Significance
Stomata are microscopic pores surrounded by two guard cells and play an important role in the uptake of CO2 for photosynthesis. Recent researches revealed that light-induced stomatal opening is mediated by at least three key components: blue light receptor phototropin, plasma membrane H+-ATPase, and plasma membrane inward-rectifying K+ channels. Here, we showed that only increasing the amount of H+-ATPase in guard cells had a significant effect on light-induced stomatal opening. Transgenic Arabidopsis plants by overexpressing H+-ATPase in guard cells exhibited enhanced photosynthesis activity and plant growth. Our results demonstrate that stomatal aperture is a limiting factor in photosynthesis and plant growth, and that overexpression of the H+-ATPase in guard cells is useful for promotion of plant growth.
Keywords: Arabidopsis thaliana, stomatal conductance, photosynthetic rate, biomass
Abstract
Stomatal pores surrounded by a pair of guard cells in the plant epidermis control gas exchange between plants and the atmosphere in response to light, CO2, and the plant hormone abscisic acid. Light-induced stomatal opening is mediated by at least three key components: the blue light receptor phototropin (phot1 and phot2), plasma membrane H+-ATPase, and plasma membrane inward-rectifying K+ channels. Very few attempts have been made to enhance stomatal opening with the goal of increasing photosynthesis and plant growth, even though stomatal resistance is thought to be the major limiting factor for CO2 uptake by plants. Here, we show that transgenic Arabidopsis plants overexpressing H+-ATPase using the strong guard cell promoter GC1 showed enhanced light-induced stomatal opening, photosynthesis, and plant growth. The transgenic plants produced larger and increased numbers of rosette leaves, with ∼42–63% greater fresh and dry weights than the wild type in the first 25 d of growth. The dry weights of total flowering stems of 45-d-old transgenic plants, including seeds, siliques, and flowers, were ∼36–41% greater than those of the wild type. In addition, stomata in the transgenic plants closed normally in response to darkness and abscisic acid. In contrast, the overexpression of phototropin or inward-rectifying K+ channels in guard cells had no effect on these phenotypes. These results demonstrate that stomatal aperture is a limiting factor in photosynthesis and plant growth, and that manipulation of stomatal opening by overexpressing H+-ATPase in guard cells is useful for the promotion of plant growth.
In the present era of global climate changes and the threat of food insufficiency, finding ways to improve the uptake of CO2 by terrestrial plants is an increasingly important problem. Stomata, key organs in the uptake of CO2, are microscopic pores surrounded by two specialized cells in the epidermis (named guard cells) and are mainly found on the leaf surface in terrestrial plants. Because the leaf surface is nearly impermeable to air and water, stomata provide the major pathway for the diffusion of CO2, O2, and water vapor between the ambient atmosphere and the interior of the leaf. This facilitation of gas exchange by stomatal opening is one of the most essential processes in plant photosynthesis and transpiration (1, 2). A recent study indicated that stomatal transpiration limited photosynthesis in rice (3). Therefore, increased stomatal opening/transpiration is expected to promote photosynthesis and thereby increase plant growth. Condon et al. (4) examined diverse wheat genotypes and showed that increased stomatal conductance, especially abaxial stomatal conductance, may have a positive effect on crop biomass. However, to our knowledge, no previous studies have determined stomatal effects on plant growth by manipulating stomatal aperture via gene regulation in guard cells, perhaps because of the difficulty in balancing the counteracting effects of taking up CO2 while losing water vapor through the stomata (5).
Light is one of the principal factors that stimulates stomatal opening, and various mechanisms underlie stomatal opening in response to different light wavelengths (6–8). Red light is thought to induce stomatal opening via photosynthesis in the mesophyll and guard cell chloroplasts, as well as the reduction of the intercellular CO2 concentration (Ci) (5, 9, 10). However, the detailed mechanisms of stomatal responses to red light are under debate (11, 12). In contrast, blue light acts as a signal and exerts the most pronounced effect on stomatal opening. The blue light receptors phototropins (phot1 and phot2) activate plasma membrane H+-ATPase through the phosphorylation of the penultimate threonine and subsequent binding of the 14-3-3 protein to the phosphorylated threonine (13–15). Blue light-activated H+-ATPase induces hyperpolarization of the plasma membrane, which allows K+ uptake through inward-rectifying K+ (K+in) channels (16–21). Accumulation of K+ induces the swelling of guard cells and stomatal opening. Thus, these three components (phototropins, plasma membrane H+-ATPase, and K+in channels) have important roles in blue light-induced stomatal opening. In addition to these components, FLOWERING LOCUS T (FT) is suggested to be a positive regulator for stomatal opening via its effect on the activation status of the plasma membrane H+-ATPase (22).
In this study, we produced transgenic plants expressing key components active in stomatal opening under the control of the strong guard cell promoter GC1 to promote stomatal opening in Arabidopsis thaliana (23). We showed that transgenic Arabidopsis plants overexpressing H+-ATPase in guard cells exhibited enhanced light-induced stomatal opening, photosynthesis, and plant growth, and that stomatal aperture is a limiting factor in photosynthesis and plant growth.
Results
Overexpression of H+-ATPase in Guard Cells Promotes Light-Induced Stomatal Opening.
