Abstract
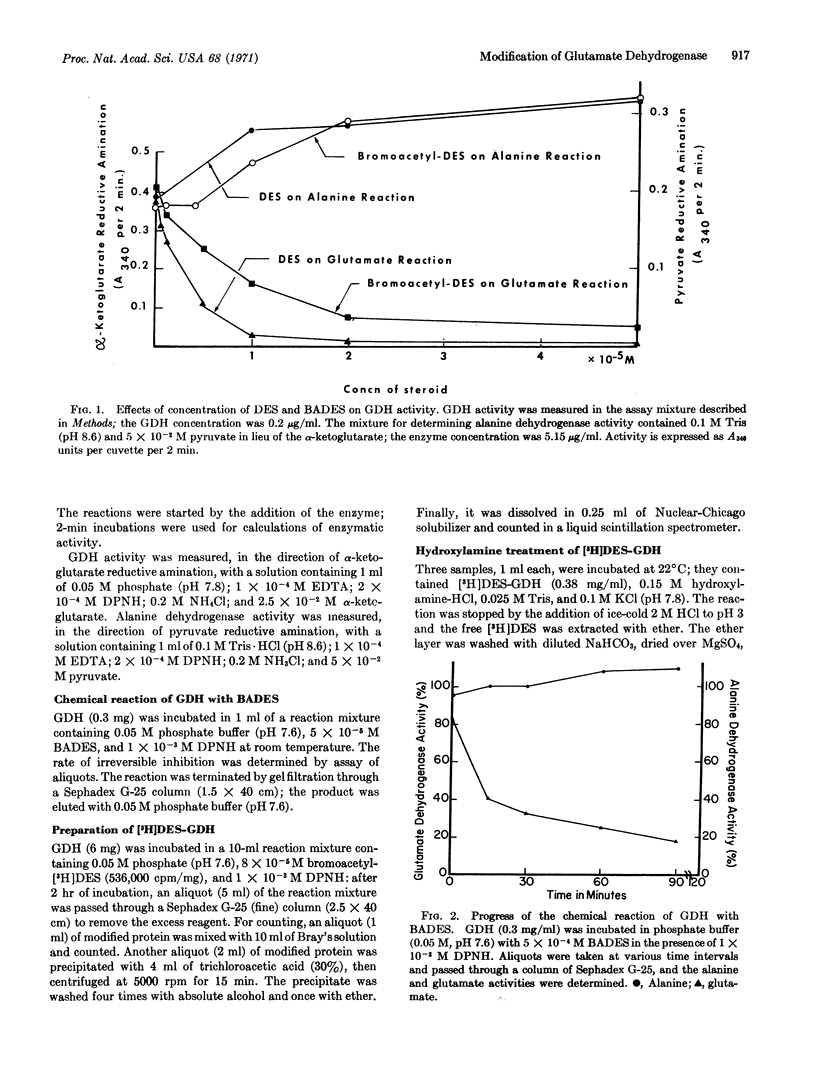
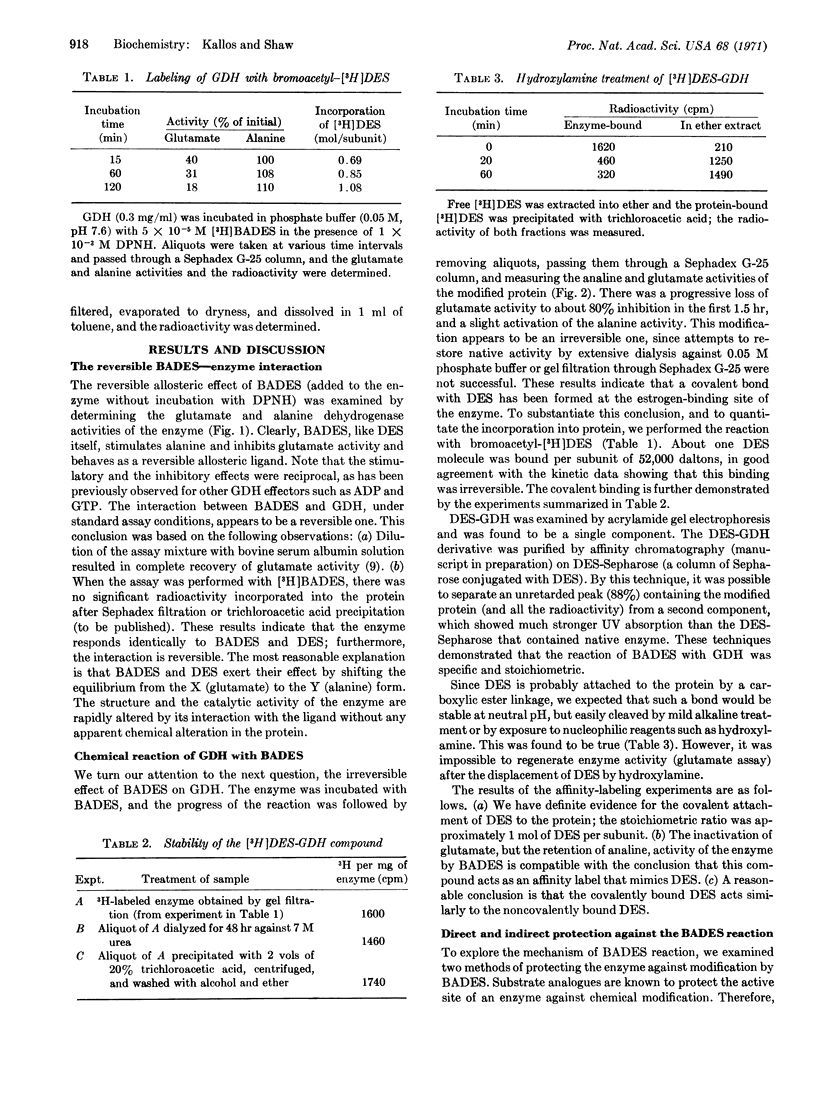
An affinity labeling reagent for the estrogenic-binding site of bovine liver L-glutamate dehydrogenase (EC 1.4.1.3) was prepared by conversion of diethylstilbestrol to its alkylating analogue, bromoacetyldiethylstilbestrol. Under standard assay conditions, the analogue acted as a reversible allosteric ligand with regulatory activity much like that of diethylstilbestrol. However, incubation of the enzyme with the alkylating agent in the presence of DPNH resulted in a permanent decrease in glutamate (X form) and an increase in alanine (Y form) activities, and in covalent attachment of diethylstilbestrol in the ratio of 1 mol per subunit (of particle weight 52,000). The brominated analogue behaved as an affinity label that mimicked the allosteric effects of diethylstilbestrol. Diethylstilbestrol protection of the enzyme against alkylation by bromoacetylated sterol suggested competition for the same binding site, while ADP protection indicated a shift of protein equilibrium into the X form. The diethylstilbestrol-enzyme compound was desensitized (relative to the native enzyme) to allosteric reagents such as ADP and GTP. The results were consistent with conformational freezing of the modified protein molecule into the Y form.