Significance
With the onset of gastrulation, sea urchin embryos deposit a calcium carbonate endoskeleton consisting of two spicules. Sea water is the source for the mineral ions, but the specific stages of the transport and deposition pathway are not well understood. This study shows that the first-formed mineral is deposited inside intracellular micrometer-size vesicles as solid nanospheres. Surprisingly, the initial deposits are distributed widely inside the embryonic cells, including epithelial cells. The possibility that the whole embryo is geared toward depositing mineral for spicule formation or other purposes, may contribute to the understanding of biomineralization processes in general.
Keywords: biomineralization, mineralization pathway, sea urchin embryonic spicule, transient precursor mineral phase, intracellular mineral deposition
Abstract
Sea urchin larvae have an endoskeleton consisting of two calcitic spicules. We reconstructed various stages of the formation pathway of calcium carbonate from calcium ions in sea water to mineral deposition and integration into the forming spicules. Monitoring calcium uptake with the fluorescent dye calcein shows that calcium ions first penetrate the embryo and later are deposited intracellularly. Surprisingly, calcium carbonate deposits are distributed widely all over the embryo, including in the primary mesenchyme cells and in the surface epithelial cells. Using cryo-SEM, we show that the intracellular calcium carbonate deposits are contained in vesicles of diameter 0.5–1.5 μm. Using the newly developed airSEM, which allows direct correlation between fluorescence and energy dispersive spectroscopy, we confirmed the presence of solid calcium carbonate in the vesicles. This mineral phase appears as aggregates of 20–30-nm nanospheres, consistent with amorphous calcium carbonate. The aggregates finally are introduced into the spicule compartment, where they integrate into the growing spicule.
During the biomineralization process, ions from the environment are transported to the mineralization site by using different strategies, such as cell penetration through specific ion channels (1), delivery by vesicles (2), and formation of a precursor amorphous mineral phase (3). The intracellular formation of a transient amorphous phase enables the mineral to be delivered efficiently to the mineralization site in a concentrated manner, thus overcoming limitations of ion transport (4).
The formation of calcite spicules by the embryonic sea urchin is a fascinating and well-documented example of a complex multistage biomineralization process. Sea urchin embryos obtain calcium directly from sea water until they can feed (5). The calcium is transported from the sea water through various outer tissue layers and is deposited inside cells. This mineral is then translocated to a delimited space inside a syncytium in which an endoskeleton composed of two spicules, each of which diffracts X-rays as a single crystal of calcite, is formed (6). The cells responsible for the formation of the syncytium are the primary mesenchyme cells (PMCs). The spicules mineralize within the syncytium space, enveloped by a thin extracellular layer (7). The PMCs are believed to control the entire biomineralization process, because spicule formation occurs in isolated and cultured PMCs (8). In the embryo, however, the calcium and carbonate ions from the sea water have to pass through various outer tissue layers. The cells of these tissues thus control the ultrastructural and chemical milieu in which mineralization by the PMCs occurs and are also involved in the process.
Calcein is a small fluorescent molecule that may be used to label calcium ions (9–12). Calcium ion labeling experiments with calcein in PMC cultures first demonstrated the formation of calcium granules inside PMCs, followed by the appearance of a fluorescent signal at the spicule tip (13). The dynamics of spicule elongation also was studied in vivo by using calcein, which labeled the surface of newly formed spicule areas (7, 14). Blocking or interfering with calcium uptake by the cells, both in vivo and in PMC cultures, shows that for the spicule to be formed, calcium first has to penetrate the cells (15–17). The manner in which calcium is transported to the PMCs is not known (7). It is known that the ectoderm cells take part in spicule mineralization, especially in determining the placement of PMCs, which in turn specify the morphology of the spicule. The involvement of ectoderm cells in calcium transport to the PMCs is not well understood, considering the fact that PMC cultures produce spicules in the absence of ectoderm cells if growth factors are introduced (8, 14, 18).
