Significance
The HA surface glycoprotein on influenza A viruses mediates viral entry into host cells. HA is highly variable and classified into 18 divergent subtypes, which cluster into two major phylogenetic groups. Antibody CR8043 has heterosubtypic neutralizing activity against group 2 viruses, including H3 viruses that currently circulate in humans. X-ray and EM structures of CR8043 Fab in complex with H3 HAs reveal that the antibody targets a conserved epitope on the HA stem. Compared with CR8020, the only other structurally characterized group 2 neutralizing antibody, CR8043 binds to HA with a different approach angle using different contact residues. The epitopes of both antibodies are very similar, which suggests that this conserved stem epitope has great potential for design of therapeutics and vaccines.
Keywords: antibody recognition, X-ray crystallography, electron microscopy
Abstract
The discovery and characterization of broadly neutralizing antibodies (bnAbs) against influenza viruses have raised hopes for the development of monoclonal antibody (mAb)-based immunotherapy and the design of universal influenza vaccines. Only one human bnAb (CR8020) specifically recognizing group 2 influenza A viruses has been previously characterized that binds to a highly conserved epitope at the base of the hemagglutinin (HA) stem and has neutralizing activity against H3, H7, and H10 viruses. Here, we report a second group 2 bnAb, CR8043, which was derived from a different germ-line gene encoding a highly divergent amino acid sequence. CR8043 has in vitro neutralizing activity against H3 and H10 viruses and protects mice against challenge with a lethal dose of H3N2 and H7N7 viruses. The crystal structure and EM reconstructions of the CR8043-H3 HA complex revealed that CR8043 binds to a site similar to the CR8020 epitope but uses an alternative angle of approach and a distinct set of interactions. The identification of another antibody against the group 2 stem epitope suggests that this conserved site of vulnerability has great potential for design of therapeutics and vaccines.
Influenza viruses are a significant and persistent threat to human health worldwide. Annual epidemics cause 3–5 million cases of severe illness and up to 0.5 million deaths (1), and periodic influenza pandemics have the potential to kill millions (2). Inhibitors against the viral surface glycoprotein neuraminidase are widely used for the treatment of influenza infections, but their efficacy is being compromised by the emergence of drug-resistant viral strains (3). Vaccination remains the most effective strategy to prevent influenza virus infection. However, protective efficacy is suboptimal in the highest risk groups: infants, the elderly, and the immunocompromised (1). Furthermore, because immunity after vaccination is typically strain-specific and influenza viruses evolve rapidly, vaccines must be updated almost annually. The antigenic composition of the vaccine is based on a prediction of strains likely to circulate in the coming year, therefore, mismatches between vaccine strains and circulating strains occur that can render the vaccine less effective (4). Consequently, there is an urgent need for new prophylactic and therapeutic interventions that provide broad protection against influenza.
Immunity against influenza viruses is largely mediated by neutralizing antibodies that target the major surface glycoprotein hemagglutinin (HA) (5, 6). Identification of antigenic sites on HA indicates that influenza antibodies are primarily directed against the immunodominant HA head region (7), which mediates endosomal uptake of the virus into host cells by binding to sialic acid receptors (8). Because of high mutation rates in the HA head region and its tolerance for antigenic changes, antibodies that target the HA head are typically only effective against strains closely related to the strain(s) by which they were elicited, although several receptor binding site-targeting antibodies with greater breadth have been structurally characterized (9–15). In contrast, antibodies that bind to the membrane-proximal HA stem region tend to exhibit much broader neutralizing activity and can target strains within entire subtypes and groups (16–25) as well as across influenza types (24). These stem-directed antibodies inhibit major structural rearrangements in HA that are required for the fusion of viral and host endosomal membranes and thus, prevent the release of viral contents into the cell (8). The stem region is less permissive for mutations than the head and relatively well-conserved across divergent influenza subtypes.
Anti-stem antibodies are elicited in some, but not all, individuals during influenza infection or vaccination (20, 26) and thus, hold great promise as potential broad spectrum prophylactic or therapeutic agents and for the development of a universal influenza vaccine (27–29). The majority of the known heterosubtypic stem binding antibodies neutralize influenza A virus subtypes belonging to group 1 (17–20, 23, 25). Furthermore, two antibodies that target a similar epitope in the HA stem, like most heterosubtypic group 1 antibodies, are able to more broadly recognize both group 1 and 2 influenza A viruses (22) or influenza A and B viruses (24). Strikingly, group 2-specific broadly neutralizing Abs (bnAbs) seem to be rare, because only one has been reported to date (21). CR8020 uniquely targets a distinct epitope in the stem in close proximity to the viral membrane at the HA base and binds lower down the stem than any other influenza HA antibody (21).
