Significance
Domestic cats are one of the most popular pets worldwide, but little is known about their domestication. This study of cats living 5,300 y ago at the agricultural village of Quanhucun, China provides the earliest known evidence for mutualistic relationships between people and cats. Isotopic data demonstrate that humans, rodents, and the cats ate substantial amounts of millet-based foods, with cats preying on grain-eating animals. One cat was old and one ate less meat and more millet than others, suggesting it scavenged leftovers or was fed. Diverse data demonstrate rodent threats to stored grain, indicating cats were advantageous to farmers, whereas food in villages was attractive to cats. These findings provide evidence for commensal processes of cat domestication.
Keywords: zooarchaeology, felid, mutualism, stable isotopes, Quanhucun site
Abstract
Domestic cats are one of the most popular pets globally, but the process of their domestication is not well understood. Near Eastern wildcats are thought to have been attracted to food sources in early agricultural settlements, following a commensal pathway to domestication. Early evidence for close human–cat relationships comes from a wildcat interred near a human on Cyprus ca. 9,500 y ago, but the earliest domestic cats are known only from Egyptian art dating to 4,000 y ago. Evidence is lacking from the key period of cat domestication 9,500–4,000 y ago. We report on the presence of cats directly dated between 5560–5280 cal B.P. in the early agricultural village of Quanhucun in Shaanxi, China. These cats were outside the wild range of Near Eastern wildcats and biometrically smaller, but within the size-range of domestic cats. The δ13C and δ15N values of human and animal bone collagen revealed substantial consumption of millet-based foods by humans, rodents, and cats. Ceramic storage containers designed to exclude rodents indicated a threat to stored grain in Yangshao villages. Taken together, isotopic and archaeological data demonstrate that cats were advantageous for ancient farmers. Isotopic data also show that one cat ate less meat and consumed more millet-based foods than expected, indicating that it scavenged among or was fed by people. This study offers fresh perspectives on cat domestication, providing the earliest known evidence for commensal relationships between people and cats.
The domestication of cats resulted in widespread adoption of cats as pets, a role for cats as rodent and small animal predators in human settlements, and expansion of the range and populations of cats around the world. Today there are more than half a billion cats worldwide. Despite the importance of cats in the modern world and their long history with humans, there is remarkably little archaeological evidence on their domestication.
Studies of mitochondrial DNA from modern wildcats and domestic cats demonstrate that ancient populations of Near Eastern wildcats (Felis silvestris lybica) were the maternal ancestors of domestic cats (1–3). A wildcat phalanx from the site of Klimonas shows that they were introduced to Cyprus 11000–10500 B.P. (all dates are reported in calibrated years before present), providing the earliest connection between humans and cats (4). The earliest cat to demonstrate a close association with humans is also from Cyprus, where a young wildcat was interred next to a human at the site of Shillourokambos ca. 9,500 y ago (5). Isolated cat bones have been found at Near Eastern sites, such as Jericho (6), but little is known about the crucial period for cat domestication between 9,000 and 4,000 y ago. Healed fractures on the forelimbs of a young swamp cat (Felis chaus) buried in a ca. 5,500-y-old grave at Hierakonpolis in Upper Egypt indicate that wildcats were actively cared for by ancient Egyptians (7, 8). However, the first evidence for domestic cats is based on Middle Kingdom Egyptian art dated to ca. 4000 B.P. (1, 6). Trade in cats was prohibited in ancient Egypt, but they were nevertheless exported to Greece around 3,000 y ago and from there to Europe (6). Cats were thought to have first appeared in China around 2,000 y ago (1). Claims for earlier cats in the region have been made (Table S1), but without precise dates or detailed biometric measurements these have been difficult to evaluate.
Current thinking emphasizes domestication as a mutualistic relationship between humans and animals, with microevolutionary changes resulting from natural and humanly directed selection in anthropogenic environments or the human niche (2, 9–12). Prey pathways to domestication have been well documented through culling of ancient goats, sheep, and cattle, and studies of age and sex profiles (10). Directed pathways are reflected in pathologies or penning indicative of management of transport animals, and most recently by evidence for ancient milking (10, 13). Commensal pathways are thought to be the route that dogs, pigs, and cats followed to domestication (10). It has been hypothesized that territorial wildcats were drawn to early agricultural villages to prey on rodents attracted to grain stores and to take advantage of year-round food sources in the human niche (5, 6, 9–12, 14, 15). Although relationships between the territorial and solitary behavior of cats and selection processes in human environments are well understood in the modern world (1, 2), archaeological evidence for commensal pathways to domestication has proven difficult to find.