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITENSKY M. W., YIELDING K. L., TOMKINS G. M. THE REVERSAL BY ORGANIC MERCURIALS OF "ALLOSTERIC" CHANGES IN GLUTAMATE DEHYDROGENASE. J Biol Chem. 1965 Feb;240:668–673. [PubMed] [Google Scholar]
- Colman R. F., Frieden C. On the role of amino groups in the structure and function of glutamate dehydrogenase. II. Effect of acetylation on molecular properties. J Biol Chem. 1966 Aug 25;241(16):3661–3670. [PubMed] [Google Scholar]
- FRIEDEN C. GLUTAMATE DEHYDROGENASE. V. THE RELATION OF ENZYME STRUCTURE TO THE CATALYTIC FUNCTION. J Biol Chem. 1963 Oct;238:3286–3299. [PubMed] [Google Scholar]
- Ginsburg A. Conformational changes in glutamine synthetase from Escherichia coli. II. Some characteristics of the equilibrium binding of feedback inhibitors to the enzyme. Biochemistry. 1969 Apr;8(4):1726–1740. doi: 10.1021/bi00832a056. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Neet K. E. The catalytic and regulatory properties of enzymes. Annu Rev Biochem. 1968;37:359–410. doi: 10.1146/annurev.bi.37.070168.002043. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Yielding K. L., Tomkins G. M. STRUCTURAL ALTERATIONS IN CRYSTALLINE GLUTAMIC DEHYDROGENASE INDUCED BY STEROID HORMONES. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1483–1488. doi: 10.1073/pnas.46.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]