In the discovery process that led to the isolation of bnAb CR8020, we recovered additional group 2-specific bnAbs. Here, we describe one such bnAb, CR8043, which recognizes a similar but nonidentical footprint on the HA as CR8020 and approaches the HA from a different angle. Furthermore, these two bnAbs are derived from different germ-line genes and, consequently, use distinct sets of interactions for HA recognition. Thus, the human immune system is able to recognize this highly conserved epitope in different ways using different germ-line genes. Hence, this valuable information can be used for the design of therapeutics and vaccines targeting this site of vulnerability in group 2 influenza A viruses that include the pandemic H3N2 subtype.
Results
Identification of bnAbs Against Group 2 Influenza A Viruses.
Memory B cells from donors vaccinated with seasonal influenza vaccine were immortalized to generate stable B-cell receptor-positive, antibody-producing memory B cells (30). We enriched for B cells that bound to recombinant H3 HA and then screened culture supernatants of reactive clones for binding to recombinant H3 HA by ELISA, which yielded 31 clones that secreted H3 HA-reactive antibodies. Among these H3-positive clones, 11 unique clones produced antibodies that neutralized A/Wisconsin/67/2005 (H3N2) in an in vitro virus neutralization assay (VNA), and for all but one, their activity was recapitulated when reformatted to recombinant IgG1 (SI Appendix, Table S1). Sequence analysis of the H3N2 neutralizing mAbs revealed that two pairs of antibodies use identical germ-line genes (CR8020 and CR8041; CR8021 and CR8038), whereas the others (including CR8043) use unique VH and VL genes (SI Appendix, Table S1). Three clones possessed heterosubtypic neutralizing activity in an in vitro VNA against H7 and/or H10 subtypes within the group 2 influenza A viruses. These antibodies include CR8020 (21) and its somatic variant CR8041 as well as CR8043. Interestingly, these three bnAbs were isolated from the same donor (SI Appendix, Table S1). Because CR8020 and CR8041 are somatic variants (SI Appendix, Fig. S1), we confined additional investigation to CR8043.
In Vitro and in Vivo Activity of CR8043 Against Group 2 Influenza Viruses.
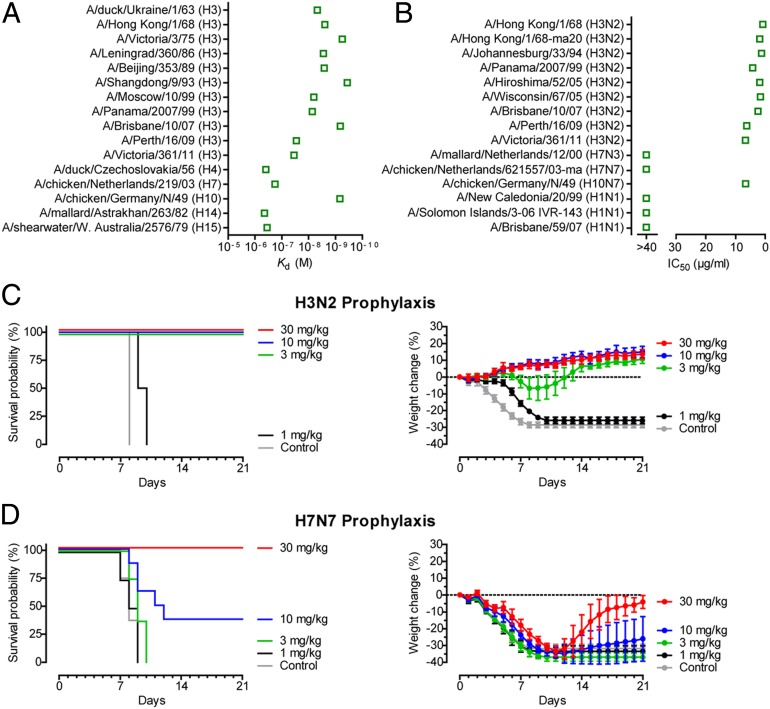
To investigate the breadth of CR8043, we tested its binding to a panel of group 2 HAs. CR8043 Fab binds with high affinity to H3 and H10 HA subtypes (mean Kd ∼ 7 nM) and accommodates ∼50 y of antigenic drift from avian (1963) to human (1968–2011) H3N2 viruses, whereas its affinity for other group 2 subtypes (H4, H7, H14, and H15) is considerably lower (Fig. 1A and Fig. 2). Accordingly, CR8043 IgG potently neutralizes H3 and H10 viruses in vitro, whereas it has no in vitro neutralizing activity against H7 viruses or group 1 H1 viruses that were included as a control (Fig. 1B).
Fig. 1.