The discovery of felid remains from Middle-late Yangshao Culture (6000–5000 B.P.) levels at the site of Quanhucun in Shaanxi, China (Fig. S1) (16) provided a rare opportunity to use archaeological data to examine early relations between people and cats. To answer the question of whether the early Chinese felids were wild or domestic and to investigate the role of cats in Yangshao villages, we undertook multidisciplinary analyses of cat bones from Quanhucun. We report here on accelerator mass spectromery (AMS)-14C dating of cat bones, biometric measurements of cat skeletal elements, and carbon and nitrogen isotope analysis of human and animal bones.
Archaeological Context
The Yangshao Culture (7000∼5000 B.P.) is one of the best-known cultures of the Chinese Neolithic and mainly distributed within the territory of Shaanxi, Shanxi, and Henan Provinces. Yangshao villages were comprised of houses, cemeteries, and settlements that were often used by large groups of people for long periods. Foxtail millet (Setaria italica) and common millet (Panicum miliaceum) were cultivated and domestic pigs (Sus scrofa) and dogs (Canis familiaris) were kept (17). The site of Quanhucun (N 34°32′53″, E 109°51′40″) (Fig. S1) is located at the village of Quanhucun, Hua County, Shaanxi Province, China. According to typological analysis of pottery, most archaeological features date to the Middle-Late Yangshao Culture (6000∼5000 B.P.) with three cultural phases (16–18). All of the archaeological contexts studied below fall within the Yangshao period.
Archaeological features and artifacts found at the site included houses, storage pits, and large quantities of pottery and floral and faunal remains, but few human burials. The crops identified by phytoliths and carbonized seeds were predominantly millets, complemented by some rice (Oryza sativa) (19). Thirty-two faunal taxa were present at the site including hare (Lepus capensis), pig (S. scrofa), dog (C. familiaris), sika deer (Cervus nippon), roe deer (Capreolus capreolus), felid (Felis sp.), tiger (Panthera tigris), and fish (Pices), as well as rodents (Cricetidae).
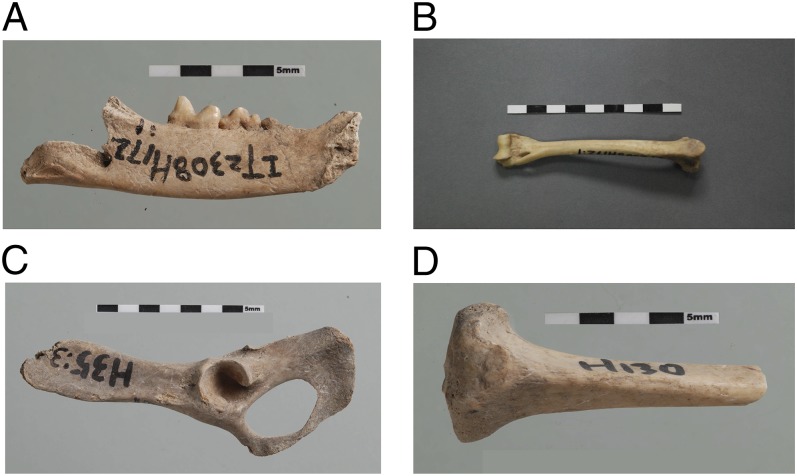
Eight felid skeletal elements are listed in Table 1 and selected elements depicted in Fig. 1. The felid bones were found in an ashy matrix in three refuse pits, H172, H35, and H130, with animal bones, pottery sherds, bone tools, and some stone tools. In addition to felids, several lines of evidence indicated the presence of rodents at Quanhucun. Bones identified to the family Cricetidae, including the common Chinese zokor (Myospalax sp.), provided direct evidence of rodents (16). The relationship of rodents to food supplies was indicated by ancient rodent burrows leading into grain-storage pits (16). Ceramic assemblages at the site also included crop storage vessels depicted in Fig. S2 and known from other Yangshao sites for their special design, which protected food-stores by making it difficult for rodents to climb into them (16).
Table 1.