Beniash et al. (19) showed that the PMCs contain electron-dense granules of amorphous calcium carbonate (ACC) about 0.5–1.5 μm in diameter. These granules were assumed to integrate into the growing spicule after fusion with the syncytium membrane. The initial crystalline deposit in the syncytium is in the form of a rhombohedral calcite crystal (20). The spicule grows by the addition of transient ACC, which then partially transforms into calcite through secondary nucleation (21, 22). The transformation from ACC to crystalline calcite is complex. Examination of the spicule surfaces with X-ray absorption spectroscopy using extended X-ray absorption fine structure (EXAFS) and X-ray photoelectron emission microscopy (X-PEEM) showed three distinct mineral phases present in adjacent sites at a scale of tens of nanometers. The initial phase, ACC1, is short-lived and presumably a hydrated ACC phase. The second phase, ACC2, is an intermediate transient form of ACC, whereas the third phase is crystalline calcite (21, 23).
Although it is known that the spicules form through secondary nucleation of ACC, many stages of the mineralization pathway are not clear. Some of the open questions are fundamental for understanding spicule mineralization, namely, what is the calcium pathway from sea water to the syncytium, do ACC deposits also form in cells that are not PMCs, and how are the ACC deposits transported into the spicule compartment? To address these questions, we studied the calcium pathways in whole embryos.
Results
SEM imaging under cryogenic conditions was performed on sea urchin embryos and larvae at different stages post fertilization. The sample preparation procedure involves only high-pressure freezing and freeze fracture of unfixed and unstained embryos. Cryo-SEM allows high-resolution imaging of biological samples as close as possible to their native state, minimizing artifacts caused by chemical procedures and drying. A cryo-SEM image of a sea urchin embryo at the late gastrula stage (Fig. 1A) shows the layer of epithelial cells, the invaginated archenteron, and the relatively large cell free space called the blastocoel. In the blastocoel, specialized PMCs are interconnected in a syncytium where the spicules are formed. Two fractured spicules (backscattered image in Fig. S1A) surrounded by PMCs are visible within the blastocoel on the two sides of the invaginated archenteron. Of particular interest is the presence of a multivesicular body adjacent to the PMCs (Fig. 1A and Fig. S1C).
Fig. 1.
Cryo-SEM micrograph of a high-pressure frozen and freeze-fractured sea urchin embryo at the late gastrula stage. (A) The spicule cross-sections are marked with arrows. The archenteron (Ar) and epithelial cells (Ep) of the embryo are fractured, showing large numbers of intracellular vesicles of 0.5–1.5 μm. Note the multivesicular body adjacent to the PMC (arrowhead), enlarged in Fig. S1C. The blastocoel is marked with an asterisk. (B) The spicule cross-section (S; BSE image of the spicule area in Fig. S1B) is adjacent to a PMC that contains many vesicles, some empty and some packed with nanospheres (arrow). (C) Enlargement of the marked vesicle in B, showing nanospheres of 20–30 nm.
Cryo-SEM observations of PMCs show intracellular deposits enclosed by membrane-delimited vesicles ranging in size from 0.5 to 1.5 μm. These vesicles contain densely packed nanospheres of 20–30 nm, reminiscent of the nanospheres of ACC observed in the forming spicule (24) (Fig. 1 B and C). Similar vesicles also are observed crossing the boundary between the cell and the spicule compartment, and in the proximity of the spicule (Fig. 2 and Fig. S2). The nanospheres produce a positive backscattered electron (BSE) signal, which implies that they are composed of a condensed phase with high electron density (Figs. S1B and S3). Because of the three-dimensionality of the sample surface, however, a positive BSE signal may be deceptive, and alone does not provide proof of the electron-dense nature of the particles. We therefore carried out calcein pulse–chase experiments to obtain a 3D overview of the pathway followed by the calcium ions and possibly to identify mineral-bearing vesicles within the PMCs.
Fig. 2.
Cryo-SEM micrograph of a high-pressure frozen and freeze-fractured sea urchin embryo at the gastrula stage showing the spicule (S) and adjacent mineral-bearing vesicles, packed with nanospheres (arrowheads). BSE image in Fig. S3.