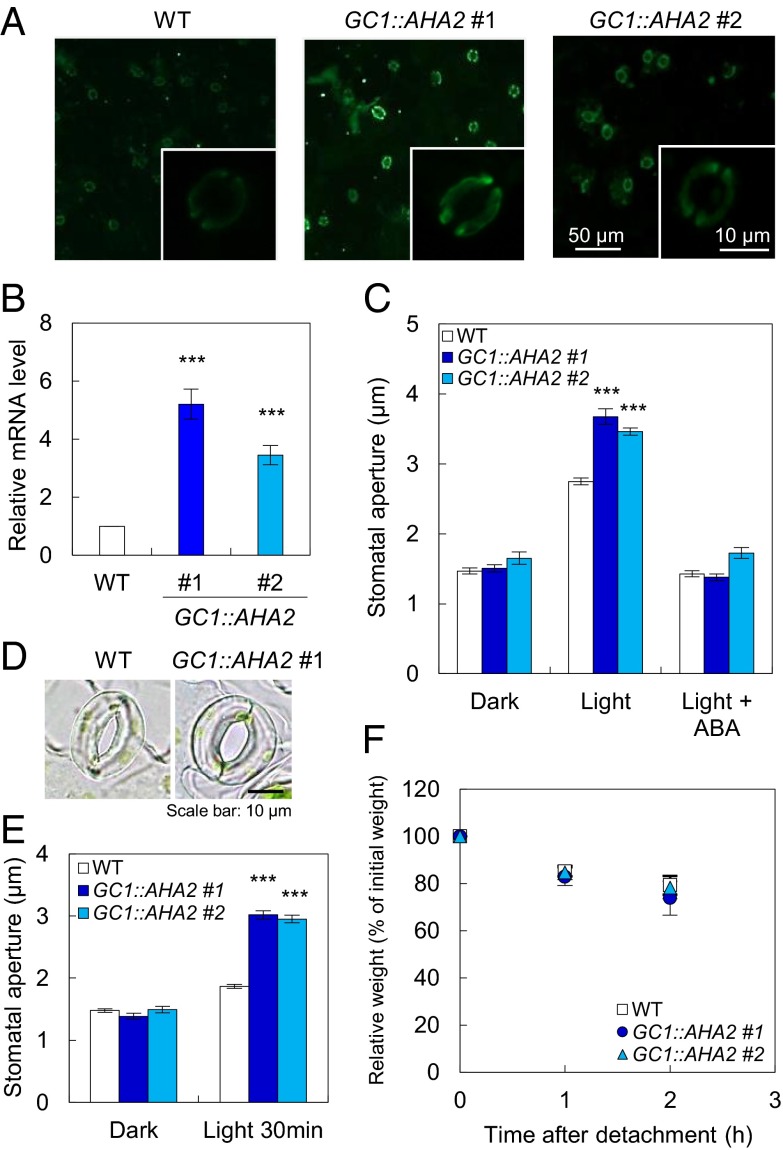
To produce transgenic plants showing enhanced stomatal opening in response to light, we attempted to express three key components of stomatal opening: PHOTOTROPIN 2 (PHOT2), an isoform of the blue light receptor phototropin (13); ARABIDOPSIS H+-ATPASE 2 (AHA2), a typical isoform of the plasma membrane H+-ATPase (24); and POTASSIUM CHANNEL IN ARABIDOPSIS THALIANA 1 (KAT1) and ARABIDOPSIS K+ TRANSPORTER 1 (AKT1), isoforms of plasma membrane K+in channels (19, 20, 25–27). We used the strong guard cell promoter GC1 (22, 23) because these proteins function exclusively within guard cells. Immunohistochemical and Western blotting analyses demonstrated that the levels of AHA2 expressed under the control of the GC1 promoter (GC1::AHA2) in guard cells of AHA2-transgenic plants were ∼1.5-fold higher than in wild-type (WT) guard cells (Fig. 1A and Fig. S1D). Quantitative (q) PCR demonstrated that AHA2 transcripts in the epidermal tissue of AHA2-transgenic plants, including guard cells, were present at levels 3.4- to 5.2-fold higher than in WT plants (Fig. 1B). We did not detect differences in the levels of AHA2 transcripts in leaves or roots (Fig. S1). Thus, we examined light-induced stomatal opening in these plants. The AHA2-transgenic plants showed ∼25% wider stomatal apertures than WT plants exposed to light illumination for 2.5 h, but these stomata closed in darkness just as in the WT (Fig. 1 C and E). We also demonstrated that stomata of the AHA2-transgenic plants opened more quickly than WT stomata over a period of 30 min of illumination (Fig. 1 D and E and Fig. S2). In contrast, the overexpression of PHOT2-GFP (GC1::PHOT2-GFP), which has been shown to restore light-induced stomatal opening in phot1 phot2, AKT1 (GC1::AKT1), and KAT1 (GC1::KAT1), had no effect on stomatal opening under these conditions, even though these components were present at increased levels in guard cells or the epidermis (Fig. S3). These results indicated that H+-ATPase, not phototropins or K+in channels, is the limiting factor in light-induced stomatal opening and that increasing the amount of H+-ATPase in guard cells increased the magnitude of stomatal opening.
Fig. 1.
Overexpression of AHA2 using the GC1 promoter promotes stomatal opening. (A) Typical fluorescence images of immunohistochemical detection of the guard cell H+-ATPase in the Arabidopsis epidermis. (For details regarding the immunohistochemical conditions, see Materials and Methods.) (B) qPCR analysis of AHA2 expression. Error bars represent the SEM (n ≥ 6). Significant differences were detected by Student t test (***P < 0.001). (C) Stomatal apertures under 2.5 h of darkness, light (50 µmol⋅m–2⋅s–1 red light and 10 µmol⋅m–2⋅s–1 blue light), or light in the presence of 20 µM ABA. (D) Typical stomata in the epidermis illuminated with light for 30 min. Light conditions were the same as in C. (E) Stomatal apertures in darkness or after 30 min of light treatment. The light conditions were the same as in C. The stomatal aperture values in C and E are the means of measurements of 25 stomata; error bars represent the SEM. Differences in stomatal aperture were detected using Student t test (***P < 0.001). (F) Kinetics of the change in fresh weight of detached rosette leaves from 4-wk-old WT and AHA2-transgenic plants. The relative weights of the leaves are presented as a percentage of the initial weight, which was the weight of each rosette leaf immediately after detachment from the plant. Data are the mean values for seven leaves; error bars represent the SD.
Note that the light-induced stomatal opening in AHA2-transgenic plants was inhibited by the plant hormone abscisic acid (ABA) to the same extent as in the WT (Fig. 1C). The weight decrease of detached leaves in AHA2-transgenic plants was also similar to that in the WT (Fig. 1F). Thus, the drought response of AHA2-transgenic plants was normal. Importantly, WT and AHA2-transgenic plants withered in the same way in response to drought stress (Fig. S4), suggesting that the drought sensitivity of AHA2-transgenic plants was similar to that of the WT.
Overexpression of H+-ATPase in Guard Cells Enhances both Stomatal Conductance and Photosynthetic Activity.