Felid skeletal elements from the site of Quanhucun showing the presence of partial skeletons with measureable elements distributed among several pit contexts
Fig. 1.
Felid specimens from the site of Quanhucun showing key body parts and the presence of an aged animal with worn dentition. (A) Left mandible with worn fourth premolar and first molar; (B) right humerus; (C) left pelvis; (D) proximal left tibia.
Results
AMS-14C Dating.
Two cat specimens from different stratigraphic layers were sampled for AMS-carbon dating (Table 2). The cat from H130:1 and that from H172:1 date to 5320∼5280 cal B.P. and 5560–5470 cal B.P., respectively, if one SD is adopted. In brief, these cats date to ca. 5,300 y ago and over a 200-y period.
Table 2.
AMS-14C data showing early direct dates for two cat specimens from the site of Quanhucun
| Lab number | Location | 14C date (B.P.) | Calibrated age (cal B.P.) |
|
| 1σ (68.2%) | 2σ (95.4%) | |||
| BA110854 | H130:1 | 4580 ± 25 | 5440∼5420 (7.6%) | 5450∼5410 (12.7%) |
| 5320∼5280 (55.7%) | 5330∼5280 (61.8%) | |||
| 5160∼5140 (4.9%) | 5170∼5130 (11.5%) | |||
| 5110∼5070 (9.4%) | ||||
| BA110855 | H172:1 | 4765 ± 30 | 5590∼5570 (8.2%) | 5590∼5460 (91.0%) |
| 5560∼5470 (60.0%) | 5380∼5330 (4.4%) | |||
The half life of 14C is 5,568 y and B.P. is referred as the date before 1950.
Zooarchaeological Analysis.
This study focused on identification and aging of cats using traditional morphological observations and biometric measurements. Eight specimens were identified [number of identifiable specimens (NISP) = 8] as felidae cf. Felis sp. A minimum number (MNI) of two individuals was calculated on the basis of two proximal left tibias. Given the distribution of the bones in pits excavated in different areas of the site, it is likely, however, that more than two individuals are represented in the study sample. The left mandible from pit H172D:1 (Fig. 1A) preserves a very worn fourth premolar and first molar (carnassial) and represents an aged animal. The biometric measurements of other intact cat skeletal elements, including the left humerus (Fig. 1B) and the left pelvis (Fig. 1C), are presented in Table 3.
Table 3.
Morphological comparison among cats from Quanhucun, ancient Egyptian (7), and European wildcats and house cats (20), showing the cats studied seemed more similar in size to domestic cats than wildcats
| Biometric parameter | House cat (mean ± standard deviation mm) | European wildcat (mean ± standard deviation mm) | Quanhucun cat (mm) | Ancient Egyptian cat Tel el-dab’a (mm) | Ancient Egyptian cats el Kab (mm) |
| Humerus GL | 96.46 ± 4.89 (n = 62) | 119.08 ± 4.89 (n = 19) | 105.6 | 112 | |
| Humerus Dp | 20.32 ± 1.31 (n = 62) | 24.66 ± 1.85 (n = 19) | 21.5 | ||
| Humerus Bd | 17.91 ± 1.16 (n = 62) | 22.18 ± 1.63 (n = 19) | 18.2 | 20.5 | |
| Humerus SD | 6.64 ± 0.68 (n = 62) | 8.04 ± 0.52 (n = 19) | 7.1 | ||
| Pelvis GL | 43.59 ± 2.59 (n = 63) | 52.7 ± 2.49 (n = 20) | 79 | ||
| Pelvis LAR | 10.96 ± 0.81 (n = 63) | 12.92 ± 0.79 (n = 20) | 11 | 13.5 | 14 |
| Pelvis SH | 10.9 ± 0.9 (n = 63) | 13.18 ± 0.94 (n = 20) | 9.5 |
Measurement codes follow von den Driesch (38). Bd, breadth of distal end; Dp, proximal end; GL, greatest length; SD, smallest breadth of diaphysis.
Asiatic wildcat skeletons, such as the Central Asian wildcat (Felis sylvstris ornata) and the Chinese desert cat (Felis sylvstris bieti), are rare in world collections. Measurements of ancient cats are also uncommon. Therefore, we compared the size of the Quanhucun felids with published data on modern European wildcats (Felis sylvestris sp.) from the western Carpathians (20), modern house cats from the Brno region in Czechoslovakia (20), and isolated cat humerus and pelvis specimens from the ancient the Egyptian sites of Tel el-dab’a and El Kab (7) (Table 3).