In vitro and in vivo binding and neutralization activity of CR8043. (A) Affinity measurements (Kd) for binding of CR8043 Fab to various H3 HAs and representative members of most of the other group 2 HA subtypes. (B) In vitro neutralization (IC50) of CR8043 IgG against a panel of influenza A viruses as determined by VNA. Prophylactic efficacy of CR8043 IgG against lethal challenge with (C) mouse-adapted H3N2 or (D) A/chicken/Netherlands/621557/2003 (H7N7) viruses. (C and D) Shown are (Left) survival curves and (Right) body weight curves of mice treated with 30, 10, 3, or 1 mg/kg CR8043 or 30 mg/kg unrelated control mAb CR3014 1 d before lethal challenge (at day 0). Body weight curves represent mean ± 95% confidence interval of the mean.
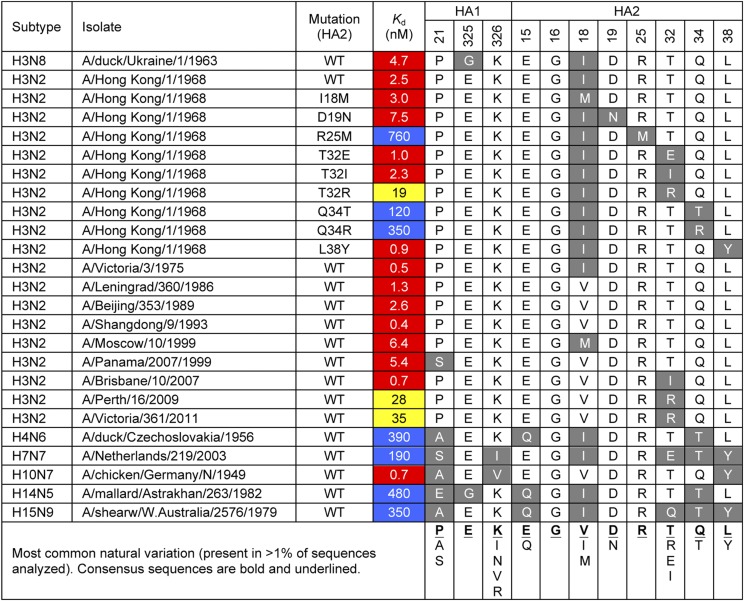
Fig. 2.
Affinity of CR8043 to a panel of HAs of natural group 2 influenza viruses or engineered mutants. The HA residues included compose the CR8043 epitope. Kd values <10 nM, <100 nM or >100 nM are colored in red, yellow or blue boxes, respectively. Residues that differ from the consensus sequence are in gray boxes.
Next, we assessed the prophylactic efficacy of CR8043 against H3N2 and H7N7 viruses using mouse challenge models. Groups of mice were challenged with a lethal dose of mouse-adapted virus 1 d after administration of varying doses of CR8043 IgG. Administration of ≥3 mg/kg CR8043 fully protected mice from death after H3N2 challenge with increased body weight at the end of the study (Fig. 1C). Despite the lack of in vitro neutralizing activity against H7 viruses, a dose of 30 mg/kg CR8043 protected all mice against lethal H7N7 challenge, and a dose of 10 mg/kg was partially protective (Fig. 1D). CR8043 did not prevent morbidity caused by H7N7, but reversal of weight loss was observed at the end of the study when the antibody was administered at 30 mg/kg (Fig. 1D).
CR8043 Binds a Conserved Epitope in the HA Stem.
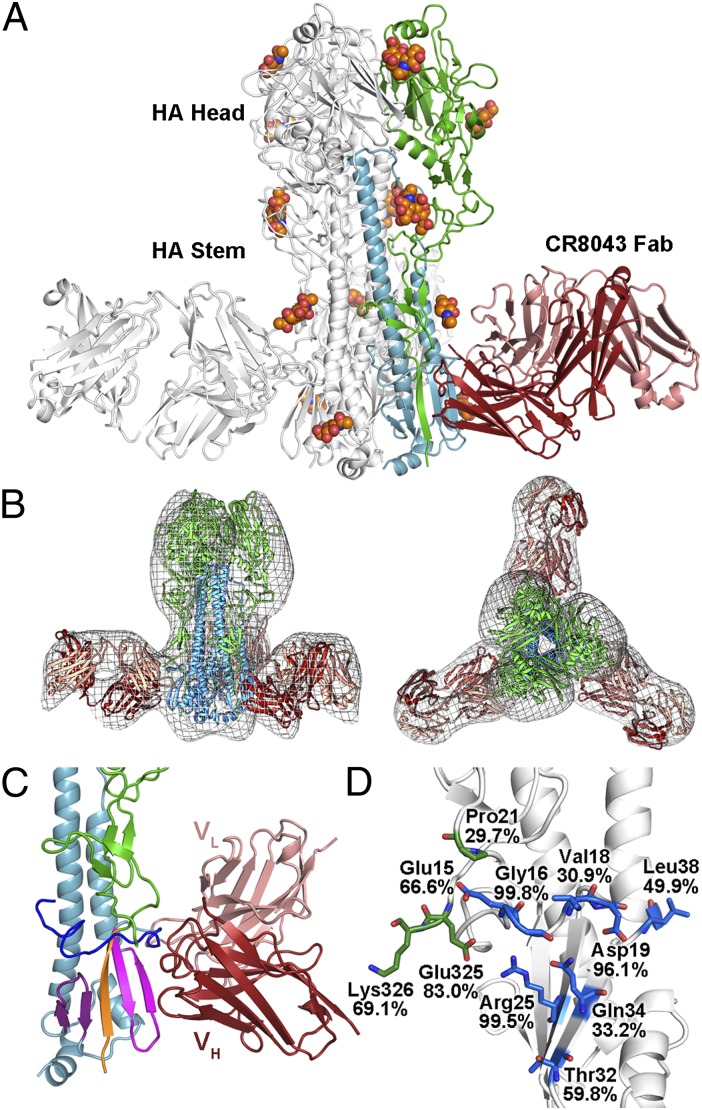
To investigate the structural basis of antibody recognition, the crystal structure of CR8043 Fab in complex with the H3N2 A/Hong Kong/1/1968 (HK68/H3) HA was determined at 4 Å resolution (SI Appendix, Table S2). Initial phases were obtained from the high-resolution starting models of HK68/H3 HA at 1.90 Å resolution (10) and CR8043 Fab, which was determined at 2.65 Å resolution (SI Appendix, Table S2). The asymmetric unit consists of an HA trimer liganded by three Fabs, which bind near the bottom of the HA stem (Fig. 3A). Furthermore, negative stain EM reconstructions of the Fab in complex with HK68/H3 HA at ∼21 Å (Fig. 3B) as well as A/Bangkok/1/1979 (H3N2) HA at ∼23 Å (SI Appendix, Fig. S2) also showed that CR8043 binds at the base of the HA stem. These findings are consistent with the observation that CR8043 does not inhibit agglutination of red blood