Significance
In social insects, selfish reproduction by workers is suppressed by “policing” behavior, whereby queens and workers identify and destroy worker-laid eggs. An alternative method of policing is to use deterrent threats to prevent offspring production in the first place. Our 7-y field experiment on wild banded mongooses, Mungos mungo, shows that selection to evade the threat of infanticide by older, socially dominant females can explain the evolution of remarkable birth synchrony in this species. The results suggest that reproduction in animal societies can be shaped by threats of punishment that remain hidden until they are triggered experimentally. It follows that coercion may be more widespread than we currently realize.
Keywords: social evolution, cooperative breeding, conflict resolution, female competition, aggression
Abstract
The evolution of cooperation in animal and human societies is associated with mechanisms to suppress individual selfishness. In insect societies, queens and workers enforce cooperation by “policing” selfish reproduction by workers. Insect policing typically takes the form of damage limitation after individuals have carried out selfish acts (such as laying eggs). In contrast, human policing is based on the use of threats that deter individuals from acting selfishly in the first place, minimizing the need for damage limitation. Policing by threat could in principle be used to enforce reproductive suppression in animal societies, but testing this idea requires an experimental approach to simulate reproductive transgression and provoke out-of-equilibrium behavior. We carried out an experiment of this kind on a wild population of cooperatively breeding banded mongooses (Mungos mungo) in Uganda. In this species, each group contains multiple female breeders that give birth to a communal litter, usually on the same day. In a 7-y experiment we used contraceptive injections to manipulate the distribution of maternity within groups, triggering hidden threats of infanticide. Our data suggest that older, socially dominant females use the threat of infanticide to deter selfish reproduction by younger females, but that females can escape the threat of infanticide by synchronizing birth to the same day as older females. Our study shows that reproduction in animal societies can be profoundly influenced by threats that remain hidden until they are triggered experimentally. Coercion may thus extend well beyond the systems in which acts of infanticide are common.
The suppression of reproductive competition in animal societies promotes the evolution and maintenance of cooperation because it ensures that helpers or workers can maximize their inclusive fitness only by maximizing the fitness of the group (1, 2). Pioneering work on social Hymenoptera has shown that worker reproduction is suppressed by “policing” behavior, whereby queens and other workers identify and destroy worker-laid eggs (3, 4). This form of insect policing serves primarily to reduce the damaging impact of individually selfish behavior (i.e., egg laying) on the fitness of the group (5). Comparative data suggest that efficient policing can, over evolutionary time, reduce to a low level the proportion of colony workers that develop their ovaries and become reproductively active (4). However, in some systems [e.g., honey bees, common wasp Vespula vulgaris, European wasp Vespula germanica (4)] workers still commit to producing eggs even when these are almost certain to be policed, suggesting that policing does not have a deterrent effect on the reproductive decisions of these individual workers. In contrast, in human societies crime or defection is policed using deterrent threats that raise the perceived costs to individuals of engaging in selfish behavior (6, 7). Individuals can then make an informed decision to refrain from selfish acts if these are likely to trigger punishment, and so the punishments themselves rarely need to be carried out (6–10). While the level of policing and transgression in insect societies is typically assumed to be genetically “hard wired” into the system [that is, determined by obligately expressed “sealed bid” strategies (11–14)], policing by threat requires that individuals are socially sensitive and responsive on a behavioral timescale to the actions (and anticipated actions) of their social partners (10).
The idea that social animals might use threats to police reproduction has been little explored to date because existing models of policing (2, 11, 12, 14–16) do not use “extensive-form” game theory, which is designed to analyze how threats influence strategic behavior (6), and because observational studies on their own cannot detect effective threats (8, 9). To reveal such threats requires an experimental approach to manipulate the status quo and break the social rules that threats are used to enforce (9, 17). In the case of reproductive competition, the influence of threats on the distribution of reproduction (or degree of reproductive skew) within groups can be tested by manipulating skew while keeping group size and composition intact. We carried out an experiment of this kind on banded mongooses, a species in which multiple females in each group contribute offspring to a communal litter (18–20), and much of the postnatal care of offspring is provided by nonbreeding males (21–24).