The greatest length of the cat humerus from Quanhucun H172D:1 is larger than that of European domestic cats, but smaller than and outside of the range of European wildcats, and smaller than the ancient domestic cat from el Kab. Other humerus measurements, such as greatest depth of the proximal end, greatest breadth of distal end, and smallest breadth of diaphysis, fit within the range of European domestic cats and are substantially smaller than those of European wildcats or the cat specimen from el Kab. This pattern is repeated in the pelvis from H35D:3. Taken together, these morphometric data are suggestive of domesticated cats. However, additional information on the range of size variation in Asian wildcats and ancient DNA evidence is needed to secure identifications. Isotopic data on foodwebs are key to understanding relations between humans and cats at Quanhucun.
Isotope Analysis.
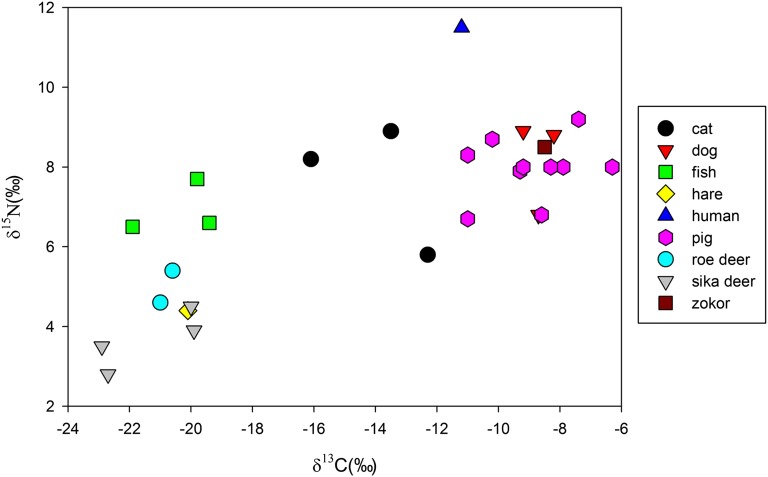
There were clear patterns in animal and human foodwebs. In Fig. 2, herbivores like sika deer, roe deer, and hare had the lowest δ13C and δ15N values, indicative of consumption of C3 plants, probably from plant leaves and C3 grasses. The mean δ13C and δ15N values of the herbivores studied here were −21.0 ± 1.3‰ (n = 7) and 4.2 ± 0.8‰ (n = 7), respectively, setting up an isotopic baseline for understanding the domestic animal diets. The average δ15N value of 6.9 ± 0.4‰ (n = 3) from the unidentified fish was higher than that of the herbivores, probably because of the longer food chain in the freshwater than in the terrestrial ecosystem (21). The δ13C and δ15N values of the pigs and dogs were quite similar, indicating that they might have shared the same food resources. As a whole, the mean δ13C value and δ15N value of domestic animals, including 10 pigs and 3 dogs, were −8.9 ± 1.3‰(n = 13) and 8.0 ± 0.8‰ (n = 13), respectively, suggesting that they consumed large quantities of C4-based protein. In addition, the spacing (3.8‰) of average δ15N values between the domestic animals and herbivores was located within the range (3∼5‰) of nitrogen isotope fractionation in trophic levels (22), implying that their diets were deprived of animal protein. This finding suggests that pig and dog diets were based on human food remains or excrement.
Fig. 2.
Scatter plot of δ13C and δ15N values for humans and other animals from Quanhucun showing that C4-based foods contributed exclusively to human, pig, and dog diets, whereas wild herbivores relied heavily on C3 diets. The δ13C and δ15N values of cats indicate that they consumed substantial C4-based protein. One cat has a particularly low δ13C value, indicating a higher than expected reliance on agricultural products.
The high δ13C value (−11.2‰) and δ15N value (11.5‰) in human bone collagen suggested that the individual consumed a large amount of C4-based animal protein. The δ13C value was also negative relative to the mean value for pigs and dogs (−8.9 ± 1.3‰, n = 13); this was probably caused by the fact that this person also consumed animal protein with lower δ13C values, such as fish.
