 1 µCi/mL l-[14C(U)]-glutamine (260 mCi/mmol) (Perkin-Elmer) or 1 µCi/mL l-[14C(U)]-arginine (274 mCi/mmol) (Perkin-Elmer), depending on the experiment. Glutamine transport into Xenopus oocytes expressing ApGLNT1 increased linearly for at least 80 min (Fig. S5). Thus, we stopped all subsequent transport assays during the linear phase at 30 min. After uptake for 30 min, oocytes were washed rapidly four times with 1 mL ice-cold transport buffer. Control experiments with water-injected oocytes were performed in parallel. Individual undamaged oocytes were transferred to scintillation vials and dissolved in 0.5 mL 5% SDS for 2 h with vigorous shaking. Uptake of labeled substrate was determined by liquid scintillation counting and corrected for background uptake into control (water-injected) oocytes.
To determine estimates of Km and Vmax transport kinetics, data were fitted to the Michaelis–Menten equation using nonlinear regression (Prism 5.0c software; GraphPad). For arginine-inhibition assays, the initial velocity of glutamine uptake was determined in the presence of increasing concentrations of arginine under standard transport assay conditions. IC50 values were obtained by fitting the data to a sigmoid concentration–inhibition equation with variable slope using Prism 5.0c software. To determine estimates of the arginine inhibition constant (Ki), glutamine transport kinetics data in the presence of inhibitor (3 mM and 7 mM arginine) were fitted to a competitive inhibition equation, and Ki was estimated by nonlinear regression using Prism 5.0c software.
Preparation of Anti-ApGLNT1 Antibody.
A monospecific anti-ApGLNT1 antibody was generated using a custom service provided by Pacific Immunology Corp. Briefly, a synthetic peptide corresponding to amino acids 28–40 of ApGLNT1 plus a C-terminal cysteine (LDNNKRGSIRTDV-C) was synthesized and conjugated to maleimide-activated keyhole limpet hemocyanin (KLH). The KLH-coupled peptide was injected into New Zealand White rabbits for antibody production. Following a standard immunization protocol, monospecific anti-ApGLNT1 antibodies were purified from rabbit serum using an affinity column with immobilized ApGLNT1 peptide.
Spatial Localization of ApGLNT1 in A. pisum Bacteriocytes.
Bacteriocytes were dissected from young adult female A. pisum (line LSR1) in 0.9% (wt/vol) NaCl and were fixed in 4% (wt/vol) formaldehyde (Thermo Scientific) overnight at 4 °C. The bacteriocytes were washed five times, for 5 min each wash, in PBS (140 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 6.5 mM Na2HPO4, pH 7.4) at room temperature and then were blocked with 5% (vol/vol) normal donkey serum (NDS; Jackson ImmunoResearch Laboratories, Inc.) in PBS with 0.3% (vol/vol) Triton X-100 (PBST) for 1 h at room temperature. Samples were incubated with primary ApGLNT1 antibody at 1:500 dilution in 5% NDS in PBST overnight at 4 °C. After incubation the bacteriocytes were washed five times, for 5 min each wash, in PBS at room temperature and were incubated with secondary Alexa-Fluor 568 donkey anti-rabbit IgG (H+L) antibody (Life Technologies) at 1:1,000 dilution in 5% NDS in PBST overnight at 4 °C. After overnight incubation the bacteriocytes were washed five times, for 5 min each wash, in PBS, and the nuclei were stained with DAPI (Life Technologies) at 300 nM for 30 min at room temperature. Bacteriocytes were mounted in 2,2′-thiodiethanol (Sigma-Aldrich) on a glass slide. Fluorescence images were acquired using a Leica TCS SP5 laser scanning confocal microscope. Control treatments were run in parallel and included localizations with peptide-preabsorbed primary antibody (using a 20-fold molar excess of peptide) and the secondary-only antibody, according to ref. 38. The localization experiment with control treatments was repeated three times with all images taken from a single confocal plane.