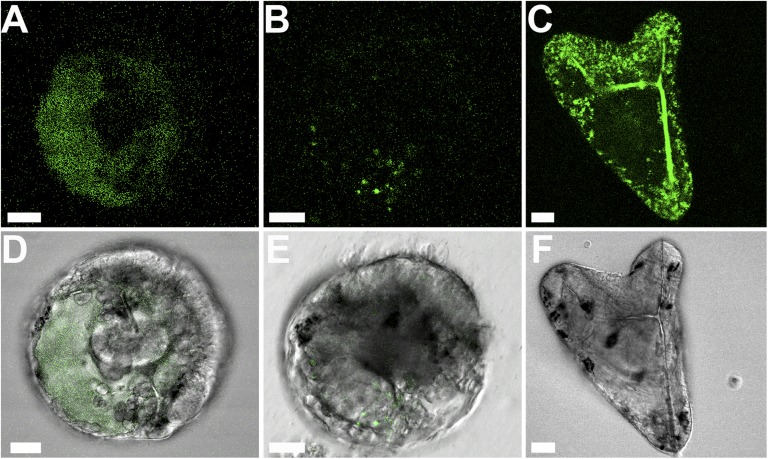
Localization of calcium in the embryonic environment was carried out using a confocal microscope monitoring calcein fluorescence. Embryos at the late gastrula stage first were exposed to a short 10-min pulse of calcein dissolved in the sea water and were observed after 1 h (Fig. 3 A and D). The calcein label is observed as a disperse cloud confined mainly within the blastocoel. After a longer calcein pulse (40 min), the calcein label appears as concentrated granules inside the epithelial cells and possibly the PMCs and other cells (Fig. 3 B and E).
Fig. 3.
Confocal micrographs of live calcein-labeled sea urchin embryos at the gastrula stage. (A–C) Green calcein fluorescence emission. (D and E) The fluorescence image in A and B is merged with the bright-field image. (F) Bright-field image of C. (A and D) The embryo received a 10-min calcein pulse at 40 h post fertilization (hpf), followed by a 1-h chase period in sea water. Calcein appears as a cloud in the blastocoel of the embryo and is not detected in an intracellular environment. One focal plane. (B and E) The embryo received a calcein pulse of 40 min at 40 hpf, followed by a 1-h chase period in sea water. Four focal planes, 2 μm apart, were stacked together. The calcein label is observed in the cellular environment in a more concentrated manner, in both the epithelial and other cells. (C and F) Embryo that was developed continuously in calcein-labeled sea water (46 hpf). Micrometer-size calcein-labeled granules are observed all over the embryo. One focal plane. Scale bars: 20 μm.
Surprisingly, when embryos were developed from fertilization continuously inside calcein-labeled sea water, calcein was detected as concentrated micrometer-sized granules all over the embryo (Fig. 3 C and F). The fluorescent label is concentrated not only in the blastocoel, where it presumably is associated with the PMCs attached to the forming spicules, but also in the epithelial cells. When chemically fixed embryos were exposed to calcein solution under the same conditions as those of the live embryos, calcein was observed inside the embryo, mostly as a dispersed cloud (Fig. S4). No label was observed in the spicule, and no extensive granular labeling occurred inside the cells. It should be taken into account that the labeling or absence thereof in the fixed embryo may be influenced by the fixation procedure and thus may not exclusively reflect passive label diffusion in vivo.
In agreement with the calcein labeling, cryo-SEM from untreated embryos reveals the presence of abundant nanosphere-containing vesicles also in the epithelial cells (Fig. 4A). The nanospheres contained in the vesicles inside the epithelial cells (Fig. 4 B–D) are within the same size range as those observed in the PMCs.
Fig. 4.
Cryo-SEM micrograph of a high-pressure frozen and freeze-fractured sea urchin embryo at the late gastrula stage. (A) The PMCs and epithelial cells (Ep) of the embryo are fractured, showing large numbers of intracellular vesicles (arrows). N, nuclei; S, spicule. (B) Epithelial cells, enlarged from A, showing fractured intracellular vesicles (arrow). (C and D) Fractured vesicle from an epithelial cell containing nanospheres (C) with corresponding BSE contrast image (D).
A definitive demonstration still is required to show that the calcein-labeled granules indeed are composed of solid calcium carbonate deposits. In particular, we need to demonstrate that calcein specifically maps the distribution of calcium and not of other divalent metal ions that also bind to calcein, such as Zn and Fe. With this in mind, we used the newly developed “airSEM” (B-nano Ltd.; www.b-nano.com), which is an SEM that operates in the atmosphere at ambient conditions (the sample is not kept under vacuum). Using this instrument, samples are shuttled directly from under the optical microscope objective to under the SEM column, which is separated from the atmospheric environment by a thin membrane that does not touch the sample. Movement between the two optical axes is done in synchronous register so that the images obtained from the SEM can be correlated directly with images from a fluorescence microscope. Unlike traditional SEMs, the airSEM allows imaging of humid samples, with no need for dehydration. For further information on the setup, see Materials and Methods.