The zokor had high δ13C (−8.5‰) and δ15N values (8.5‰), quite similar to the pigs and dogs, demonstrating a similar diet. The δ13C values of the cats ranged from −16.1‰ to −12.3‰ and averaged −14.0 ± 1.1‰ (n = 3), which indicated that substantial C4-based foods were included in their diets. The δ15N values ranged from the 5.8‰ to 8.9‰ and averaged 7.6 ± 0.9‰ (n = 3). One cat had a particularly high δ13C value (−12.3‰) and low δ15N value (5.8‰), suggesting that its diet was composed of more C4-based plant protein than that of the other cats (Fig. 2).
Discussion
Millet Agriculture and Rodent Threats to Stored Grains.
Interpretation of the isotopic data is based upon the ancient distribution of C3 and C4 vegetation in north China. Carbon isotopic ratios of the soil organic matter in paleosols of the loess plateau indicate that C3 vegetation was dominant during the Holocene (23, 24). Plants with C4 photosynthetic pathways include those from the Poaceae, Cyperaceae, and Amaranthaceae families (25). Millet domestication originated in ancient North China over 10,000 y ago (26, 27) and millets were the only C4 crop cultivated widely 6000∼2100 B.P. in the Guanzhong Basin (17, 19). The δ13C value of foxtail millet is −12.5‰ and that of common millet is −13.1‰ (28). Therefore, the δ13C values of human or animal collagen with strong C4 signals are considered to be caused by direct or indirect consumption of millet grains, millet by-products or millet refuse (29).
Isotopic analyses of human collagen suggest that by the Yangshao period millets became the main dietary resource for people living in North China (29–31). Similar isotopic values for domestic pigs, dogs, and humans from a number of Yangshao sites indicate that pigs and dogs fed on millet byproducts, human leftovers, garbage, or feces (29, 30, 32). The results from the Quanhucun study fit into this larger context of developed millet agriculture in North China. The isotopic signature of the wild herbivores, including sika deer, roe deer, and hare, shows that they relied mainly on C3 plants, supporting the assumption that the vegetation surrounding the site was largely made up of C3 plants. The high carbon isotopic values of human, pig, and dog bones indicate that millet-based foods contributed a great deal to human and animal diets.
The highly developed millet agriculture, together with millet food preparation and storage, attracted rodent commensals to Quanhucun (9, 10, 33). Wild birds may also have eaten millets in fields. However, a specific rodent threat to grain stores at the site was demonstrated by ancient rodent bones, burrows discovered in grain storage pits, and use of ceramic grain storage vessels with unique angles and textures specifically designed to exclude rodents (16). Furthermore, the common Chinese zokor had a high δ13C value (−8.5‰), indicative of the consumption of millet products or prepared foods. High δ13C values have also been observed in bone collagen of common Chinese zokor (−11.6‰) and Norway rat (Rattus norvegicus) (−9.3‰) from the contemporary Wuzhangguoliang site (32), demonstrating that rodents that ate grain stores or crops were widely distributed in Yangshao villages.
Commensalism and Cat Domestication.
Together, the spread of millet farming and commensal rodent populations attracted cats and provided incentives for farming communities to support them. The cat population at Quanhucun survived for several hundred years, with one of the individuals that we studied living to a considerable age, suggesting a favorable environment for cat survival. One animal stands out from the others, with a high δ13C value (−12.3‰) and low δ15N value (5.8‰), suggesting that it ate large quantities of agricultural products and did not rely as heavily as expected on rodents or other small animals for food. These data are intriguing, raising the possibility that this cat was unable to hunt and scavenged for discarded human food or that it was looked after and fed by people.
These findings provide unique direct information on commensal relations, selection processes, and mechanisms of cat domestication in the human niche. Cats depended on diverse sources of food available to them in the village of Quanhucun. Nitrogen isotopes indicated that they ate meat, but despite cats being obligate carnivores, at least one individual consumed a significant amount of grain. Another survived into old age. These results suggest that cats may have played a variety of roles in the settlement, ranging from mutualistic hunters and scavengers to encouraged animals or even pets. Advantages for humans in this relationship are clear, given the demonstrated rodent threats to stored grain and food security. For cats, human settlements provided year-round food. Both environmental and weak directed selection acted on cats in the human environment at Quanhucun. There was selection for cats that were successful hunters and scavengers in built environment and for tameness and affiliative behavior toward humans. Mutualistic domesticatory relationships between people and cats ultimately resulted in population expansion and humanly facilitated dispersal of cats across the world.