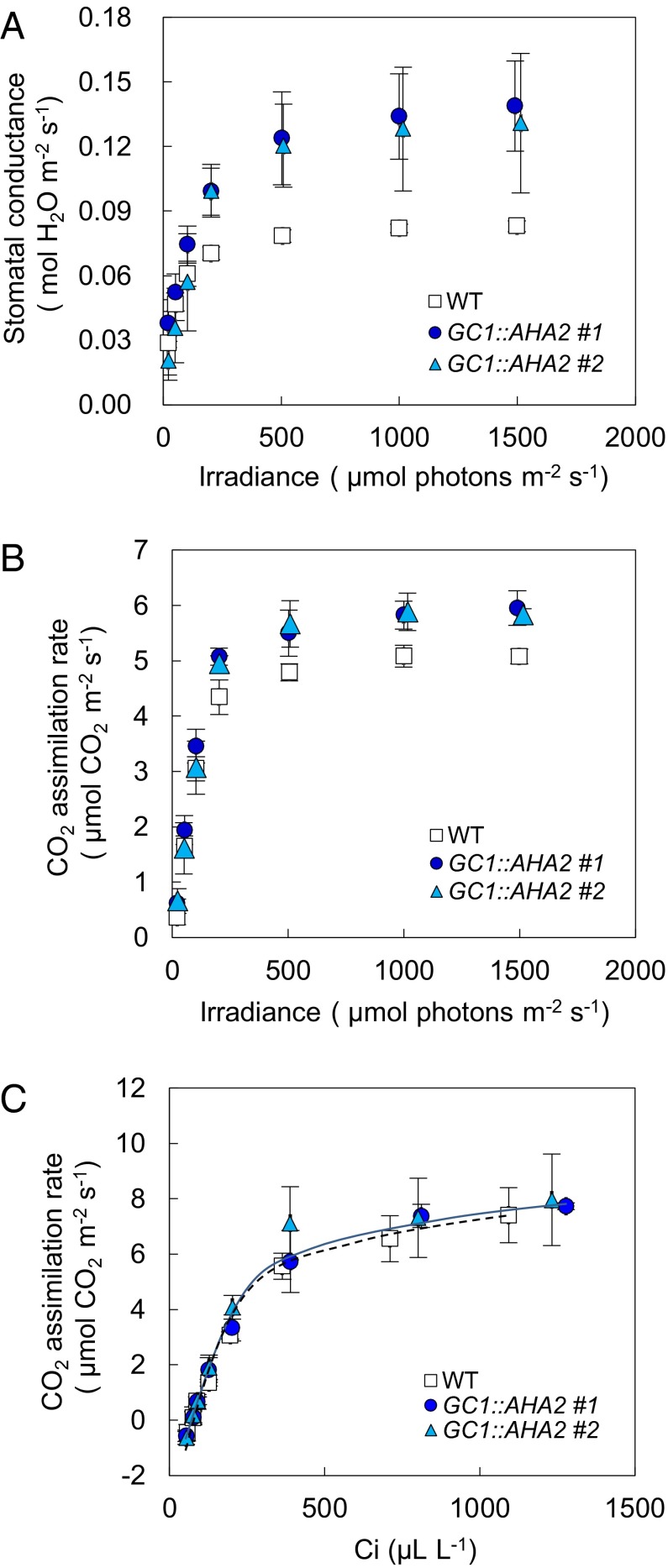
We examined stomatal conductance and photosynthetic activity (CO2 assimilation rate) in detail in intact leaves of AHA2-transgenic plants using a gas-exchange system. Stomatal conductance and the CO2 assimilation rate were significantly higher in AHA2-transgenic plants than in WT plants at photon flux densities ≥200 µmol⋅m–2⋅s–1 (P < 0.05) (Fig. 2 A and B and Table S1). The increments of stomatal conductance of the AHA2-transgenic plants to that of the WT were getting higher with the increasing of light intensities, resulting in decreased water-use efficiency (the ratio between the photosynthetic rate and the transpiration rate) in the AHA2-transgenic plants compared with WT plants (Table S1). The gas-exchange parameters of AHA2-transgenic plants of line #2 were somewhat variable, probably because of the relatively low overexpression of AHA2 (Fig. 1B and Fig. S1B). To determine whether the higher photosynthetic rate of the AHA2-transgenic plants was attributable to the increased stomatal opening, we examined the response curve between the CO2 assimilation rate and the leaf intercellular CO2 concentration using a gas-exchange system under saturating-light conditions (Fig. 2C). The two curves were almost coincident with each other. According to Farquhar et al. (28), these results indicate that both the Rubisco carboxylation capacity and the electron transport capacity were similar in the WT and transgenic plants, but only the stomatal conductance was greater in the AHA2-transgenic plants (Fig. 2C). These results indicate that the enhanced stomatal opening in AHA2-transgenic plants contributes to the increased photosynthetic rate.
Fig. 2.
Gas-exchange properties of AHA2-transgenic plants. (A and B) Light responses of stomatal conductance (A) and the CO2 assimilation rate (B) in WT and AHA2-transgenic plants. Measurements were conducted at 380 µL⋅L–1 CO2; the leaf temperature and leaf chamber relative humidity were maintained at 24 °C and 40–50% (Pa/Pa), respectively. (C) Relationship between the CO2 assimilation rate and the intercellular CO2 concentration in WT and AHA2-transgenic plants. Measurements were conducted in white light of ∼750 µmol⋅m–2⋅s–1. The leaf temperature and relative humidity in the leaf chamber were maintained at 24 °C and 40–50% (Pa/Pa), respectively. Data are the means of measurements on three different plants; error bars represent the SD and are not shown if smaller than the symbols. White squares, WT plants; dark blue circles and light blue triangles, AHA2-transgenic plants.
Overexpression of H+-ATPase in Guard Cells Promotes Plant Productivity Under Conditions of Sufficient Light.