At our study site in Uganda banded mongooses live in mixed-sex groups of around 20 adults, plus offspring, and groups breed on average four times per year (25). At any one time the study population consists of 10–13 groups. Each group contains a cohort of one to five older, dominant adult females (typically age 4+ y) plus one to eight younger subordinate adult females (typically 1–3 y old). Older females are classed as socially dominant because they aggressively evict younger females from the group when the number of adult females grows large (20); in contrast, younger females do not evict older breeders. Multiple females reproduce in each breeding attempt (mean = 3.4, range 1–12). On average 74% (± 29%; mean ± SE) of dominant females and 49% (± 38%) of subordinate females in each group become pregnant in each breeding attempt (n = 107 attempts). Pregnant females give birth together to a communal litter in an underground den, usually on the same day [i.e., in 64% of 294 communal litters (26)].
Female banded mongooses could in principle gain an advantage in pup–pup competition by giving birth a few days before other breeders in the group (26). However, when females do give birth asynchronously (i.e., on different days), the offspring of first-birthing females almost always die within the first few days, whereas the offspring of last-birthing females almost always survive (26). This dependence of immediate postnatal survival on the pregnancy status of other breeding females in the group is a signature of female infanticide (27) and tallies with direct observations of female infanticide in this system (26) (Methods). Overall, early-life pup survivorship in asynchronous communal litters is approximately half that of pups in synchronous litters (26), and females that conceive particularly early appear to extend gestation to achieve birth synchrony with other females in their group (28). We hypothesize, therefore, that females synchronize birth to the same day to escape the threat of infanticide, either because birth synchrony removes temporal and spatial cues to maternity in communal litters (18, 28), or because females that have just given birth are mechanistically inhibited from killing offspring (26). We tested our hypothesis by inducing banded mongooses to reproduce out of synchrony using short-acting contraceptives.
Our design consisted of three experiments. In Exp. 1 we suppressed reproduction in all subordinate females and allowed all dominant females to breed (Methods). This experiment tested whether dominant females suffer fitness costs when subordinates also breed, and whether subordinates kill litters when they have not given birth themselves. In Exp. 2 we suppressed all subordinates and all except a single dominant female breeder, thereby mimicking the high skew pattern of reproduction seen in other cooperatively breeding mongooses [e.g., meerkats Suricata suricatta (29), and dwarf mongooses Helogale parvula (30)]. This experiment tested whether single dominant females stand to gain from fully monopolizing reproduction, as assumed by most reproductive skew models (31–35). In Exp. 3 we suppressed all dominant female breeders and left subordinate females to breed. This experiment tested how dominant females exercise reproductive control over subordinate reproduction, and in particular whether dominant females kill litters when they have not given birth themselves. In all cases we compared breeding attempts in which we suppressed females using contraceptive (“EXP” attempts) with control breeding attempts in the same group immediately before and after the treatment (labeled “PRE” and “POST” breeding attempts, respectively). The design of our experiment and the resulting number of dominant and subordinate breeders in PRE, EXP, and POST breeding attempts is illustrated in the top row of Fig. 1.
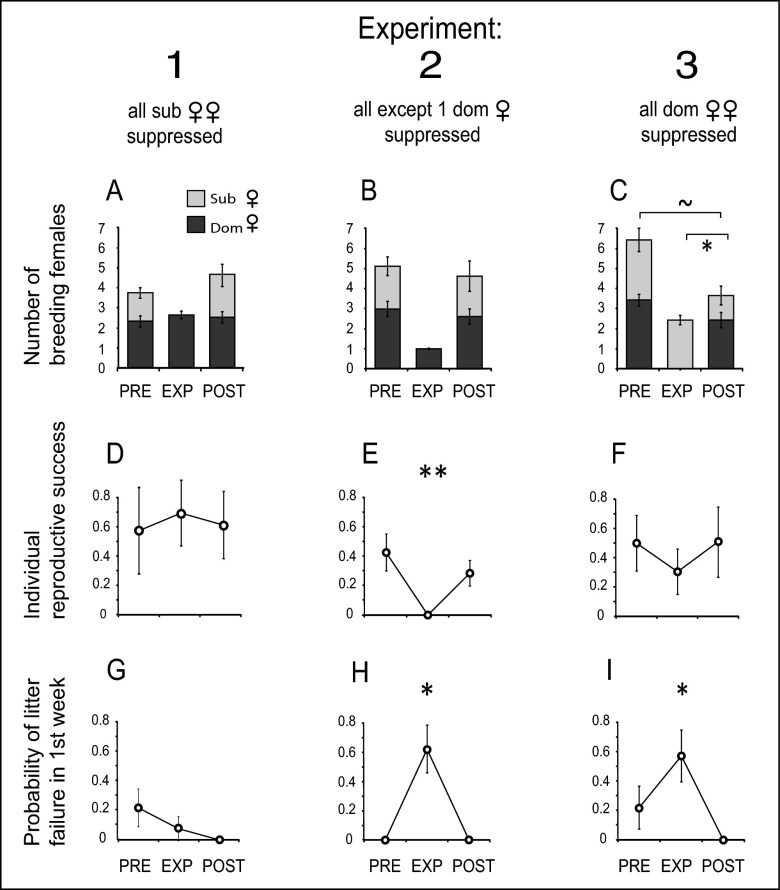
Fig. 1.