Our study demonstrates the presence of cats in an early agricultural village in China dating back to ca. 5,300 y ago. This finding is outside of the range of F. s. lybica, which mitochondrial DNA studies of contemporary felids indicate was the ancestor of all domestic cats. The date is also more than 3,000 y earlier than domestic cats were previously thought to have appeared in China (1). This finding is unexpected and raises questions regarding trajectories of cat domestication and spread.
If Near Eastern wildcats were the ancestors of domestic cats and cats appeared in north China more than 5,000 y ago, it is reasonable to suppose that cats might have been transported from western Asia via complex exchange and trade networks that played a similar role in the spread of other animals (sheep and cattle) to eastern Asia (34, 35). However, there is as yet no consensus on the timing of and processes involved in the appearance of these domesticates in China (34, 36). On the other hand, Asian wildcats (F. s. ornata and F. s. bieti) (37) may have interbred with domestic cats or even been locally domesticated. Examining these issues will require future studies of ancient DNA.
Taken together, isotopic and archaeological data from Quanhucun provide complementary information on foodwebs among people, millets, rodents, and cats, and evidence for commensal and mutualistic relations between humans and cats in an early agricultural village. This site provides unique evidence for cats feeding within human foodwebs and supports hypotheses for commensal pathways to cat domestication.
Materials and Methods
Biometric Measurements of Felid Bones and Teeth.
The felid skeletal elements were carefully examined and the morphological parameters of some intact or partly intact skeletal elements were documented. Measurements were taken using calipers and following protocols and codes set forth in von den Driesch (38). The measurements were also compared with previously published biometric data (5, 14) to determine whether the cats studied here fit within the range of variability of wild or domestic cats. The results are illustrated in Table 2.
AMS-14C Dating.
Two pieces of felid bone from two separate pits were selected for AMS-14C dating at Peking University. The IntCal04 calibration curve and the OxCal v3.10 calibration program were used for date calibration (39, 40). Results are presented in Table 3.
Collagen Extraction and Stable Isotopic Measurements.
Animal bones of several taxa, including hare, zokor, cat, dog, domestic pig, silka deer, roe deer, and unidentified fish, as well as one human bone were selected for carbon and nitrogen isotopic analysis. The color and quality of preservation of specimens was consistent and secure archaeological contexts were sampled. Sample information in detail is listed in Table S2.
The extraction of bone collagen was undertaken according to the following protocol. After bone contaminants from outer and inner surfaces were removed, the bones were decalcified in 0.5 mol/L HCl and refreshed every 2 d until the bone was soft and no bubbles were produced. The residues were washed with deionized water to neutrality, rinsed in 0.125 mol/L NaOH for 20 h, and washed again with deionized water. The remains were rinsed in 0.001 mol/L HCl and gelatinized at 70 °C for 48 h. After filtration, the residues were freeze-dried to obtain gelatinized collagen.
The carbon and nitrogen contents (weight% C and N) of collagen and C and N stable isotopes were measured with a Finnigan MAT Delta plus equipped with a Cario elemental analyzer. The standard used to measure the contents of C and N was C8H9NO. IAEA-N-1 and USGS 24 were used to normalize N2 (AIR as standard) and CO2 (PDB as standard) in steel bottles, respectively. The analytical precision for δ13C and δ15N value was 0.1‰ and 0.2‰, respectively. The contents of C and N and their stable isotopic data are shown in Table S2.
Generally, the bone collagen discussed below had an average C content of 47.7 ± 4.7%, an average N content of 15.7 ± 1.4%, and atomic C/N ratios in the range of 2.9–3.6, similar to those of modern bones (41% C content, 15% N content, and 2.9–3.6 C/N ratio) (41, 42), suggesting that all samples retained their in vivo isotopic signatures.
Supplementary Material
Acknowledgments
We thank our reviewers for their insightful comments and suggestions for improving the manuscript. This work was supported by the Chinese Academy of Science Strategic Priority Research Program (Grant XDA05130303), the Chinese Academy of Sciences and Max-Planck Society Partnership Group Project, and National Science Foundation in China (41373018).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311439110/-/DCSupplemental.