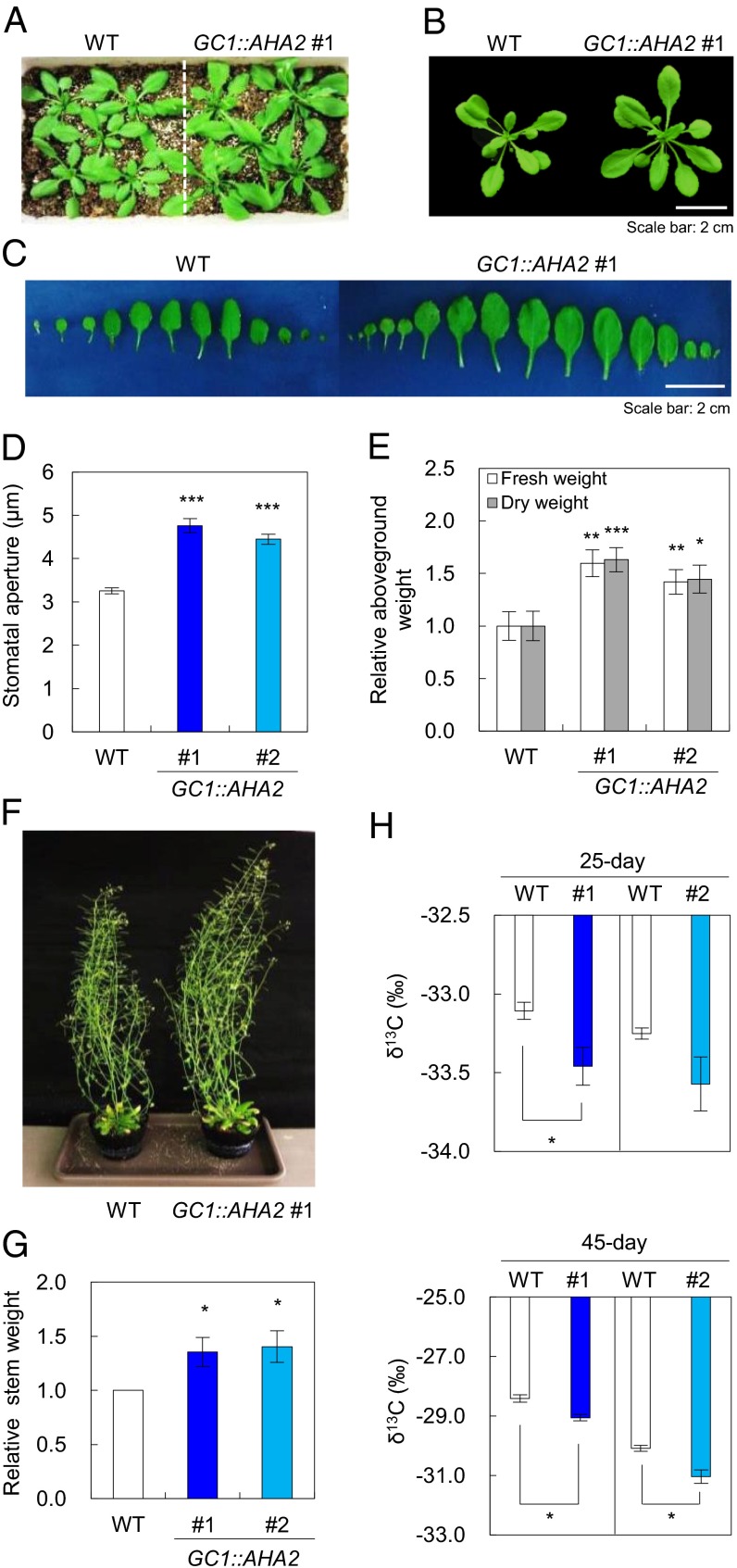
We examined the growth characteristics of AHA2-transgenic plants (Fig. 3). Notably, the AHA2-transgenic plants, which exhibited wider stomatal aperture, produced larger and increased numbers of rosette leaves with ∼42–63% greater fresh and dry weights than WT plants when the plants were grown under a light intensity of 200 µmol⋅m–2⋅s–1 for 25 d. Moreover, the dry weights of total flowering stems in 45-d-old AHA2-transgenic plants, including seeds, siliques, and flowers, were ∼36–41% greater than those of the WT under the same growth conditions. The number of siliques per AHA2-transgenic plant was much greater than for WT plants, although the dry weights of individual siliques from AHA2-transgenic plants were comparable to those from WT plants (Fig. S5). In contrast, the stomatal density and index and the size of AHA2-transgenic plants were comparable to those of WT plants (Table 1). These results indicate that the increased productivity was not attributable to differences in stomatal morphology or differentiation and that the increased light-induced stomatal opening promoted plant growth.
Fig. 3.
Phenotypic characterization of AHA2-transgenic plants. (A and B) Phenotypes of WT and AHA2-transgenic plants grown under high light conditions (200 µmol⋅m–2⋅s–1) for 25 d. (C) Rosette and juvenile leaves of WT and AHA2-transgenic plants grown under high light conditions. (D) Stomatal aperture under high light conditions (200 µmol⋅m–2⋅s–1) in 25-d-old plants. Error bars represent the SEM (n = 25). Significant differences in stomatal aperture were detected using Student t test (***P < 0.001). (E) Relative aboveground fresh and dry weights of 25-d-old plants. (F) Phenotypes of WT and AHA2-transgenic plants grown under high light conditions for 50 d. (G) Relative dry stem weights of 45-d-old plants. Fresh and dry weights are the means of measurements of more than five plants; error bars represent the SEM. (H) Carbon isotope ratio (δ13C) of WT and AHA2-transgenic plants at 25 d (Upper) and 45 d (Lower); #1 and #2 represent the AHA2-transgenic lines GC1::AHA2 #1 and #2, respectively. Error bars represent the SEM (n = 3–4 plants). Differences were detected by Student t test (*P < 0.05; **P < 0.005; ***P < 0.001).
Table 1.
Stomatal density, index, and size of WT and GC1::AHA2 transgenic plants
| Adaxial / Abaxial | WT | GC1::AHA2 #1 | GC1::AHA2 #2 |
| Stomatal density, mm−2 | |||
| Adaxial | 88.5 ± 13.8 | 84.8 ± 12.8 | 87.0 ± 12.2 |
| Abaxial | 130.2 ± 18.8 | 129.5 ± 25.4 | 122.0 ± 15.6 |
| Stomatal index | |||
| Adaxial | 0.36 ± 0.05 | 0.37 ± 0.05 | 0.37 ± 0.05 |
| Abaxial | 0.30 ± 0.06 | 0.29 ± 0.03 | 0.30 ± 0.03 |
| Stomatal size, µm | |||
| Adaxial | 24.94 ± 1.94 | 24.83 ± 2.34 | 24.59 ± 1.97 |
| Abaxial | 23.28 ± 2.16 | 23.54 ± 2.66 | 23.20 ± 1.76 |
Stomatal density, index, and size were calculated according to a previous method (12).