Results of the three suppression experiments. (A–C) Number of dominant and subordinate breeders in synchronous breeding attempts before treatment with contraceptive (PRE), the treatment breeding attempt (EXP), and the breeding attempt subsequent to the treatment (POST). (D–F) Individual reproductive success (measured as the number of pups reared to independence) of: (D) dominant females that reproduced in Exp. 1; (E) the single dominant female left untreated in Exp. 2; (F) subordinate females in Exp. 3. (G–I) Probability of whole-litter failure in the first week after birth. Symbols: ∼ P = 0.06, *P < 0.05, **P = 0.012; asterisks refer to statistical tests across all three categories: in D–F, Friedman tests; in G–I, GLMM. Exp. 1: n = 12 breeding attempts in each of PRE, EXP, POST; Exp. 2: n = 8; Exp. 3: n = 9. Bars show SE.
Results and Discussion
Experimental suppression of subordinate females in Exp. 1 reduced the mean number of breeders per group from 3.83 (± 0.50) in PRE breeding attempts to 2.67 (± 0.50) in EXP breeding attempts [generalized linear mixed-model (GLMM): χ22 = 9.95, P = 0.017] (Fig. 1A). The number of breeding females in the POST breeding attempts recovered to 4.63 (± 0.52). The reproductive success of dominant females (number of pups surviving to independence, assigned using microsatellites) was not significantly different in PRE, EXP, and POST breeding attempts, suggesting that dominant females do not suffer immediate fitness costs in the current litter when subordinates reproduce (Friedman test: χ22 = 0.79, P = 0.59) (Fig. 1D).
In Exp. 2, when we suppressed all breeders except a single dominant female (Fig. 1B), that female had lower reproductive success than she did in the control breeding attempts before and after the manipulation (Friedman test: χ22 = 4.75, P = 0.048; post hoc Wilcoxon paired tests: PRE vs. EXP: W = 0, P = 0.063; POST vs. EXP: W = 0, P = 0.063), and significantly lower reproductive success than the average reproductive success of all dominant females in PRE and POST breeding attempts (X22 = 7.75, P = 0.012; PRE vs. EXP: W = 0, P = 0.035; POST vs. EXP: W = 0, P = 0.035) (Fig. 1E). The cause of this reduced reproductive success was a sharp spike in the probability of litter failure within the first week [GLMM: χ22 = 8.82, P = 0.027] (Fig. 1H).
In Exp. 3, when all dominant females in the group were suppressed, on average 2.33 ± 0.29 subordinate females reproduced, which was not significantly different from the number of reproductive subordinates in PRE breeding attempts (3.00 ± 0.58; paired t test: t8 = 0.89, P = 0.40) (Fig. 1C). The reproductive success of these subordinates was not significantly different from PRE and POST breeding attempts (Friedman test: χ22 = 1.31, P = 0.25) (Fig. 1F). However, the timing of pup mortality differed markedly between treatments: in EXP breeding attempts there was again a sharp spike in the probability of whole-litter failure in the first week after birth in experimental litters (GLMM: χ22 = 7.24, P = 0.024) (Fig. 1I). Why this spike in early-life litter failure (Fig. 1I) did not translate into significantly lower overall reproductive success of subordinate females (Fig. 1F) is unclear: it may be that pups in EXP litters that did survive the first week were unusually robust, or that our sample size was too small to detect the signal of first week mortality given other later influences on pup survival to 3 mo. Finally, the number of subordinates that reproduced in the POST breeding attempt (1.22 ± 0.47) was lower than the number of reproductive subordinates in both EXP breeding attempts (paired t test: t8 = 2.48, P = 0.038) and PRE breeding attempts (paired t test, t8 = 2.19, P = 0.06) (Fig. 1C), suggesting that our experimental suppression of dominants deterred subordinates from attempting to reproduce in the subsequent breeding attempt.