References
- 1.Driscoll C, Juliet C, Andrew C, O’Brien S. The taming of cats. Sci Am. 2009;300:68–75. [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll CA, Macdonald DW, O’Brien SJ. From wild animals to domestic pets, an evolutionary view of domestication. Proc Natl Acad Sci USA. 2009;106(Suppl 1):9971–9978. doi: 10.1073/pnas.0901586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll CA, et al. The Near Eastern origin of cat domestication. Science. 2007;317(5837):519–523. doi: 10.1126/science.1139518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigne JD, et al. First wave of cultivators spread to Cyprus at least 10,600 y ago. Proc Natl Acad Sci USA. 2012;109(22):8445–8449. doi: 10.1073/pnas.1201693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vigne JD, Guilaine J, Debue K, Haye L, Gérard P. Early taming of the cat in Cyprus. Science. 2004;304(5668):259. doi: 10.1126/science.1095335. [DOI] [PubMed] [Google Scholar]
- 6.Faure E, Kitchener A. An archaeological and historical review of the relationships between felids and people. Anthrozoos. 2009;22(3):221–238. [Google Scholar]
- 7.Linseele V, Van Neer W, Hendrick S. Evidence for early cat taming in Egypt. J Archaeol Sci. 2007;34(12):2081–2090. [Google Scholar]
- 8.Linseele V, Van Neer W, Hendrick S. Early cat taming in Egypt: A correction. J Archaeol Sci. 2008;35(9):2672–2673. [Google Scholar]
- 9.Tchernov E. Commensal animals and human sedentism in the Middle East. In: Clutton-Brock J, Grigson C, editors. Animal and Archaeology. Oxford: British Archaeological Reports, International Series; 1984. pp. 91–115. [Google Scholar]
- 10.Zeder M. Pathways to animal domestication. In: Damania A, Gepts P, editors. Harlan II: Biodiversity in Agriculture: Domestication, Evolution and Sustainability. Davis: Univ of California; 2012. pp. 227–259. [Google Scholar]
- 11.Vigne JD. The origins of animal domestication and husbandry: A major change in the history of humanity and the biosphere. C R Biol. 2011;334(3):171–181. doi: 10.1016/j.crvi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Smith B. A cultural niche construction theory of initial domestication. Biol Theory. 2012;6(3):260–271. [Google Scholar]
- 13.Outram AK, et al. The earliest horse harnessing and milking. Science. 2009;323(5919):1332–1335. doi: 10.1126/science.1168594. [DOI] [PubMed] [Google Scholar]
- 14.Baldwin J. Notes and speculations on domestication of cat in Egypt. Anthropos. 1975;70(3-4):428–448. [Google Scholar]
- 15.Todd N. An ecological behavioral genetic model for the domestication of the cat. Carnivore. 1978;1:52–60. [Google Scholar]
- 16.Wang W. Cats, mice and human settlement life: Opinions on cat bones excavated from Quanhucun site. Archaeology and Cultural Relics. 2010;1:22–25. Chinese. [Google Scholar]
- 17.The Institute of Archaeology, Chinese Academy of Social Sciences . Chinese Archaeology: Neolithic. Beijing: Chinese Social Sciences Press; 2010. pp. 206–269. (in Chinese) [Google Scholar]
- 18.The Archaeology Department of the Beijing University Institute of Archaeology Chinese Academy of Social Sciences Institute of Archaeology Chinese Academy of Social Sciences . Huaxian Quanhucun: Archaeological Excavations at the Yellow River Reservoirs Report No. 6. Beijing: Science Press; 2003. pp. 109–118. (in Chinese) [Google Scholar]
- 19.Zhang J, et al. Phytolith evidence of millet agriculture during 6000∼2100 aBP in the Guanzhong Basin, China. Quaternary Sciences. 2010;30(2):287–297. Chinese. [Google Scholar]
- 20.O’Connor T. Wild or domestic? biometric variation in the cat Felis silvestris Schreber. Int J Osteoarchaeol. 2007;17(6):581–595. [Google Scholar]
- 21.Richards MP, Pettitt PB, Stiner MC, Trinkaus E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc Natl Acad Sci USA. 2001;98(11):6528–6532. doi: 10.1073/pnas.111155298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges R, Reynard L. Nitrogen isotopes and the trophic level of humans in archaeology. J Archaeol Sci. 2007;34:1240–1251. [Google Scholar]