Continuously calcein-labeled, fixed, and sectioned embryos embedded in a wet agarose gel produced in sea water first were observed under the fluorescence microscope and then were shuttled under the SEM objective at the precise location imaged in the light microscope. Fig. 5 compares the fluorescence image obtained from the section (Fig. 5C) with the distribution of heavy atoms using backscattered electrons (Fig. 5A), and with the calcium map using energy dispersive spectroscopy (EDS) (Fig. 5B), all in the exact same area. A comparison of the images shows that the calcein signal is associated with calcium rather than with other ions or with free calcein (Fig. 5D). The differences between the images mainly are a result of the fact that the depth of sampling of the three techniques is different. Fluorescence detects signals from the whole slice (100 μm), whereas EDS and backscattering under the conditions used detect signals to a depth of up to about 3 μm.
Fig. 5.
Correlative airSEM image of a ≤100-μm slice of a fixed embryo at ambient conditions showing the BSE signal (A), calcium EDS map (B), and calcein fluorescence (C). (D) The correlation between the calcium EDS signal (B) and calcein fluorescence (C) is shown in the superimposition of the two images. Yellow, colocalized signals; green and red, fluorescence and calcium EDS signals, appearing separately. Slight deformation of the surface, resulting from electron beam damage, may have decreased the actual colocalization area.
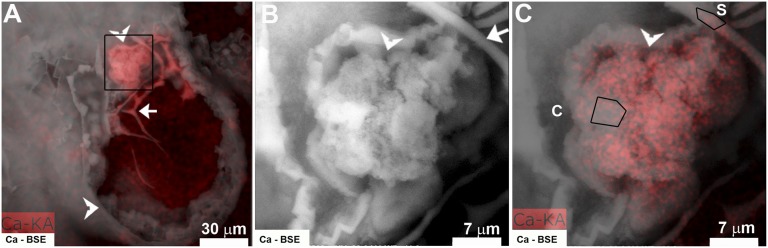
To show that the calcium deposits are of solid calcium carbonate, we again used the airSEM to image a 50-μm slice of a fixed embryo at higher resolution. A good correlation between the backscattered electron signal and the calcium EDS map was observed (Fig. 6 A and C). Quantitative elemental EDS analyses were performed in two different areas within the same image (Fig. 6C): a part of the spicule, which is composed of solid CaCO3, and a group of cells that because of their location near the outer embryonic surface and the spicule, probably consist of PMCs and epithelial cells. The amount of calcium measured in the spicule, 6.3 wt %, is practically identical to the amount measured in the intracellular compartment, 6.2 wt % (Table 1; related EDS spectra are shown in Fig. S5). Control areas with a low calcein label and high backscattering signal give calcium amounts <2% (Fig. S6). This observation shows that solid calcium carbonate is present within the cells. At both locations, the calcium content is lower than the expected calcium concentration of pure CaCO3 (40 wt %) because of the presence of other elements in the sample area, especially Na and Cl from sea water and C and O from the agarose and the cell components. The calcium concentration measured in the cellular area is much higher than any value that could be obtained from concentrated cytoplasm or sea water.
Fig. 6.
BSE image (white) and calcium EDS map (red) of a fixed and sliced embryo taken with the airSEM. (A) Whole embryo, with calcium EDS map (red) and BSE signal (white) superimposed. The spicule is marked with an arrow, and the membrane enveloping the embryo is marked with arrowheads. (B) Enlargement of the square-labeled area in A, containing a part of the spicule (arrow) and cell group (arrowhead); BSE image. (C) The same region as in B, with calcium EDS map (red) superimposed. EDS quantitative analysis of the marked areas is presented in Table 1. C, cell group; S, spicule.
Table 1.