The spike in early-life litter failure when one or more dominant females were suppressed (Fig. 1 H and I) supports our hypothesis that dominant females kill communal litters that are certain not to contain their own young, and hence that subordinates can evade infanticide by synchronizing birth to the same day as dominants. In contrast, suppression of subordinate females did not trigger whole-litter infanticide in the first week after birth (Fig. 1G). As in some other social mammals (27, 36, 37) and joint-nesting birds (38–40), female banded mongooses appear to kill offspring that are produced before, but not after, their own (26). Our experiments suggest that it is the presence or absence of reliable cues to maternity, rather than the pregnancy status of dominant females per se, that is the main determinant of this pattern. Although dominant banded mongoose females did not suffer immediate fitness costs when subordinates added to the communal litter (Fig. 1D), these females do have an incentive to kill the litters of other breeders when maternity is clear because this removes competitors for their next breeding attempt. Pups are more likely to survive to independence (3 mo old) if there are no older pups present in the group when they are born (GLMM: χ21 = 4.53, P = 0.037) (Fig. 2A), and those pups that do survive are almost 10% heavier (LMM: χ21 = 4.06, P = 0.048) (Fig. 2B).
Fig. 2.
Potential fitness benefits of whole-litter infanticide. Because banded mongoose groups breed on average four times a year, many communal litters (36%) are born into groups containing older dependent pups (i.e., <3 mo old) from the previous breeding attempt. Newborn offspring that do not overlap with dependent older pups show: (A) improved survival to 3 mo (n = 160 pups) and (B) higher body weight at 3 mo (n = 237 pups) compared with newborn offspring in groups that contain dependent pups. Body weight at 3 mo is a predictor of survival to adulthood and age at first reproduction (53). *P < 0.05; bars show predicted means ± SE from mixed-effects models controlling for repeated measures among litters and groups.
We did not find evidence that patterns of reproduction reflect reproductive incentives to avert the threat of dispersal or eviction, as assumed by most “transactional” models of reproductive skew (31–35). None of our suppression experiments had any detectable effect on the stability of social groups. Suppression of dominant females (Exps. B and C) did not lead to an increased probability of eviction of subordinate females or any other group members. Eviction was only observed during one breeding attempt in the 51 breeding attempts (PRE, EXP, and POST) that were involved in these two experiments. Our long-term data suggest that subordinate females do not exercise preemptive reproductive restraint to avoid eviction in the manner assumed by the restraint transactional model of skew (33). Suppressed subordinates were not more likely to disperse, as no subordinate females were observed to disperse in either experimental or control breeding attempts. In fact, banded mongoose females have never been observed to leave their natal group voluntarily in our population in 18 y of study (25). Although transactional skew models have been valuable in focusing attention on threats as a means of reproductive suppression, our results support theoretical arguments (9, 32, 41) that threats of infanticide or physical attack, rather than threats of dispersal or eviction, are most relevant to the outcome of reproductive conflict in cooperative animal societies.
We have shown that manipulating the distribution of reproduction among females triggers large changes in the rate and timing of early-life pup mortality, consistent with whole-litter infanticide. This finding adds to evidence from a range of social mammals that females compete for reproduction by killing the offspring of rival breeders (42), and supports the proposal that policing plays a fundamental role in the evolution of cooperation in vertebrates as well as insects (43). However, the finding also suggests that reproductive coercion may extend well beyond the systems in which infanticide is common. Hidden threats of policing can deter subordinates from reproducing in the first place (44–46), or select for counterstrategies [such as synchronous birth (26, 37), egg-laying (47), or egg-mimicry (48)] to render threats noncredible (i.e., unprofitable for a threatening individual to carry out, in the event that a transgression occurs). In both cases, threats of infanticide influence reproductive behavior even though no offspring are killed.