cells by the viruses that it neutralizes (SI Appendix, Table S3), and that CR8043 competes with CR8020 for binding to H3 HA but not with the anti-head mAb CR8057 (SI Appendix, Fig. S3). CR8043 targets a highly conserved epitope in the HA stem that consists of the fusion peptide and the β-sheet of HA2 preceding the A helix (Fig. 3 C and D and SI Appendix, Table S4). On average, a total of 1,056 Å2 is buried at the interface (506 Å2 on HA and 550 Å2 on the Fab), where the heavy and light chains contribute to 74% and 26% of the Fab buried surface area, respectively. Despite the moderate resolution, the main chains for each component were clearly observed and could be traced in the electron density (SI Appendix, Fig. S4).
Fig. 3.
CR8043 binds an epitope in the HA stem close to the virus membrane. (A) Crystal structure of CR8043 Fab in complex with HK68/H3 HA. One Fab-HA protomer of the trimeric complex is colored with HA1 in green, HA2 in blue, the Fab heavy chain in red, and the Fab light chain in pink. Glycans are shown as spheres (carbon in orange, oxygen in red, and nitrogen in blue). (B, Left) Side and (B, Right) top views of negative stain EM reconstructions (gray mesh) of CR8043 Fab in complex with HK68/H3 HA with the crystal structure of the complex docked into the EM density. (C) Zoomed-in view of the interaction between CR8043 and HK68/H3 HA. The coloring is similar to A but with the fusion peptide in blue and the three segments of the β-sheet colored purple, orange, and pink (they derive from the C terminus of HA2, the N terminus of HA1, and the N terminus of HA2, respectively). (D) Sequence conservation of the CR8043 epitope across all group 2 HAs. Epitope residues are shown as sticks [carbon in green (HA1) or blue (HA2), oxygen in red, and nitrogen in dark blue], and the weighted percent identity of each residue with the group 2 consensus sequence is indicated, which is not always identical to the residue at that position for HK68/H3.
CR8043 Binding Specificities and Structural Comparison with CR8020.
Mutants that escaped from neutralization by CR8043 harbored mutations at the base of the HA stem (Arg25Met and Gln34Arg on HA2) near the central region of the epitope (SI Appendix, Table S5). These mutations, along with the natural variant Gln34Thr, severely weaken the interaction (Fig. 2), and hence, the specific residue at position 34 in HA2 seems to clearly separate binders (Gln34) from nonbinders (Thr34). Accordingly, Thr34 is conserved in >98% of strains from the H4, H7, H14, and H15 subtypes and thus, likely accounts for the reduced efficacy of CR8043 against these subtypes. In addition, although the Thr32Arg mutation found in recent H3N2 strains slightly decreases binding (∼10-fold) by CR8043 Fab (Fig. 2), these viruses are still potently neutralized by CR8043 (Fig. 1B).