EDS elemental analysis of the spicule and cell areas
| Element | Spicule, wt % | Cells, wt % |
| Oxygen | 27 ± 12 | 35 ± 15 |
| Carbon | 18 ± 9 | 16 ± 8 |
| Sodium | 22.1 ± 5.4 | 18.4 ± 4.7 |
| Chlorine | 18.8 ± 2.4 | 12.4 ± 1.7 |
| Nitrogen | 6 ± 4 | 10 ± 6 |
| Calcium | 6.3 ± 0.7 | 6.2 ± 0.7 |
| Magnesium | 1.0 ± 0.3 | 0.9 ± 0.3 |
| Phosphorus | 0.08 ± 0.09 | 0.15 ± 0.10 |
Discussion
We monitored aspects of the calcium pathway in sea urchin embryos from the sea water until incorporation in the spicules. We show that calcein-labeled calcium first appears dispersed inside the blastocoel, where the calcium most likely still is in the form of free ions. Calcium is later observed as concentrated micrometer-size intracellular granules, not only inside PMCs, which are known to play a crucial role in spicule mineralization, but also in epithelial cells. These granules are composed of solid calcium carbonate nanospheres that most likely are ACC. The calcitic spicule is composed of nanospheres of the same size and shape (24), indicating that the intracellular nanosphere-bearing granules are part of the mineralization pathway and eventually are integrated into the spicule.
The fact that calcium is first observed dispersed in the blastocoel implies that calcium penetrates from sea water into the embryo through or between the epithelial cells and subsequently is deposited intracellularly in the form of solid calcium carbonate deposits inside vesicles. Based on Beniash et al. (19), we assume that these intracellular calcium carbonate deposits are composed of ACC. Surprisingly, these mineral deposits are formed not only in the PMCs, but also in epithelial cells. This raises the question: what is the function of the calcium carbonate deposits inside the epithelial cells?
One possible function of the epithelial mineral deposits is that they are a reservoir of calcium and carbonate ions needed by the embryo for other purposes, or conceivably for future shell and tooth formation after the larva undergoes metamorphosis. Another possibility is that some of this mineral is a reservoir for spicule formation. In this case, it would have to be transported through the blastocoel to the syncytium, which might occur in the form of ions after the extruded ACC dissolves. An alternative possibility is that the granules might be transported as such through the blastocoel. We did observe multivesicular bodies (Fig. 1A and Fig. S1C) composed of vesicles of the same size as the mineral-bearing intracellular vesicles. These unusual noncellular structures may be transporting mineral from the epithelial cells to the syncytium, where spicule formation occurs. It is interesting to note that Ettensohn and Malinda (25) demonstrated in vivo that photoablation of a distinctive region of ectoderm resulted in inhibition of spicule elongation, suggesting that a local ectoderm–PMC interaction is required for spicule growth. Our results indicate that the interactions between the ectoderm cells and the spicule compartment also may involve actual calcium transport.
The PMCs presumably take up dissolved ions from the blastocoel. It has been shown in culture that they do possess calcium ion pumps (15). Adding manganese in vivo reduced calcium uptake into the PMCs and prevented spicule formation, possibly because of manganese and calcium competition on the same ion pumps (16). Interestingly, calcium was detected as calcein-labeled intracellular granules even in Mn-exposed embryos (16). This might be the result of cellular calcium uptake that is not in the form of ions, possibly through particle endocytosis.
We do not know how the mineral in the vesicles of the PMCs is translocated into the membrane-bound spicule formation compartment. Using cryo-SEM, we have observed vesicles located in the interface between a PMC and the spicule compartment that appear to be heading toward the spicule (Fig. S2). We also have observed spicules in the process of being formed surrounded by micrometer-sized mineral granules resembling the intracellular granules (Fig. 2). These observations are consistent with the mineral granules being translocated in the solid state from inside the cells into the spicule formation compartment, as suggested by Beniash et al. (19) and Ingersoll et al. (26).
Stable and transient amorphous mineral phases formed by many organisms consist of nanospheres (27, 28). It is possible that the nanospheres themselves are derived from smaller building blocks, such as stable ion clusters, which were shown to exist even in undersaturated calcium carbonate solutions (29, 30). In the case of the disordered calcium phosphate precursor phase in zebrafish fin rays, the nanospheres also appear in intracellular membrane-delimited vesicles, which are delivered to the mineralization site (2). In vitro studies of the involvement of nanospheres in calcite single-crystal growth from biogenic ACC exposed to water show that 20–30-nm nanospheres are translocated to the surface of a growing crystal, where they crystallize (28). Analogously, in the case of the growing embryonic spicule, after delivery of the vesicle-packed mineral, the disaggregated nanospheres presumably then crystallize as a result of contact with other already-crystalline nanospheres to ultimately form a single crystal (21, 22).
From the discussion above, a conceivable scenario for a general ion pathway emerges. The ions first are concentrated, and the mineral is deposited in both specialized and nonspecialized cells. The mineral then is transported to the mineralization site, where it is incorporated as such into the developing mineralized tissue.