Supplementary Material
Acknowledgments
We thank Helen M. Bramlett and Jessie Truettner for the use of radiochemical laboratory facilities; Ana Castro and Ben Sherman for oocyte preparation; Rebecca P. Duncan and Athula H. Wikramanayake for useful discussion and critical reading of the manuscript; and two anonymous reviewers whose comments helped improve the final manuscript. H.F. was supported by a University of Miami Maytag Fellowship. This work was supported by National Science Foundation Award 1121847 (to A.C.C.W. and D.R.G.P.) and National Institutes of Health Award DC011091 (to C.W.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306068111/-/DCSupplemental.
References
- 1.Lodwig EM, et al. Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature. 2003;422(6933):722–726. doi: 10.1038/nature01527. [DOI] [PubMed] [Google Scholar]
- 2.Prell J, et al. Legumes regulate Rhizobium bacteroid development and persistence by the supply of branched-chain amino acids. Proc Natl Acad Sci USA. 2009;106(30):12477–12482. doi: 10.1073/pnas.0903653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poole P, Allaway D. Carbon and nitrogen metabolism in Rhizobium. Adv Microb Physiol. 2000;43(43):117–163. doi: 10.1016/s0065-2911(00)43004-3. [DOI] [PubMed] [Google Scholar]
- 4.Newton WE. Nitrogen fixation in perspective. In: Pedrosa FO, Hungria M, Yates MG, Newton WE, editors. Nitrogen Fixation: From Molecules to Crop Productivity. Vol 38. Academic Publishers, New York: Kluwer; 2000. pp. 3–8. [Google Scholar]
- 5.Moran NA, Baumann P. Bacterial endosymbionts in animals. Curr Opin Microbiol. 2000;3(3):270–275. doi: 10.1016/s1369-5274(00)00088-6. [DOI] [PubMed] [Google Scholar]
- 6.Baumann P, Moran NA, Baumann L. The Prokaryotes, a Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications. New York: Springer; 2000. [Google Scholar]
- 7.Moran NA, Telang A. Bacteriocyte-associated symbionts of insects - A variety of insect groups harbor ancient prokaryotic endosymbionts. Bioscience. 1998;48(4):295–304. [Google Scholar]
- 8.Buchner P. Endosymbiosis of Animals with Plant Microorganisms. New York: Interscience Publishers; 1965. [Google Scholar]
- 9.Shigenobu S, Wilson ACC. Genomic revelations of a mutualism: The pea aphid and its obligate bacterial symbiont. Cell Mol Life Sci. 2011;68(8):1297–1309. doi: 10.1007/s00018-011-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas GH, et al. A fragile metabolic network adapted for cooperation in the symbiotic bacterium Buchnera aphidicola. BMC Syst Biol. 2009;3:24. doi: 10.1186/1752-0509-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407(6800):81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 12.Bermingham J, et al. Impact of host developmental age on the transcriptome of the symbiotic bacterium Buchnera aphidicola in the pea aphid (Acyrthosiphon pisum) Appl Environ Microbiol. 2009;75(22):7294–7297. doi: 10.1128/AEM.01472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moran NA, Dunbar HE, Wilcox JL. Regulation of transcription in a reduced bacterial genome: Nutrient-provisioning genes of the obligate symbiont Buchnera aphidicola. J Bacteriol. 2005;187(12):4229–4237. doi: 10.1128/JB.187.12.4229-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reymond N, et al. Different levels of transcriptional regulation due to trophic constraints in the reduced genome of Buchnera aphidicola APS. Appl Environ Microbiol. 2006;72(12):7760–7766. doi: 10.1128/AEM.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viñuelas J, et al. Conservation of the links between gene transcription and chromosomal organization in the highly reduced genome of Buchnera aphidicola. BMC Genomics. 2007;8:143. doi: 10.1186/1471-2164-8-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen AK, Moran NA. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci USA. 2011;108(7):2849–2854. doi: 10.1073/pnas.1013465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson ACC, et al. Genomic insight into the amino acid relations of the pea aphid, Acyrthosiphon pisum, with its symbiotic bacterium Buchnera aphidicola. Insect Mol Biol. 2010;19(Suppl 2):249–258. doi: 10.1111/j.1365-2583.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 18.McLean D, Houk E. Phase contrast and electron microscopy of the mycetocytes and symbiotes of the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 1973;19(3):625–629. [Google Scholar]