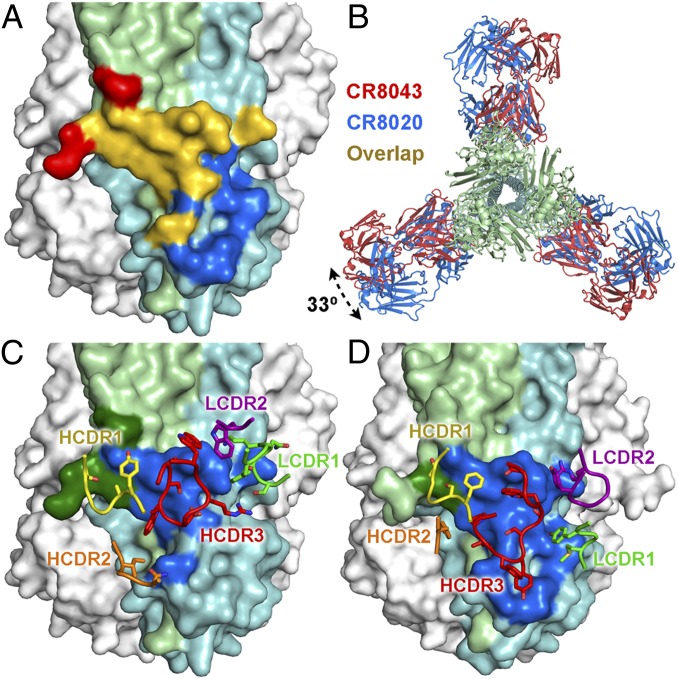
Although the binding sites of CR8043 and CR8020 overlap considerably (Fig. 4A), their sensitivities to epitope mutations are distinct, supporting the notion that the bnAbs have unique modes of binding. For instance, the Arg25Met mutation that escapes CR8043 has no effect on the in vitro neutralizing activity of CR8020, whereas the Gln34Arg mutation allows viruses to escape from both bnAbs (SI Appendix, Table S5). In addition, viruses containing the mutations Asp19Asn or Gly33Glu are not neutralized by CR8020 but are still neutralized by CR8043, albeit somewhat less potently (SI Appendix, Table S5).
Fig. 4.
Comparison of CR8043 and CR8020 contacts on HA. (A) CR8043 (red and yellow) and CR8020 (blue and yellow) footprints mapped onto the HA surface, with HA1 colored in light green and HA2 colored in light blue. The yellow areas indicate the overlap in the epitopes. (B) Top view overlay of the crystal structures of the CR8043 (red) and CR8020 (blue; Protein Data Bank ID code 3SDY) Fabs in complex with HK68/H3 showing the different angle of approach to the HA. The interactions of (C) CR8043 or (D) CR8020 CDRs with HA. The antibody footprints are illustrated in dark green (HA1) and dark blue (HA2), and the contacting CDRs are shown as sticks.
The differing sensitivity of CR8043 and CR8020 to epitope mutations can be explained, in part, by a 33° rotation in the antibody approach angles to the HA surface (Fig. 4B) and may also be a consequence of a six-residue insertion in light chain complementarity-determining region 1 (LCDR1) in CR8043, which is derived from the VK4–01 germ-line gene (vs. VK3–20 for CR8020). Furthermore, CR8043 and CR8020 are derived from the VH1–3 and VH1–18 germ lines, respectively, and subsequently, they have distinct paratopes (Fig. 4 C and D). However, some structural similarities can be observed between CR8043 and CR8020, such as close overlap and identity of the contact residues of their heavy chain complementarity determining region 1 (HCDR1) loops (SI Appendix, Fig. S5A), as well as overlap of HCDR3 residues TrpH98 of CR8043 with the hydrophobic ProH97 and LeuH98 of CR8020 (SI Appendix, Fig. S5B). In addition, LCDR1 TyrL32 of CR8043 has a similar aromatic interaction with the HA as LCDR2 TyrL49 of CR8020 (SI Appendix, Fig. S5C).
CR8043 Prevents HA Conformational Changes and HA Maturation.