Conclusions
We demonstrate using confocal microscopy, cryo-SEM, and airSEM that during the process of calcium carbonate deposition in sea urchin embryos, calcium is first introduced into the blastocoel in a dispersed form. The mineral then appears inside PMCs, as well as inside epithelial cells, as membrane-bound micrometer-size granules. The granules finally are introduced into the spicule compartment, where they presumably disaggregate into nanospheres and crystallize by secondary nucleation.
Materials and Methods
Sea Urchin Embryonic/Larval Culture.
Ripe cultured adult sea urchins Paracentrotus lividus were produced and supplied by the Israel Oceanographic and Limnological Research Institute. Spawning was induced by injecting 1 mL of 1 M KCl solution into the coelomic cavity. The egg suspension was collected, washed, and kept in sea water containing 30 mg/L penicillin (Sigma–Aldrich) and 15 mg/L streptomycin (Sigma–Aldrich) at 18 °C. Sperm was kept at 4 °C undiluted. The fertilization was carried out up to 24 h after spawning by sperm dilution in sea water and rapid mixing with the egg suspension inside sea water containing 30 mg/L penicillin and 15 mg/L streptomycin. The culture was kept at 18 °C with gentle shaking (100 rpm). The developmental stage of the embryo was determined using a light microscope with normal and polarized light. The research involving sea urchins is approved by the Israel Oceanographic and Limnological Research, National Center for Mariculture.
Calcein Labeling.
Calcein, 150 μg/mL (Sigma–Aldrich), was dissolved in sea water containing 30 mg/L penicillin and 15 mg/L streptomycin and filtered in a 0.22-μm sterile Corning filter system. For pulse–chase experiments, the embryos were transferred from sea water to calcein-labeled sea water for 10 or 40 min (pulse period) at 40 h post fertilization, washed in sea water, and transferred to unlabeled sea water for 1 h (chase period). For continuous labeling, the embryos were developed from fertilization inside the calcein-labeled sea water at 18 °C with gentle shaking (100 rpm). Approximately 46 h post fertilization, the embryos were washed with sea water.
For the control experiment, embryos 46 h after fertilization were fixed for 1 h in sea water containing 2.5% (wt/vol) glutaraldehyde and 2.5% (wt/vol) paraformaldehyde. The fixed embryos were washed in sea water and exposed to the same calcein solution as the live embryos for 40 min. The embryos were washed with sea water and imaged in the confocal microscope after a 1-h chase period in sea water.
A drop of the embryo suspension was put on a glass-bottom Petri plate, followed by a drop of 5% (wt/vol) agarose gel (SeaKem) in sea water solution to confine the embryos spatially in a hydrated environment.
Confocal Imaging.
The calcein fluorescence emission and transmitted light bright-field images were obtained by using a confocal laser scanning microscope, Olympus FluoView 1000, with 20× and 40× objective lens magnification. The laser wavelength was (excitation) 488 nm, and emission was taken with a 520–550-nm band-pass filter. A Z series of both the transmitted and fluorescence images was collected for each specimen with 2-μm steps.
Cryo-SEM Imaging.
Embryos during the late gastrula stage, ∼40 h post fertilization, were high-pressure frozen: 10 μL of the embryo suspension was sandwiched between two metal discs (3-mm diameter, 0.1-mm cavities) and cryoimmobilized in a high-pressure freezing device (HPM10; Bal-Tec). The frozen samples were kept in liquid nitrogen and transferred by using a vacuum cryotransfer device (VCT 100; Leica Microsystems) to a freeze-fracture device (BAF 60; Leica Microsystems). Samples were freeze-fractured at −120 °C, etched for 10 min at −105 °C, and coated with 2.5 nm platinum/carbon by double-axis rotary shadowing. Samples were observed at −120 °C in an Ultra 55 SEM (Zeiss) by using a secondary electron in-lens detector and a backscattered electron in-lens detector (5 kV). Image brightness and contrast levels were adjusted by using Adobe Photoshop.
airSEM Imaging.