- 23.Liu W, Ning Y, An Z, Lu H, Cao Y. The response of organic carbon stable isotopes from modern soil and palaeosol in loess plateau to the vegetation. Sciences in China: Series D. 2002;32(10):830–836. Chinese. [Google Scholar]
- 24.Liu L, Zhou X, Yu Y, Guo Z. Soil organic carbon isotopic evidence to natural vegetation in loess plateau. Quaternary Sciences. 2011;31(3):506–513. Chinese. [Google Scholar]
- 25.Yin L, Li M. A study on the geographic distribution and ecology of C4 plants in China: C4 plant distribution in China and their relation with regional climatic condition. Acta Ecol Sin. 1997;17(4):350–363. Chinese. [Google Scholar]
- 26.Lu H, et al. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc Natl Acad Sci USA. 2009;106(18):7367–7372. doi: 10.1073/pnas.0900158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, et al. Early millet use in northern China. Proc Natl Acad Sci USA. 2012;109(10):3726–3730. doi: 10.1073/pnas.1115430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Q, et al. Carbon isotope fractionation during low temperature carbonization of foxtail and common millets. Org Geochem. 2011;42(7):713–719. [Google Scholar]
- 29.Barton L, et al. Agricultural origins and the isotopic identity of domestication in northern China. Proc Natl Acad Sci USA. 2009;106(14):5523–5528. doi: 10.1073/pnas.0809960106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechenkina E, Ambrose S, Ma X, Benfer R., Jr Reconstructing northern Chinese Neolithic subsistence practices by isotopic analysis. J Archaeol Sci. 2005;32:1176–1189. [Google Scholar]
- 31.Guo Y, Hu Y, Gao Q, Wang C, Richards M. Human dietary analysis in Jiangzhai site. Acta Anthropologica Sinica. 2011;30(2):149–157. Chinese. [Google Scholar]
- 32.Guan L, et al. Carbon and nitrogen isotopic analysis of animal bones from Wuzhuangguoliang site, Shaanxi. Quarternary Sciences. 2008;28(6):1160–1165. Chinese. [Google Scholar]
- 33.Weissbrod L. Biological indicators of occupation intensity: An environmental ethnoarchaeology of Maasai settlements. In: Dean R, editor. The Archaeology of Anthropogenic Environments. Carbondale, IL: Center for Archaeological Investigations; 2010. pp. 295–320. [Google Scholar]
- 34.Flad R, Yuan J, Li S. Zooarchaeological evidence for animal domestication in northwest China. In: Maden D, Chen F, Gao X, editors. Late Quaternary Climate Change and Human Adaptation in Arid China. Amsterdam: Elsevier Science & Technology; 2007. pp. 167–203. [Google Scholar]
- 35.Yuan J. Zooarchaeological study on the domestic animals in ancient China. Quaternary Sciences. 2010;30(2):298–307. [Google Scholar]
- 36. Cai D, Sun Y (2012) On the origin of the ancient DNA of domestic animals in China. Research on the Frontier Archaeology eds Center for Frontier Archaeology of Jilin University (Science Press, Beijing) pp 445–455. Chinese.
- 37.Agnarsson I, Kuntner M, May-Collado LJ. Dogs, cats, and kin: A molecular species-level phylogeny of Carnivora. Mol Phylogenet Evol. 2010;54(3):726–745. doi: 10.1016/j.ympev.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 38.von den Driesch A. Guide to the Measurement of Animal Bones from Archaeological Sites. Peabody Museum Bulletin No. 1. Cambridge, MA: Harvard Univ; 1976. [Google Scholar]
- 39.Reimer P, et al. IntCal04 Terrestrial radiocarbon age calibration, 26-0 ka BP. Radiocarbon. 2004;46:1029–1058. [Google Scholar]
- 40.Ramsey C. 2005. Available at http://c14.arch.ox.ac.uk/oxcal3/oxcal.htm. Accessed November 29, 2013.
- 41.DeNiro M. Post-mortem preservation of alteration of in vivo bone collagen isotope ratios in relation to paleodietary reconstruction. Nature. 1985;317:806–809. [Google Scholar]
- 42.Ambrose S. Preparation and characterization of bone and tooth collagen for stable carbon and nitrogen isotope analysis. J Archaeol Sci. 1990;17(4):431–451. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.