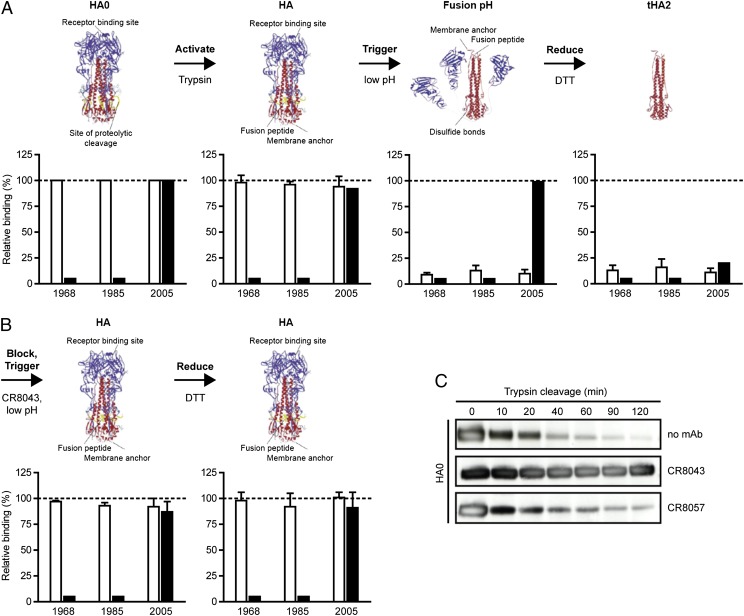
Because CR8043 and CR8020 target a similar epitope, we hypothesized that CR8043 has a similar mechanism of action as CR8020 and stabilizes the pre-fusion HA conformation. We analyzed binding of CR8043 to different HA forms and assessed if CR8043 could prevent the major conformational rearrangements of the fusion machinery that are triggered at low pH. CR8043 bound to both the uncleaved precursor (HA0) and mature (HA) forms of the surface-expressed H3 HAs of A/Hong Kong/1/1968, A/Hong Kong/24/1985, and A/Wisconsin/67/2005 (Fig. 5A). However, the antibody did not bind to the low pH, post-fusion conformation (Fig. 5A), indicating that CR8043 recognizes a native pre-fusion epitope and not the rearranged conformation that arises during the fusion process. In contrast, binding of CR8057 to the HA head of the 2005 H3 strain was only lost after DTT-mediated dissociation of HA1 from HA2 (Fig. 5A). When CR8043 was bound to mature HA before low pH treatment, the antibody remained bound after exposure to low pH (Fig. 5B), indicating that CR8043 inhibits the pH-dependent HA rearrangements that trigger fusion. In addition, pre-binding of CR8043 prevents reduction of the HA and the subsequent dissociation of HA1 from HA2, because CR8057 binding was preserved after DTT treatment (Fig. 5B).
Fig. 5.
CR8043 inhibits the fusogenic conformational changes in HA and blocks proteolytic activation of HA0. (A) FACS binding of CR8043 (open bars) and the head binding control mAb CR8057 (solid bars) to various conformations of surface-expressed H3 HAs of A/Hong Kong/1/1968, A/Hong Kong/24/1985, or A/Wisconsin/67/2005. The various conformations are indicated above the corresponding graphs and were as follows: uncleaved precursor (HA0); neutral pH, cleaved (HA); fusion pH, cleaved (pH 4.9); and trimeric HA2 (tHA2). Binding is expressed as the percentage of binding to untreated HA (HA0). Data represent mean + SD of three independent experiments. Ribbon diagrams are modified from ref. 38. (B) FACS binding of CR8043 (open bars) and CR8057 (solid bars) to surface-expressed H3 HAs as above, except that mAb CR8043 was added before exposure of the cleaved HAs to pH 4.9. (C) Immunoblot of trypsin digestion over time of uncleaved (HA0), soluble H3 HA, which was pretreated without mAb, with CR8043, or with CR8057 and detected using a polyclonal serum against H3 HA.
In addition to inhibiting conformational changes in HA that are required for membrane fusion, some influenza antibodies can neutralize infection by preventing cleavage of immature HA0 into HA1 and HA2 (21, 22) Indeed, CR8043, but not CR8057, prevented proteolytic activation of HA0 (Fig. 5C), thereby blocking the progression of influenza infection.
Discussion
The discovery of human bnAbs that recognize nearly invariant epitopes of influenza A viruses has provided new tools and opportunities for the development of therapies or vaccines. Although several heterosubtypic group 1 influenza A anti-stem antibodies have been characterized (16–20, 23, 25) as well as antibodies that cross the groups 1 and 2 barrier (22, 24), heterosubtypic group 2 antibodies have been less common, and only bnAb CR8020 has been structurally characterized to date (21). In this study, we have identified another bnAb, CR8043, which was isolated from the same donor as CR8020 and also has broadly neutralizing activity against group 2 virus subtypes. Surprisingly, the antibodies are derived from different germ lines [CR8043 and CR8020 derive from VH1–3 and VH1–18 germ-line genes, respectively, which are each estimated to be present in 6% of the human antibody repertoire (31)]. However, these antibodies contact similar epitopes at the base of the HA stem. Moreover, they both interfere with virus infectivity by inhibiting HA0 maturation as well as the pH-triggered conformational rearrangements in HA that are required for membrane fusion.
CR8043 protects mice against lethal challenge with H3 and H7 viruses but does not neutralize H7 viruses in vitro. This paradoxical effect has also been observed with bnAb CR9114, which protects mice against lethal challenge with influenza B viruses, despite a lack of in vitro neutralizing activity against this genus (24). Presumably, in both cases, antibody effector functions mediated by the antibody Fc domain, such as antibody-dependent cellular cytotoxicity for bnAb FI6 (22) or complement-dependent cytotoxicity, contribute to protection (32).