- 19.Baumann P, et al. Genetics, physiology, and evolutionary relationships of the genus Buchnera: Intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- 20.Koga R, Meng X-Y, Tsuchida T, Fukatsu T. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface. Proc Natl Acad Sci USA. 2012;109(20):E1230–E1237. doi: 10.1073/pnas.1119212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price DRG, Duncan RP, Shigenobu S, Wilson ACC. Genome expansion and differential expression of AATs at the aphid/Buchnera symbiotic interface. Mol Biol Evol. 2011;28(11):3113–3126. doi: 10.1093/molbev/msr140. [DOI] [PubMed] [Google Scholar]
- 22.Duncan RP, Nathanson L, Wilson ACC. Novel male-biased expression in paralogs of the aphid slimfast nutrient AAT expansion. BMC Evol Biol. 2011;11:253. doi: 10.1186/1471-2148-11-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakabachi A, et al. Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera. Proc Natl Acad Sci USA. 2005;102(15):5477–5482. doi: 10.1073/pnas.0409034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald SJ, Lin GG, Russell CW, Thomas GH, Douglas AE. The central role of the host cell in symbiotic nitrogen metabolism. Proc Biol Sci. 2012;279(1740):2965–2973. doi: 10.1098/rspb.2012.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poliakov A, et al. Large-scale label-free quantitative proteomics of the pea aphid-Buchnera symbiosis. Mol Cell Proteomics. 2011;10(6):007039. doi: 10.1074/mcp.M110.007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki T, Ishikawa H. Production of essential amino acids from glutamate by mycetocyte symbionts of the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 1995;41(1):41–46. [Google Scholar]
- 27.Sandstrom J, Pettersson J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J Insect Physiol. 1994;40(11):947–955. [Google Scholar]
- 28.International Aphid Genomics Consortium Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 2010;8(2):e1000313. doi: 10.1371/journal.pbio.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umbarger HE. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science. 1956;123(3202):848. doi: 10.1126/science.123.3202.848. [DOI] [PubMed] [Google Scholar]
- 30.Doy CH, Pittard AJ. Feedback control of tryptophan biosynthesis. Nature. 1960;185(4717):941–942. doi: 10.1038/185941b0. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature. 2012;490(7420):361–366. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 32.Desai TA, Rao CV. Regulation of arabinose and xylose metabolism in Escherichia coli. Appl Environ Microbiol. 2010;76(5):1524–1532. doi: 10.1128/AEM.01970-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehead LF, Day DA. The peribacteroid membrane. Physiol Plant. 1997;100(1):30–44. [Google Scholar]
- 34.Prell J, et al. Role of symbiotic auxotrophy in the Rhizobium-legume symbioses. PLoS ONE. 2010;5(11):e13933. doi: 10.1371/journal.pone.0013933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266(30):19867–19870. [PubMed] [Google Scholar]
- 37.Yao SYM, Cass CE, Young JD. The Xenopus oocyte expression system for the cDNA cloning and characterization of plasma membrane transport proteins. In: Baldwin SA, editor. Membrane Transport. Oxford: Oxford Univ Press; 2000. pp. 47–78. [Google Scholar]
- 38.Burry RW. Controls for immunocytochemistry: An update. J Histochem Cytochem. 2011;59(1):6–12. doi: 10.1369/jhc.2010.956920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.