Plants with higher stomatal conductance fix more 12C than 13C, making the ratio of 13C/12C (δ13C) less than in plants with lower stomatal conductance (29). To confirm that the wider stomatal aperture of the AHA2-transgenic plants contributed to plant growth, we examined δ13C in WT and AHA2-transgenic plants. The AHA2-transgenic plants had a lower δ13C than WT plants under well-watered conditions (Fig. 3H), although the δ13C values for AHA2-transgenic line #2 plants seemed to vary widely over a 25-d period. In contrast, no difference in stomatal aperture was observed between WT and AHA2-transgenic plants under mild drought conditions, and no observed changes in weight or δ13C were detected between WT and AHA2-transgenic plants (Fig. S6). These results also suggest that increases in the biomass of AHA2-transgenic plants are dependent primarily on stomatal aperture.
Note that when AHA2-transgenic plants were grown under a light intensity of 80 µmol⋅m–2⋅s–1, the differences in plant growth between AHA2-transgenic and WT plants were small (Table 2). Intercellular CO2 concentration may still affect the photosynthetic rate because the limiting electron transport is partitioned between carboxylation-related and oxygenation-related reactions (28). However, under a relatively low experimental light intensity, the photosynthetic electron-transport rate, rather than stomatal aperture size, was most likely the limiting factor for photosynthesis (30). Therefore, AHA2-transgenic plants showed superior growth under relatively high light intensity (200 µmol⋅m–2⋅s–1). Further investigations are needed to examine the light dependency of plant growth in AHA2-transgenic plants. In contrast, the growth of PHOT2-, KAT1-, and AKT1-transgenic plants was similar to that of the WT, even under a light intensity of 200 µmol⋅m–2⋅s–1 (Table 2).
Table 2.
Plant growth of WT and transgenic plants
| Plants | Fresh weight, % | Dry weight, % |
| High light (25-d) | ||
| WT | 100 | 100 |
| GC1::AHA2 #1 | 159.7 ± 12.7† | 163.1 ± 11.6† |
| GC1::AHA2 #2 | 142.1 ± 11.7‡ | 144.6 ± 13.3* |
| GC1::PHOT2-GFP #3 | 99.3 ± 7.3 | 95.6 ± 6.5 |
| GC1::AKT1 #1 | 78.4 ± 7.5 | 78.0 ± 8.0 |
| GC1::KAT1 #2 | 101.5 ± 26.2 | 106.5 ± 27.6 |
| GC1::FT-GFP #1 | 94.5 ± 13.5 | 102.0 ± 13.7 |
| GC1::AHA2-P68S #1 | 99.7 ± 6.4 | 100.0 ± 6.3 |
| High light (45-d) | ||
| WT | 100 | |
| GC1::AHA2 #1 | not detected | 135.6 ± 13.3* |
| GC1::AHA2 #2 | not detected | 140.6 ± 14.6* |
| Low light (25-d) | ||
| WT | 100 | 100 |
| GC1::AHA2 #1 | 107.4 ± 26.2 | 112.8 ± 15.4 |
Differences were detected by Student t test (*P < 0.05; †P < 0.005; ‡P < 0.001). The dry weights of high light (45-d) plants refer to the final stem weight presented in Fig. 3. High light, photon flux density at 200 µmol⋅m−2⋅s−1; low light, photon flux density at 80 µmol⋅m−2⋅s−1.
Constitutive Open Stomata Phenotype Has No Effect on Plant Growth.
In this study, WT AHA2 was overexpressed in guard cells. The stomata of the AHA2-transgenic plants showed increased stomatal opening in response to light, but closed normally in darkness and in response to ABA (Fig. 1). Thus, we examined stomatal phenotype and plant growth in the transgenic plants, which exhibited a constitutive open stomata phenotype. We produced AHA2-P68S-transgenic plants (GC1::AHA2-P68S), which were presumed to have constitutive high levels of active H+-ATPase as a result of a Pro68-to-Ser point mutation in the first transmembrane domain (31). Indeed, the stomata of the AHA2-P68S-transgenic plants were constantly open with a wide aperture under both light and dark conditions and even in the presence of ABA (Fig. S7A). However, no enhanced plant growth was observed in the AHA2-P68S-transgenic plants (Fig. S7 B and C). We also examined FT-transgenic plants (GC1::FT-GFP) because FT is a positive regulator of stomatal opening (22). Stomata of FT-transgenic plants were constantly open with a larger aperture than the stomata of WT plants under light and dark conditions but closed in response to ABA (22). However, the growth of FT-transgenic plants was comparable to that of WT plants (Table 2). These results indicate that a constitutive open stomata phenotype has no effect on plant growth.
Discussion
In this study, we produced A. thaliana transgenic plants overexpressing the important components in light-induced stomatal opening, such as the plasma membrane H+-ATPase, phot2, and K+in channels, using the strong guard cell promoter GC1. Only increasing the amount of plasma membrane H+-ATPase in guard cells had a significant effect on light-induced stomatal opening (Figs. 1 and 3D), indicating that the plasma membrane H+-ATPase is the limiting factor in the stomatal opening process. We suspect that this effect is attributable to the electrical properties of plasma membrane K+in channels. The voltage–current relationship of the plasma membrane K+in channels shows that the activity of K+in channels is hyperpolarization-dependent (18). Therefore, increasing the levels of guard cell H+-ATPase, which promotes hyperpolarization of the plasma membrane in response to blue light (16), effectively induces K+ uptake into guard cells and stomatal opening. In addition, AHA2-transgenic plants showed enhanced photosynthetic activity and increased growth in leaves, seeds, siliques, and flowers under relatively high light intensity (200 µmol⋅m–2⋅s–1) (Figs. 2 and 3, Table 2, and Table S1). In their examination of diverse wheat varieties using carbon or oxygen isotopes, Farquhar and coworkers demonstrated that stomatal conductance may have positive effects on crop biomass (4, 32). In this study, we manipulated stomatal opening by means of gene regulation in guard cells to promote plant growth. Our results show that manipulation of the plasma membrane H+-ATPase is very useful not only for promoting stomatal opening but also for enhancing photosynthesis and plant growth. In support of our results, control of H+-ATPase translocation to the plasma membrane by PATROL1, which is involved in intercellular membrane traffic, was recently reported to affect stomatal opening, photosynthesis, and plant growth (33).