The results presented here show that CR8043 uses a unique paratope and angle of approach to target a highly conserved epitope on the stem of group 2 influenza HAs that is overlapping but not identical to the CR8020 epitope. A similar trend in HA recognition by heterosubtypic group 1 bnAbs, which bind to a highly conserved epitope higher up on the HA stem (18, 19), is also developing. Although several of these group 1 bnAbs initially came from the VH1–69 family, human and mouse antibodies from other germ-line families also target a similar but not identical stem epitope (22, 25). Comparisons of their crystal structures have identified some similarities in their binding interactions, such as conservation in aromatic interactions, despite using different CDR loops and completely different angles of approach (25). In addition, access to a conserved epitope using different modes of binding and varied angles of approach is an emerging theme for glycan-dependent antibody recognition of HIV-1 Env (33) and has defined a supersite of vulnerability on HIV-1. This concept is emulated here for influenza virus, where stem antibodies approach the epitope in different ways and use distinct interactions but have functionally similar modes of neutralization, as also observed for bnAbs to the HA receptor binding site (9–11, 13–15).
Group 2-specific bnAbs may be therefore more robustly elicited than previously assumed (34), and it remains to be seen if additional group 2 bnAbs target the same epitope (35). As such, these findings stress the importance of the CR8043/CR8020 epitope for neutralization of group 2 influenza A viruses and as an emerging target to guide the development of broader spectrum influenza therapies and vaccines. Although the epitopes of both antibodies are largely overlapping, from the vaccine design perspective, the CR8043 epitope may be more attractive than the CR8020 epitope, because it is mostly contained within a linear stretch of HA2 amino acids 15–38. It may be possible to mimic the optimal conformation for presentation by a protein scaffold, such as with the recent, successful grafting of the CD4 binding site from gp120 onto an unrelated protein scaffold (36, 37). Thus, CR8043 sheds light on the potential rational design of broader spectrum influenza vaccines.
Materials and Methods
Influenza A group 2 neutralizing antibodies were isolated from healthy donors 7 d after vaccination with a seasonal influenza vaccine. B-cell receptor-positive memory B cells were immortalized and selected for binding to recombinant Allophycocyanin-labeled H3 HA. B-cell supernatants were then tested for HA binding by ELISA and in vitro neutralizing activity against H3N2 by VNA. H3N2-neutralizing antibodies were subsequently reformatted into human IgG1 and evaluated for binding against H1, H3, and H7 HA by ELISA and FACS as well as in vitro neutralizing activity against several influenza A viruses by VNA. Prophylactic efficacy studies of CR8043 in female SPF 129 X1/SvJ or BALB/c mice were performed by i.v. injecting varying concentrations of CR8043 1 d before intranasal lethal challenge with H3N2 or H7N7 viruses, respectively. Animals were monitored for survival and weighed daily. Recombinant CR8043 Fab and HA were generated in baculovirus for binding studies and X-ray and EM structural studies. Kd values were determined by biolayer interferometry using an Octet RED instrument (ForteBio) by immobilizing biotinylated HA on streptavidin biosensors and then dipping into varying concentrations of CR8043 Fab. CR8043 Fab was added to HK68/H3 in a 3.2:1 molar excess to achieve three Fabs per trimer, and the complex was purified from unbound Fab by gel filtration. Crystals were grown by sitting drop vapor diffusion, and crystals were cryoprotected and flash cooled in liquid nitrogen. Diffraction data were collected at the Stanford Synchrotron Radiation Lightsource (SSRL), and the structures were determined by molecular replacement. Reconstructions of CR8043-HA complexes were determined by negative stain EM. Additional detailed information is described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ozcan Sahin, Freek Cox, and the Protein Sciences Team of the Crucell Vaccine Institute for excellent assistance; Lisette Cornelissen for assistance with the animal experiments; Leo Poon and Malik Peiris for the generation of escape mutants; Miriam Bujny, David Zuijdgeest, Gerrit Jan Weverling, Robyn Stanfield, Jean-Philippe Julien, and Leopold Kong for fruitful discussions; and Henry Tien of the Robotics Core at the Joint Center for Structural Genomics for automated crystal screening. We also thank the staff at beamlines 11-1 and 12-2 at SSRL for beamline support. SSRL is a Directorate of Stanford Linear Accelerator Center National Accelerator Laboratory and an Office of Science User Facility operated for the US Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the Department of Energy Office of Biological and Environmental Research; National Institutes of Health, National Center for Research Resources, Biomedical Technology Program Grant P41RR001209; and the National Institute of General Medical Sciences. This work has been funded in part by Crucell Holland B.V.; National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services Contract HHSN272200900060C; National Institutes of Health Molecular Evolution Training Program Grant GM080209 (to P.S.L.); and National Institutes of Health Grant R56 AI099275 (to I.A.W.). The EM data were collected at the National Resource for Automated Molecular Microscopy at The Scripps Research Institute, which is supported by the National Institutes of Health through P41 Program Grant RR017573 at the National Center for Research Resources. This work is The Scripps Research Institute manuscript number 24039.