Embryos continuously developed in calcein-labeled sea water were chemically fixed using 4% paraformaldehyde in sea water for 1 h, washed with sea water, and mounted in 7% agarose gel dissolved in sea water. The mounted sample was sliced using a vibratome to a thickness of 50–100 μm, and the slices were put on a glass slide and imaged using the airSEM within 2 h. The airSEM (B-nano Ltd.) operates in a direct correlative manner: the optical and SEM microscopes are located on a single platform, both in upright geometry, and the sample is shuttled between the two optical axes with accurate registration. Embryos first were imaged under the optical microscope for sample orientation and fluorescent imaging. Epifluorescent images of the calcein fluorescence (Ex': BP460-495; Em': BA510-550) were acquired using a 20× objective. The sample then was shuttled to the optical axes of the SEM, and the same area was imaged at a matching field of view by using backscattered electrons (beam energy: 30 kV; probe current: 1000 pA). It is noteworthy that working in air, the backscattered electron image does not suffer from charge-induced problems, as occurs in conventional SEM microscopes operating under vacuum.
Elemental analysis and mapping were carried out at the same location by an EDS detector placed on the same optical axes of the SEM microscope. At all stages, the sample was held at ambient conditions, with no exposure to the vacuum environment. No additional processing was performed on the sample.
Supplementary Material
Acknowledgments
We thank Vladimir Kiss and Reinat Nevo for their help with confocal microscopy and Eugenia Klein, Elena Kartvelishvily, and the Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging at the Weizmann Institute of Science for their help and guidance. The research was supported by a German Research Foundation grant within the framework of the Deutsch–Israelische Projektkooperation and a Department of Energy award (DE-FG02-07ER15899). L.A. is the incumbent of the Dorothy and Patrick Gorman Professorial Chair of Biological Ultrastructure, and S.W. is the incumbent of the Dr. Trude Burchardt Professorial Chair of Structural Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312833110/-/DCSupplemental.
References
- 1.Volk GM, Goss LJ, Franceschi VR. Calcium channels are involved in calcium oxalate crystal formation in specialized cells of Pistia stratiotes L. Ann Bot (Lond) 2004;93(6):741–753. doi: 10.1093/aob/mch092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahamid J, et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc Natl Acad Sci USA. 2010;107(14):6316–6321. doi: 10.1073/pnas.0914218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowenstam HA, Weiner S. Transformation of amorphous calcium phosphate to crystalline dahillite in the radular teeth of chitons. Science. 1985;227(4682):51–53. doi: 10.1126/science.227.4682.51. [DOI] [PubMed] [Google Scholar]
- 4.Addadi L, Raz S, Weiner S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv Mater. 2003;15(12):959–970. [Google Scholar]
- 5.Nakano E, Okazaki K, Iwamatsu T. Accumulation of radioactive calcium in larvae of the sea urchin Pseudocentrotus depressus. Biol Bull. 1963;125(1):125–132. [Google Scholar]
- 6.Berman A, et al. Intercalation of sea urchin proteins in calcite: Study of a crystalline composite material. Science. 1990;250(4981):664–667. doi: 10.1126/science.250.4981.664. [DOI] [PubMed] [Google Scholar]
- 7.Wilt FH. Biomineralization of the spicules of sea urchin embryos. Zoolog Sci. 2002;19(3):253–261. doi: 10.2108/zsj.19.253. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki K. Spicule formation by isolated micromeres of the sea urchin embryo. Am Zool. 1975;15(3):567–581. [Google Scholar]
- 9.Wallach DFH, Surgenor DM, Soderberg J, Delano E. Preparation and properties of 3,6-dihydroxy-2,4-bis-[N-N'-di-(carboxymethyl)-aminomethyl] fluoran. Anal Chem. 1959;31(3):456–460. [Google Scholar]
- 10.Moran AL. Calcein as a marker in experimental studies of newly-hatched gastropods. Mar Biol. 2000;137(5-6):893–898. [Google Scholar]
- 11.Du SJ, Frenkel V, Kindschi G, Zohar Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev Biol. 2001;238(2):239–246. doi: 10.1006/dbio.2001.0390. [DOI] [PubMed] [Google Scholar]