Current models of policing (2, 11, 12, 14–16) do not allow for threats to influence reproductive strategies because they focus on the coevolution of genetically determined (sealed bid) levels of investment in selfishness and policing, with no scope for flexible responses on a behavioral timescale. Consequently, although policing may ultimately select for lower levels of selfishness (4, 43), this response occurs only on an evolutionary timescale; in the short term, it cannot influence how selfishly an individual group member behaves. In contrast, the use of a threat implies a sequential, two-step process (8, 41), in which individuals are responsive on a behavioral timescale to the actions of their social partners (10). This type of behavioral responsiveness is known to be widespread in animal societies (9, 10, 49, 50) and is evident from our experiments (Fig. 1 C, E, H, and I). Modeling policing by threat requires an extensive-form game theoretical approach in which the sequence of behavioral decision-making is made explicit. In the SI Text we develop a simple extensive-form model of policing by threat. In this type of model, threats of infanticide are frequently hidden in the sense that they can select for reproductive suppression or strategies to evade policing even though acts of infanticide are rarely observed. The model predicts that because the cost of producing offspring is generally much higher in vertebrates compared with insects, there is greater potential for policing by threats rather than acts of infanticide in the former than the latter. Because threats are carried out only when they are ineffective as a deterrent (20), or triggered experimentally (ref. 17 and the present study), policing by threat is an efficient and probably systematically underestimated force shaping reproduction and cooperation in animal societies.
Methods
Study Population.
The research was carried out under a permit from Uganda Wildlife Authority and Uganda National Council for Science and Technology, and all methods approved by the ethical review panel of the University of Exeter. Data were collected from 11 groups of banded mongooses, living on and around Mweya Peninsula in Queen Elizabeth National Park Uganda (0°12′S; 29°53′E), between November 2005 and January 2013. Descriptions of habitat, climate, and study population are provided elsewhere (18). All individuals were marked with color-coded plastic collars or unique shave marks, and groups were visited every 3 d to determine group composition (or daily when birth was imminent).
Infanticide typically occurs in the den, so is rarely observed in our population. Between November 1995 and April 2008 we observed within-group infanticide on 24 occasions, all within 1 wk of birth (26). In 16 of these cases dead pups were observed at or close to the natal den, with bite marks and wounds to the head or body, but we could not identify the group members that may have inflicted these wounds. In the remaining eight cases one or more adults were observed eating dead pups; in all cases these “pup eaters” included one or more dominant females. Evictions were defined as cases when adult individuals left their group for at least 1 d as a consequence of aggression from other group members (21). Individuals that were observed away from their group with no signs of aggression who did not return were recorded as having dispersed.
Contraceptive Treatment.
Experimental reproductive suppression in females was achieved using subcutaneous injection of synthetic progesterone (5 mg/kg medroxyprogesterone acetate; brand name Depo-provera) immediately after the birth of a communal litter (mean 5.33 ± 3.46 d after birth), and hence before postpartum estrus. Females were caught and anesthetized using the methods described in ref. 51. This procedure allowed us to successfully block reproduction for a single breeding attempt in 115 females (52 dominants and 63 subordinates) in 29 EXP breeding attempts (Exp. 1, n = 12 experiments in 8 groups conducted between 2006 and 2010; Exp. 2, n = 8 experiments in 6 groups conducted between 2006 and 2010; Exp. 3, n = 9 experiments in 5 groups conducted between 2008 and 2012). Adult females received an average of 1.8 treatments over the 7 y of study (range 1–5). By the time that pups in the EXP breeding attempts were born (10–11 wk postestrus) there was no significant difference between treated females (n = 5) and untreated females (n = 8) in progesterone [mean ± SE = 265.5 ± 123.3 ng/g (treated) and 124.9 ± 15.8 ng/g (untreated); t test: t12 = −1.13, P = 0.32] or estrogen [median = 30.3, interquartile range 25.8–45.4 ng/g (treated) and median = 41.2, interquartile range 18.5–53.3 ng/g (untreated); Mann–Whitney U test: U = 18, P = 0.83] levels, as measured from fecal metabolites. For details of fecal sample collection and hormone analyses, see the SI Text. On average 50 (± 10%) of treated females conceived in the breeding attempt following the treatment litter, which did not differ significantly from the number of untreated females who conceived (57 ± 11%; GLMM: χ22 = 0.45, P = 0.50, controlling for a significant influence of dominance status; χ22 = 5.49, P = 0.019, n = 106 females in 17 experimental breeding attempts).
Statistical Analysis.
All statistical analyses were conducted in Genstat 14 (VSN International). To investigate the influence of the experimental treatment on female reproductive success, we used genetic analysis to determine the number of pups born to each female that survived to independence at 3 mo. Maternity was assigned with ≥95% confidence using a panel of 20 microsatellites following the methodology reported in ref. 52. Because female reproductive success was not normally distributed, we conducted a Friedman’s test to assess the effect of treatment (PRE, EXP, POST). Each experiment was assigned a unique identity number, which was included as a blocking factor to ensure experimental litters were compared with the appropriate control litters. To assess whether treatment influenced whether or not litters failed in the first week after birth, we fitted the probability of litter failure (1 = failed, 0 = survived) as the binomial response term in a GLMM. Litters were assumed to have failed if the group left no babysitters for more than 3 d (26). Group size (all group members >3 mo of age) and rainfall 60 d before birth (in millimeters) were fitted as covariates in all GLMMs and experimental number was included as a random term.