Footnotes
Conflict of interest statement: R.H.E.F., E.J.M.S., J.J., W.K., M.J., H.J.W.M.K., C.O., E.C.M.B.-v.d.L., T.K., R.V., and J.G. are employees of Crucell Holland B.V.. M.J.K., A.Q.B., T.B., and H.S. are employees of AIMM Therapeutics.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structures have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4NM4 and 4NM8). The reconstruction data reported have been deposited in the Electron Microscopy Data Bank, www.emdatabank.org (codes 5793 and 5794).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319058110/-/DCSupplemental.
References
- 1.World Health Organization (WHO) Fact Sheet 211: Influenza. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Potter CW. A history of influenza. J Appl Microbiol. 2001;91(4):572–579. doi: 10.1046/j.1365-2672.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson I, et al. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin Infect Dis. 2009;48(4):389–396. doi: 10.1086/596311. [DOI] [PubMed] [Google Scholar]
- 4.Carrat F, Flahault A. Influenza vaccine: The challenge of antigenic drift. Vaccine. 2007;25(39–40):6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69(4):2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- 8.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 9.Whittle JR, et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci USA. 2011;108(34):14216–14221. doi: 10.1073/pnas.1111497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekiert DC, et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489(7417):526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee PS, et al. Heterosubtypic antibody recognition of the influenza virus hemagglutinin receptor binding site enhanced by avidity. Proc Natl Acad Sci USA. 2012;109(42):17040–17045. doi: 10.1073/pnas.1212371109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsibane T, et al. Influenza human monoclonal antibody 1F1 interacts with three major antigenic sites and residues mediating human receptor specificity in H1N1 viruses. PLoS Pathog. 2012;8(12):e1003067. doi: 10.1371/journal.ppat.1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu R, et al. A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nat Struct Mol Biol. 2013;20(3):363–370. doi: 10.1038/nsmb.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt AG, et al. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc Natl Acad Sci USA. 2013;110(1):264–269. doi: 10.1073/pnas.1218256109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong M, et al. Antibody recognition of the pandemic H1N1 influenza virus hemagglutinin receptor binding site. J Virol. 2013;87(22):12471–12480. doi: 10.1128/JVI.01388-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap AK, et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci USA. 2008;105(16):5986–5991. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Throsby M, et al. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3(12):e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sui J, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekiert DC, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120(5):1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekiert DC, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333(6044):843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corti D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 23.De Marco D, et al. A non-VH1-69 heterosubtypic neutralizing human monoclonal antibody protects mice against H1N1 and H5N1 viruses. PLoS One. 2012;7(4):e34415. doi: 10.1371/journal.pone.0034415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreyfus C, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337(6100):1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyfus C, Ekiert DC, Wilson IA. Structure of a classical broadly neutralizing stem antibody in complex with a pandemic H2 influenza virus hemagglutinin. J Virol. 2013;87(12):7149–7154. doi: 10.1128/JVI.02975-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekiert DC, Wilson IA. Broadly neutralizing antibodies against influenza virus and prospects for universal therapies. Curr Opin Virol. 2012;2(2):134–141. doi: 10.1016/j.coviro.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337(6091):183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250(1):180–198. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwakkenbos MJ, et al. Generation of stable monoclonal antibody-producing B cell receptor-positive human memory B cells by genetic programming. Nat Med. 2010;16(1):123–128. doi: 10.1038/nm.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnaout R, et al. High-resolution description of antibody heavy-chain repertoires in humans. PLoS One. 2011;6(8):e22365. doi: 10.1371/journal.pone.0022365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton DR. Antibodies, viruses and vaccines. Nat Rev Immunol. 2002;2(9):706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 33.Kong L, et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol. 2013;20(7):796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margine I, et al. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol. 2013;87(8):4728–4737. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang TT, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6(2):e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azoitei ML, et al. Computation-guided backbone grafting of a discontinuous motif onto a protein scaffold. Science. 2011;334(6054):373–376. doi: 10.1126/science.1209368. [DOI] [PubMed] [Google Scholar]
- 37.Azoitei ML, et al. Computational design of high-affinity epitope scaffolds by backbone grafting of a linear epitope. J Mol Biol. 2012;415(1):175–192. doi: 10.1016/j.jmb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skehel JJ, Wiley DC. Influenza haemagglutinin. Vaccine. 2002;20(Suppl 2):S51–S54. doi: 10.1016/s0264-410x(02)00131-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.