Note that other key components of light-induced stomatal opening investigated here (phototropins and K+in channels) had no effect on stomatal opening, photosynthesis, or plant growth under the conditions of this study (Fig. S3 and Table 2). These results suggest that the amounts of endogenous phototropins and K+in channels in guard cells are sufficient and are not limiting factors in light-induced stomatal opening. This is consistent with data suggesting that native K+in channel activity levels are high and predicted not to be rate-limiting for stomatal opening (18, 19, 34). Overexpression of PHOT2 in guard cells may enhance blue light sensitivity because blue light sensitivity in etiolated seedlings is suggested to depend on the expression level of phot2 (35). Thus, PHOT2-transgenic plants may perform better under relatively low light intensity than under relatively high light intensity. Further investigations are needed to clarify this point.
Recently, the rice mutant slac1, a drought-sensitive open-stomata mutant, has been shown to have increased rates leaf photosynthesis (3). However, the slac1 mutation had no effect on plant growth because of an increased sensitivity to drought stress. Consistent with this finding, AHA2-P68S transgenic plants, which have constitutive open-stomata and ABA-insensitive phenotypes, did not show enhanced plant growth (Fig. S7). In addition, the stomata of FT-transgenic plants (GC1::FT-GFP), which have a normal ABA response, were constantly open with a wide aperture under both light and dark conditions (22). However, the growth of FT-transgenic plants was comparable to that of WT plants (Table 2). These results indicate that a lack of sensitivity to drought or increased water loss at night may negatively impact biomass accumulation. Further studies on the mechanisms underlying the relationship between nighttime water loss and plant growth are required. Therefore, the stomata of AHA2-transgenic plants, which exhibit increased stomatal opening in light but also close normally in darkness and in response to ABA (Fig. 1C), contribute to enhanced photosynthesis and plant growth. Application of this strategy in crops and fuel plants is expected to contribute greatly to the promotion of plant production and a sustainable low-carbon society.
Materials and Methods
Plant Materials and Growth Conditions.
Arabidopsis thaliana gl1 [Columbia (Col), carrying the homozygous recessive gl1], used here as the WT, was the background ecotype of all transgenic plants. Plants used for experiments in gene expression, stomatal aperture, and gas exchange were grown in soil under a 16-h fluorescent light (80 µmol⋅m–2⋅s–1)/8-h dark cycle at 24 °C in 55–70% (Pa/Pa) humidity in a growth room. Plants used for biomass productivity measurements were grown under the same environmental conditions, except the light intensity was ∼80 µmol⋅m–2⋅s–1 (low light conditions) and 200 µmol⋅m–2⋅s–1 (high light conditions).
Construction of Transformation Vectors and Transformation of Plants.
The plasmid vectors used for plant transformation were constructed according to a previous method (22). In brief, a genomic DNA fragment spanning 1,702 to 1 bp upstream of the start codon of GC1 (At1g22690) flanked by HindIII and XbaI sites was used to replace the corresponding region of CaMV35S in pPZP211 (pPZP211-GC1). Before HindIII digestion, a single-nucleotide substitution (1,453 C to G) was introduced into the GC1 fragment by site-directed mutagenesis. cDNA fragments of AHA2 (At4g30190), PHOT2 (At5g58140), KAT1 (At5g46240), and AKT1 (At2g26650) were amplified with the following oligonucleotide primers: 5′-CGGGATCCGAGATGTCGAGTCTCGAAGATATCAAGAAC-3′ and 5′-CGGGATCCCTACACAGTGTAGTGACTGGG-3′ for AHA2; 5′-GCCTCTAGAGTTATGGGGATGGAGAGGCCAAGAGCCC-3′ and 5′-CATGCCATGGCGAAGAGGTCAATCTCCAAGTCCG-3′ for PHOT2; 5′-GCCTCTAGAAAGATGTCGATCTCTTGGACTCG-3′ and 5′-GCCTCTAGATCAATTTGATGAAAAATACAAATGATCACC-3′ for KAT1; and 5′-GCCTCTAGAGTGATGAGAGGAGGGGCTTTGTTATGC-3′ and 5′-GCCTCTAGATTAAGAATCAGTTGCAAAGATGAGATGATC-3′ for AKT1. The amplified DNA fragments were inserted into pPZP211-GC1 using BamHI, XbaI, or NcoI. The stop codon of the PHOT2 coding sequence in pPZP211-GC1::PHOT2 was replaced with a gene encoding synthetic green fluorescent protein with an S65T mutation (GFP). A single amino acid substitution (P68S) was introduced into pPZP211-GC1::AHA2 by site-directed mutagenesis using the following primers: 5′-GGGGTTTATGTGGAATTCACTTTCATGGGTCATGG-3′ and 5′-CCATGACCCATGAAAGTGAATTCCACATAAACCCC-3′. The construction of GC1::FT-GFP was described previously (22). All of the plant transformation plasmid vectors were introduced into Agrobacterium tumefaciens (GV3101), which was then used to transform plants using a standard method (22). F3 homozygous plants were used for the experiments.
Reverse transcription–PCR and qPCR.