- 12.Tester CC, et al. Time-resolved evolution of short- and long-range order during the transformation of amorphous calcium carbonate to calcite in the sea urchin embryo. Adv Funct Mater. 2013;23(34):4185–4194. [Google Scholar]
- 13.Wilt FH, Killian CE, Hamilton P, Croker L. The dynamics of secretion during sea urchin embryonic skeleton formation. Exp Cell Res. 2008;314(8):1744–1752. doi: 10.1016/j.yexcr.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guss KA, Ettensohn CA. Skeletal morphogenesis in the sea urchin embryo: Regulation of primary mesenchyme gene expression and skeletal rod growth by ectoderm-derived cues. Development. 1997;124(10):1899–1908. doi: 10.1242/dev.124.10.1899. [DOI] [PubMed] [Google Scholar]
- 15.Hwang SP, Lennarz WJ. Studies on the cellular pathway involved in assembly of the embryonic sea urchin spicule. Exp Cell Res. 1993;205(2):383–387. doi: 10.1006/excr.1993.1101. [DOI] [PubMed] [Google Scholar]
- 16.Pinsino A, Roccheri MC, Costa C, Matranga V. Manganese interferes with calcium, perturbs ERK signaling, and produces embryos with no skeleton. Toxicol Sci. 2011;123(1):217–230. doi: 10.1093/toxsci/kfr152. [DOI] [PubMed] [Google Scholar]
- 17.Farach MC, Valdizan M, Park HR, Decker GL, Lennarz WJ. Developmental expression of a cell-surface protein involved in calcium uptake and skeleton formation in sea urchin embryos. Dev Biol. 1987;122(2):320–331. doi: 10.1016/0012-1606(87)90297-1. [DOI] [PubMed] [Google Scholar]
- 18.Knapp RT, Wu C-H, Mobilia KC, Joester D. Recombinant sea urchin vascular endothelial growth factor directs single-crystal growth and branching in vitro. J Am Chem Soc. 2012;134(43):17908–17911. doi: 10.1021/ja309024b. [DOI] [PubMed] [Google Scholar]
- 19.Beniash E, Addadi L, Weiner S. Cellular control over spicule formation in sea urchin embryos: A structural approach. J Struct Biol. 1999;125(1):50–62. doi: 10.1006/jsbi.1998.4081. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki K, Inoue S. Crystal property of the larval sea urchin spicule. Dev Growth Differ. 1976;18(4):413–434. doi: 10.1111/j.1440-169X.1976.00413.x. [DOI] [PubMed] [Google Scholar]
- 21.Politi Y, et al. Transformation mechanism of amorphous calcium carbonate into calcite in the sea urchin larval spicule. Proc Natl Acad Sci USA. 2008;105(45):17362–17366. doi: 10.1073/pnas.0806604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner S, Addadi L. Crystallization pathways in biomineralization. Annu Rev Mater Res. 2011;41:21–40. [Google Scholar]
- 23.Politi Y, et al. Structural characterization of the transient calcium carbonate amorphous precursor phase in sea urchin embryos. Adv Funct Mater. 2006;16(10):1289–1298. [Google Scholar]
- 24.Addadi L, Vidavsky N, Weiner S. Transient precursor amorphous phases in biomineralization. In the footsteps of Heinz A. Lowenstam. Z Kristallogr. 2012;227(11):711–717. [Google Scholar]
- 25.Ettensohn CA, Malinda KM. Size regulation and morphogenesis: A cellular analysis of skeletogenesis in the sea urchin embryo. Development. 1993;119(1):155–167. doi: 10.1242/dev.119.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Ingersoll EP, McDonald KL, Wilt FH. Ultrastructural localization of spicule matrix proteins in normal and metalloproteinase inhibitor-treated sea urchin primary mesenchyme cells. J Exp Zoolog A Comp Exp Biol. 2003;300(2):101–112. doi: 10.1002/jez.a.10316. [DOI] [PubMed] [Google Scholar]
- 27.Mahamid J, Sharir A, Addadi L, Weiner S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc Natl Acad Sci USA. 2008;105(35):12748–12753. doi: 10.1073/pnas.0803354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gal A, et al. Calcite crystal growth by a solid-state transformation of stabilized amorphous calcium carbonate nanospheres in a hydrogel. Angew Chem Int Ed Engl. 2013;52(18):4867–4870. doi: 10.1002/anie.201210329. [DOI] [PubMed] [Google Scholar]
- 29.Pouget EM, et al. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science. 2009;323(5920):1455–1458. doi: 10.1126/science.1169434. [DOI] [PubMed] [Google Scholar]
- 30.Gebauer D, Völkel A, Cölfen H. Stable prenucleation calcium carbonate clusters. Science. 2008;322(5909):1819–1822. doi: 10.1126/science.1164271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.