To investigate whether the presence of older pups influences pup survival to 3 mo, we used a GLMM and fitted whether or not each pup survived as the binomial response term (1 = survived, 0 = died). Whether or not older pups were present in the group was fitted as the main term of interest and other factors likely to influence pup survival were included as fixed effects: group size, rainfall in the 3 mo after birth (in millimeters), pup sex, and litter size. Group and litter were fitted as random effects. This analysis was conducted on 160 pups from 121 litters in 11 groups. To investigate whether the presence of older pups influences pup weight at 3 mo, we weighed pups between 90 and 100 d of age by encouraging them to step onto an electronic weighing balance. The mean weight of 237 pups from 75 litters in 8 groups was fitted as the response term in a LMM, which included the same fixed effects and random terms as outlined above.
Supplementary Material
Acknowledgments
We thank the Uganda Wildlife Authority for permission to conduct our research; the Wardens of Queen Elizabeth National Park for logistical support; Francis Mwanguhya, Solomon Kyabulima, Kenneth Mesigwe, Robert Businge, Corsin Müller, Neil Jordan, Bonnie Metherell, Roman Furrer, Jennifer Sanderson, and David Jansen for help in the field; Sue Walker and Rebecca Purcell at Chester Zoo Endocrinology Unit for help with hormonal analyses; and Nick Davies, Jeremy Field, Kevin Foster, Andy Young, and three anonymous referees for comments on the manuscript. This study was funded by the Natural Environment Research Council of the United Kingdom and The Royal Society.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312626111/-/DCSupplemental.
References
- 1.Alexander RD. The Biology of Moral Systems. New York: Aldine de Gruyter; 1987. [Google Scholar]
- 2.Frank SA. Perspective: Repression of competition and the evolution of cooperation. Evolution. 2003;57(4):693–705. doi: 10.1111/j.0014-3820.2003.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Ratnieks FLW, Visscher KP. Worker policing in the honeybee. Nature. 1989;342(6251):796–797. [Google Scholar]
- 4.Wenseleers T, Ratnieks FLW. Enforced altruism in insect societies. Nature. 2006;444(7115):50. doi: 10.1038/444050a. [DOI] [PubMed] [Google Scholar]
- 5.Ratnieks FLW, Wenseleers T. Evolution. Policing insect societies. Science. 2005;307(5706):54–56. doi: 10.1126/science.1106934. [DOI] [PubMed] [Google Scholar]
- 6.Binmore KG. Game Theory and the Social Contract. Vol 2: Just Playing. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- 7.Paternoster R. How much do we really know about criminal deterrence? J Crim Law Criminol. 2010;100(3):765–823. [Google Scholar]
- 8.Cant MA, Johnstone RA. How threats influence the evolutionary resolution of within-group conflict. Am Nat. 2009;173(6):759–771. doi: 10.1086/598489. [DOI] [PubMed] [Google Scholar]
- 9.Cant MA. The role of threats in animal cooperation. Proc Biol Sci. 2011;278(1703):170–178. doi: 10.1098/rspb.2010.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cant MA, Young AJ. Resolving social conflict among females without overt aggression. Philos Trans R Soc Lond B Biol Sci. 2013;368(1631):20130076. doi: 10.1098/rstb.2013.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenseleers T, Helanterä H, Hart AG, Ratnieks FLW. Worker reproduction and policing in insect societies: An ESS analysis. J Evol Biol. 2004;17(5):1035–1047. doi: 10.1111/j.1420-9101.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Wenseleers T, Hart AG, Ratnieks FLW. When resistance is useless: Policing and the evolution of reproductive acquiescence in insect societies. Am Nat. 2004;164(6):E154–E167. doi: 10.1086/425223. [DOI] [PubMed] [Google Scholar]
- 13.Bonckaert W, van Zweden JS, d’Ettorre P, Billen J, Wenseleers T. Colony stage and not facultative policing explains pattern of worker reproduction in the Saxon wasp. Mol Ecol. 2011;20(16):3455–3468. doi: 10.1111/j.1365-294X.2011.05200.x. [DOI] [PubMed] [Google Scholar]