Total RNA was extracted from epidermal fragments using the RNeasy Plant Mini Kit (Qiagen). Epidermal fragments from fully expanded rosette leaves, whole leaves, and roots were isolated from 4- to 6-wk-old plants as described previously (36). First-strand cDNAs were synthesized from total RNA using the PrimeScript II First Strand cDNA Synthesis Kit using oligo(dT) primers (Takara). cDNA fragments of AHA2 and TUB2 were amplified with the following oligonucleotide primers: 5′-GGGGAATTCATGTCGAGTCTCGAAG-3′ and 5′-GGGGAATTCTACACAGTGTAGTGAC-3′ for AHA2 and 5′-CATTGTTGATCTCTAAGATCCGTG-3′ and 5′-TACTGCTGAGAACCTCTTGAG-3′ for TUB2. All PCRs were performed for 30 cycles.
qPCR was performed using Power SYBR Green PCR Master Mix and the StepOne Real-Time PCR system (Applied Biosystems) as described previously (37). For gene-specific amplification of the AHA2 transcripts, the following primer sets were used: 5′-TTGTTGAACGTCCTGGAGCA-3′ and 5′-AATTCC CAGTTGGCGTAAACC-3′. Relative quantification was performed using the comparative cycle threshold method, and the relative amount of PCR product amplified using the above primer sets was normalized to the TUB2 gene fragment as an internal control amplified using the primers 5′-AAACTCACTACCCCCAGCTTTG-3′ and 5′-CACCAGACATAGTAGCAGAAATCAAGT-3′.
Immunohistochemical Detection of Plasma Membrane H+-ATPase in Guard Cells.
Immunohistochemical detection was performed according to a previous method (36) with modifications. Fully expanded rosette leaves of WT and AHA2-transgenic plants were harvested and then cut into small pieces. The pieces were fixed with 4% (wt/vol) formaldehyde freshly prepared from paraformaldehyde in fixation buffer [50 mM Pipes⋅NaOH (pH 7.0), 5 mM MgSO4, 5 mM EGTA] for 1 h at room temperature. The fixed samples were washed with PBS (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4), destained with 100% methanol at 37 °C, and then washed with Milli-Q water (Millipore) at room temperature. The destained samples were attached to slide glasses, sealed with cover glasses, and then frozen and thawed five times with liquid nitrogen. After removing the cover glasses, the samples were completely dried overnight. On the second day, the samples were digested on the slide glasses with 4% (wt/vol) Cellulase Onozuka R-10 (Yakult) and 0.5% (wt/vol) Macerozyme R-10 (Yakult) in PBS for 1 h at 37 °C. After digestion, the samples were washed with PBS and permeabilized with 3% (vol/vol) Nonidet P-40 (Nonidet P-40; MP Biomedicals) and 10% (vol/vol) dimethyl sulfoxide (Wako) for 30 min at room temperature. The samples were then washed with PBS and blocked in 3% (wt/vol) BSA Fraction V (Sigma) in PBS for 1 h at room temperature. Thereafter, the samples were incubated with anti–H+-ATPase antibody at a dilution of 1:5,000 in PBS with 3% (wt/vol) BSA for 6 h at 37 °C or overnight at 4 °C. The samples were washed with PBS and incubated with Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) at a dilution of 1:500 in PBS with 3% (wt/vol) BSA at 37 °C for 3 h in darkness. After a wash with PBS, each specimen was sealed under a cover glass with 50% (vol/vol) glycerol. The specimens were observed under a fluorescence microscope (BX50; Olympus) with a narrow excitation bandpass filter set for Alexa Fluor 488, model nos. BP460–480HQ BA495–540HQ (U-MGFPHQ; Olympus), using a Hg arc lamp as a source of excitation light. Fluorescent images were collected using a CCD camera system (DP72; Olympus) and processed using DP2-BSW software (Olympus). To estimate fluorescence intensities, all images were taken at identical exposure times. The fluorescence intensities of stomata were quantified using ImageJ (National Institutes of Health).
Measurement of Stomatal Aperture.
Stomatal apertures were measured according to a previous method (38) with some modifications. Epidermal tissues isolated from overnight dark-adapted 3- to 4-wk-old plants were incubated in basal buffer [5 mM MES⋅bis-Tris propane (pH 6.5), 50 mM KCl, and 0.1 mM CaCl2]. For inhibition of light-induced stomatal opening by ABA, epidermal tissues were incubated under blue/red light [blue light (Stick-B-32; EYELA) at 10 µmol⋅m–2⋅s–1 superimposed on background red light (LED-R; EYELA) at 50 µmol⋅m–2⋅s–1] for 2.5 h at 24 °C in the presence or absence of 20 µM ABA. Stomatal apertures in the abaxial epidermis were measured microscopically. Stomatal apertures are presented as the mean of 25 stomata with SEM. The results were confirmed by blind reassessment by another researcher.
Gas-Exchange Measurements.
Gas-exchange measurements were performed using the LI-6400 system (Li-Cor), and parameters were calculated with the software supplied by the manufacturer. White light was provided by a fiberoptic illuminator with a halogen projector lamp (15 V/150 W; Moritex) as a light source. The power supply (MHAB-150W; Moritex) was used to power the lamp. The light was attenuated with a series of optical crown glass metallic neutral density filters (Newport Japan, Hakuto). The flow rate, leaf temperature, and relative humidity were kept constant at 500 µmol⋅s–1, 24 °C, and 40–50% (Pa/Pa), respectively. For light response curves, the conditions were the same, and after the initial 30 min of dark adaptation, the intensity of the white light (20, 50, 100, 200, 500, 1,000, and 1,500 µmol⋅m–2⋅s–1) was increased in steps of more than 20-min duration (stable statue). For CO2 response curves, leaves were first acclimated at 400 µL⋅L–1 CO2 under saturating white-light conditions (∼750 µmol⋅m–2⋅s–1) for 40 min, and then the CO2 concentration was increased stepwise from 50 to 1,500 µL⋅L–1 CO2 at intervals of ∼20 min.
Carbon Isotope Ratio Analyses.
Carbon isotopes were measured with a system combining an elemental analyzer (Vario Micro; Elementar) and a stable isotope ratio mass spectrometer (IsoPrime) (39). Dried tissue from 25-d or 45-d rosette leaves was used for the carbon isotope analysis. Different transgenic plant lines and WT plants (control) were grown in different planters or in the different period. Carbon isotope ratio (δ13C, ‰) was obtained in δ-notation, where δ = Rsample/Rstandard – 1 and Rsample and Rstandard are the isotope ratios of the plant sample and the PeeDee belemnite, respectively (39, 40).
Statistical Analysis.