- 14.Wenseleers T, Helanterä H, Alves DA, Dueñez-Guzmán E, Pamilo P. Towards greater realism in inclusive fitness models: The case of worker reproduction in insect societies. Biol Lett. 2013;9(6):20130334. doi: 10.1098/rsbl.2013.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank SA. Mutual policing and repression of competition in the evolution of cooperative groups. Nature. 1995;377(6549):520–522. doi: 10.1038/377520a0. [DOI] [PubMed] [Google Scholar]
- 16.El Mouden C, West SA, Gardner A. The enforcement of cooperation by policing. Evolution. 2010;64(7):2139–2152. doi: 10.1111/j.1558-5646.2010.00963.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong MYL, Buston PM, Munday PL, Jones GP. The threat of punishment enforces peaceful cooperation and stabilizes queues in a coral-reef fish. Proc Biol Sci. 2007;274(1613):1093–1099. doi: 10.1098/rspb.2006.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cant MA. Social control of reproduction in banded mongooses. Anim Behav. 2000;59(1):147–158. doi: 10.1006/anbe.1999.1279. [DOI] [PubMed] [Google Scholar]
- 19.Gilchrist JS. Reproductive success in a low skew, communal breeding mammal: The banded mongoose, Mungos mungo. Behav Ecol Sociobiol. 2006;60(6):854–863. [Google Scholar]
- 20.Cant MA, Hodge SJ, Bell MB, Gilchrist JS, Nichols HJ. Reproductive control via eviction (but not the threat of eviction) in banded mongooses. Proc Biol Sci. 2010;277(1691):2219–2226. doi: 10.1098/rspb.2009.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cant MA. Patterns of helping effort in co-operatively breeding banded mongooses (Mungos mungo) J Zool. 2003;259(2):115–121. [Google Scholar]
- 22.Gilchrist JS. Pup escorting in the communal breeding banded mongoose: Behavior, benefits, and maintenance. Behav Ecol. 2004;15(6):952–960. [Google Scholar]
- 23.Gilchrist JS, Russell AF. Who cares? Individual contributions to pup care by breeders vs non-breeders in the cooperatively breeding banded mongoose (Mungos mungo) Behav Ecol Sociobiol. 2007;61(7):1053–1060. [Google Scholar]
- 24.Nichols HJ, et al. Food availability shapes patterns of helping effort in a cooperative mongoose. Anim Behav. 2012;83(6):1377–1385. [Google Scholar]
- 25.Cant MA, Vitikainen E, Nichols HJ. Demography and social evolution of banded mongooses. Adv Stud Behav. 2013;45:407–445. [Google Scholar]
- 26.Hodge SJ, Bell MB, Cant MA. Reproductive competition and the evolution of extreme birth synchrony in a cooperative mammal. Biol Lett. 2011;7(1):54–56. doi: 10.1098/rsbl.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young AJ, Clutton-Brock TH. Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol Lett. 2006;2(3):385–387. doi: 10.1098/rsbl.2006.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilchrist JS. Female eviction, abortion, and infanticide in banded mongooses (Mungos mungo): Implications for social control of reproduction and synchronized parturition. Behav Ecol. 2006;17(4):664–669. [Google Scholar]
- 29.Clutton-Brock TH, et al. Cooperation, control, and concession in meerkat groups. Science. 2001;291(5503):478–481. doi: 10.1126/science.291.5503.478. [DOI] [PubMed] [Google Scholar]
- 30.Creel SR, Waser PM. Failures of reproductive suppression in dwarf mongooses (Helogale parvula): Accident or adaption. Behav Ecol. 1991;2(1):7–15. [Google Scholar]
- 31.Vehrencamp SL. A model for the evolution of despotic versus egalitarian societies. Anim Behav. 1983;31(3):667–682. [Google Scholar]
- 32.Reeve HK, Ratnieks FLW. Queen-queen conflicts in polygynous societies: Mutual tolerance and reproductive skew. In: Keller L, editor. Queen Number and Sociality in Insects. Oxford: Oxford Univ Press; 1993. pp. 45–85. [Google Scholar]
- 33.Johnstone RA, Cant MA. Reproductive skew and the threat of eviction: A new perspective. Proc Biol Sci. 1999;266(1416):275–279. [Google Scholar]