Significance was determined according to Student t test using Microsoft Excel. Two-sided tests were performed for homoscedastic matrices.
Supplementary Material
Acknowledgments
We thank Dr. Julian I. Schroeder for critical reading of the manuscript. We also thank Ms. Maki Hayashi and Mr. Eigo Ando for technical advice on immunohistochemical analysis and Dr. Koji Takahashi and Ms. Yuki Hayashi for technical advice on quantitative PCR. This work was supported, in part, by the Advanced Low Carbon Technology Research and Development Program from the Japan Science and Technology Agency and Grants-in-Aid 21227001 and 22119005 (to T.K.) and 21114007 (to I.T.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.M.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305438111/-/DCSupplemental.
References
- 1.Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annu Rev Plant Physiol. 1982;33:317–345. [Google Scholar]
- 2.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424(6951):901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 3.Kusumi K, Hirotsuka S, Kumamaru T, Iba K. Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J Exp Bot. 2012;63(15):5635–5644. doi: 10.1093/jxb/ers216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condon AG, Richards RA, Farquhar GD. Carbon isotope discrimination is positively correlated with grain yield and dry matter production in field-grown wheat. Crop Sci. 1987;27(5):996–1001. [Google Scholar]
- 5.Roelfsema MRB, Hedrich R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005;167(3):665–691. doi: 10.1111/j.1469-8137.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 7.Shimazaki K, Doi M, Assmann SM, Kinoshita T. Light regulation of stomatal movement. Annu Rev Plant Biol. 2007;58:219–247. doi: 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita T, Hayashi Y. New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. Int Rev Cell Mol Biol. 2011;289:89–115. doi: 10.1016/B978-0-12-386039-2.00003-1. [DOI] [PubMed] [Google Scholar]
- 9.Sharkey TD, Ogawa T. In: Stomatal Function, Stomatal Responses to Light. Zeiger E, Farquhar G, Cowan I, editors. Stanford, CA: Stanford Univ Press; 1987. pp. 195–208. [Google Scholar]
- 10.Vavasseur A, Raghavendra AS. Guard cell metabolism and CO2 sensing. New Phytol. 2005;165(3):665–682. doi: 10.1111/j.1469-8137.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- 11.Baroli I, Price GD, Badger MR, von Caemmerer S. The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiol. 2008;146(2):737–747. doi: 10.1104/pp.107.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Noguchi K, Terashima I. Photosynthesis-dependent and -independent responses of stomata to blue, red and green monochromatic light: Differences between the normally oriented and inverted leaves of sunflower. Plant Cell Physiol. 2011;52(3):479–489. doi: 10.1093/pcp/pcr005. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita T, et al. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414(6864):656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T, Shimazaki Ki. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 1999;18(20):5548–5558. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita T, Shimazaki K. Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol. 2002;43(11):1359–1365. doi: 10.1093/pcp/pcf167. [DOI] [PubMed] [Google Scholar]
- 16.Assmann SM, Simoncini L, Schroeder JI. Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba L. Nature. 1985;318(6043):285–287. [Google Scholar]
- 17.Shimazaki K, Iino M, Zeiger E. Blue light-dependent proton extrusion by guard cell protoplasts of Vicia faba. Nature. 1986;319(6051):324–326. [Google Scholar]
- 18.Schroeder JI, Raschke K, Neher E. Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA. 1987;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak JM, et al. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiol. 2001;127(2):473–485. [PMC free article] [PubMed] [Google Scholar]
- 20.Szyroki A, et al. KAT1 is not essential for stomatal opening. Proc Natl Acad Sci USA. 2001;98(5):2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder JI, Hedrich R, Fernandez JM. Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature. 1984;312(5992):361–362. [Google Scholar]
- 22.Kinoshita T, et al. FLOWERING LOCUS T regulates stomatal opening. Curr Biol. 2011;21(14):1232–1238. doi: 10.1016/j.cub.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods. 2008;4:6. doi: 10.1186/1746-4811-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno K, Kinoshita T, Inoue S, Emi T, Shimazaki K. Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 2005;46(6):955–963. doi: 10.1093/pcp/pci104. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1992;89(9):3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258(5088):1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 27.Sentenac H, et al. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256(5057):663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- 29.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–537. [Google Scholar]
- 30.Ogren E, Evans JR. Photosynthetic light-response curves: I. The influence of CO2 partial pressure and leaf inversion. Planta. 1993;189(2):182–190. [Google Scholar]
- 31.Merlot S, et al. Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 2007;26(13):3216–3226. doi: 10.1038/sj.emboj.7601750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbour MM, Fischer RA, Sayre KD, Farquhar GD. Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain yield in irrigated wheat. Aust J Plant Physiol. 2000;27(7):625–637. [Google Scholar]
- 33.Hashimoto-Sugimoto M, et al. A Munc13-like protein in Arabidopsis mediates H+-ATPase translocation that is essential for stomatal responses. Nat Commun. 2013;4:2215. doi: 10.1038/ncomms3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichida AM, Pei ZM, Baizabal-Aguirre VM, Turner KJ, Schroeder JI. Expression of a Cs+-resistant guard cell K+ channel confers Cs+-resistant, light-induced stomatal opening in transgenic arabidopsis. Plant Cell. 1997;9(10):1843–1857. doi: 10.1105/tpc.9.10.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue S, et al. Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol. 2011;156(1):117–128. doi: 10.1104/pp.111.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayashi M, Inoue S, Takahashi K, Kinoshita T. Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol. 2011;52(7):1238–1248. doi: 10.1093/pcp/pcr072. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K, Hayashi K, Kinoshita T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012;159(2):632–641. doi: 10.1104/pp.112.196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inoue S, et al. Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc Natl Acad Sci USA. 2008;105(14):5626–5631. doi: 10.1073/pnas.0709189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tazoe Y, Noguchi K, Terashima I. Effects of growth light and nitrogen nutrition on the organization of the photosynthetic apparatus in leaves of a C4 plant, Amaranthus cruentus. Plant Cell Environ. 2006;29(4):691–700. doi: 10.1111/j.1365-3040.2005.01453.x. [DOI] [PubMed] [Google Scholar]
- 40.Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436(7052):866–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.