- 34.Reeve HK, Emlen ST, Keller L. Reproductive sharing in animal societies: Reproductive incentives or incomplete control by dominant breeders? Behav Ecol. 1998;9(3):267–278. [Google Scholar]
- 35.Johnstone R, Cant MA. Models of reproductive skew: Outside options and the resolution of reproductive conflict. In: Hager R, Jones CB, editors. Reproductive Skew in Vertebrates. Cambridge, United Kingdom: Cambridge Univ Press; 2009. pp. 3–23. [Google Scholar]
- 36.Konig B. Components of lifetime reproductive success in communally and solitarily nursing house mice—A laboratory study. Behav Ecol Sociobiol. 1994;34(4):275–283. [Google Scholar]
- 37.Schradin C, Kinahan AA, Pillay N. Cooperative breeding in groups of synchronously mating females and evolution of large testes to avoid sperm depletion in african striped mice. Biol Reprod. 2009;81(1):111–117. doi: 10.1095/biolreprod.108.075838. [DOI] [PubMed] [Google Scholar]
- 38.Mumme RL, Koenig WD, Pitelka FA. Reproductive competition in the communal acorn woodpecker—Sisters destroy each others eggs. Nature. 1983;306(5943):583–584. [Google Scholar]
- 39.Vehrencamp SL. Relative fecundity and parental effort in communally nesting anis, Crotophaga sulcirostris. Science. 1977;197(4301):403–405. doi: 10.1126/science.197.4301.403. [DOI] [PubMed] [Google Scholar]
- 40.Vehrencamp SL, Quinn JS. Joint laying systems. In: Koenig W, Dickinson J, editors. Ecology and Evolution of Cooperative Breeding in Birds. Cambridge, United Kingdom: Cambridge Univ Press; 2004. pp. 177–196. [Google Scholar]
- 41.Hager R, Johnstone RA. Infanticide and control of reproduction in cooperative and communal breeders. Anim Behav. 2004;67(5):941–949. [Google Scholar]
- 42.Clutton-Brock T, Huchard E. Social competition and its consequences in female mammals. J Zool. 2013;289(3):151–171. [Google Scholar]
- 43.Ratnieks FLW, Wenseleers T. Altruism in insect societies and beyond: Voluntary or enforced? Trends Ecol Evol. 2008;23(1):45–52. doi: 10.1016/j.tree.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Saltzman W, Digby LJ, Abbott DH. Reproductive skew in female common marmosets: What can proximate mechanisms tell us about ultimate causes? Proc Biol Sci. 2009;276(1656):389–399. doi: 10.1098/rspb.2008.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett NC, Faulkes CG. African Mole Rats: Ecology and Eusociality. Cambridge, United Kingdom: Cambridge Univ Press; 2000. [Google Scholar]
- 46.Young AJ, Monfort SL, Clutton-Brock TH. The causes of physiological suppression among female meerkats: A role for subordinate restraint due to the threat of infanticide? Horm Behav. 2008;53(1):131–139. doi: 10.1016/j.yhbeh.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Vehrencamp SL. Evolutionary routes to joint-female nesting in birds. Behav Ecol. 2000;11(3):334–344. [Google Scholar]
- 48.Martin SJ, Beekman M, Wossler TC, Ratnieks FLW. Parasitic Cape honeybee workers, Apis mellifera capensis, evade policing. Nature. 2002;415(6868):163–165. doi: 10.1038/415163a. [DOI] [PubMed] [Google Scholar]
- 49.McNamara JM, Houston AI, Barta Z, Osorno JL. Should young ever be better off with one parent than with two? Behav Ecol. 2003;14(3):301–310. [Google Scholar]
- 50.McNamara JM, Gasson CE, Houston AI. Incorporating rules for responding into evolutionary games. Nature. 1999;401(6751):368–371. doi: 10.1038/43869. [DOI] [PubMed] [Google Scholar]
- 51.Jordan NR, Mwanguhya F, Kyabulima S, Ruedi P, Cant MA. Scent marking within and between groups of wild banded mongooses. J Zool. 2010;280(1):72–83. [Google Scholar]
- 52.Nichols HJ, Bell MBV, Hodge SJ, Cant MA. Resource limitation moderates the adaptive suppression of subordinate breeding in a cooperatively breeding mongoose. Behav Ecol. 2012;23(3):635–642. [Google Scholar]
- 53.Hodge SJ, et al. Maternal weight, offspring competition and the evolution of communal breeding. Behav Ecol. 2009;20(4):729–735. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.