Significance
The quest for an optimal xylose pathway in yeast is of utmost importance along the way to realizing the potential of lignocellulosic biomass conversion into fuels and chemicals. An often-overlooked aspect of this catabolic pathway is the molecular transporter of this sugar. Here we demonstrate that sugar transport preference and kinetics can be rewired through the programming of a specific sequence motif. The result is a study to rationally alter the sugar preference of a protein through defined sequence-level modifications. In these cases, primary hexose transporters were rewired into xylose transporters.
Keywords: metabolic engineering, xylose metabolism, protein engineering, transporter engineering
Abstract
Utilization of exogenous sugars found in lignocellulosic biomass hydrolysates, such as xylose, must be improved before yeast can serve as an efficient biofuel and biochemical production platform. In particular, the first step in this process, the molecular transport of xylose into the cell, can serve as a significant flux bottleneck and is highly inhibited by other sugars. Here we demonstrate that sugar transport preference and kinetics can be rewired through the programming of a sequence motif of the general form G-G/F-XXX-G found in the first transmembrane span. By evaluating 46 different heterologously expressed transporters, we find that this motif is conserved among functional transporters and highly enriched in transporters that confer growth on xylose. Through saturation mutagenesis and subsequent rational mutagenesis, four transporter mutants unable to confer growth on glucose but able to sustain growth on xylose were engineered. Specifically, Candida intermedia gxs1 Phe38Ile39Met40, Scheffersomyces stipitis rgt2 Phe38 and Met40, and Saccharomyces cerevisiae hxt7 Ile39Met40Met340 all exhibit this phenotype. In these cases, primary hexose transporters were rewired into xylose transporters. These xylose transporters nevertheless remained inhibited by glucose. Furthermore, in the course of identifying this motif, novel wild-type transporters with superior monosaccharide growth profiles were discovered, namely S. stipitis RGT2 and Debaryomyces hansenii 2D01474. These findings build toward the engineering of efficient pentose utilization in yeast and provide a blueprint for reprogramming transporter properties.
Molecular transporter proteins facilitate monosaccharide uptake and serve as the first step in catabolic metabolism. In this capacity, the preferences, regulation, and kinetics of these transporters ultimately dictate total carbon flux (1–3); and optimization of intracellular catabolic pathways only increases the degree to which transport exerts control over metabolic flux (4, 5). Thus, monosaccharide transport profiles and rates are important design criteria and a driving force to enable metabolic engineering advances, ultimately resulting in a biorefinery concept whereby biomass is converted via microbes into a diverse set of molecules (6–10). Among possible host organisms, Saccharomyces cerevisiae is an emerging industrial organism with well-developed genetic tools and established industrial processes and track record (11–16). However, S. cerevisiae lacks an endogenous xylose catabolic pathway and thus is unable to natively use the second most abundant sugar in lignocellulosic biomass. Decades of research have been focused on improving xylose catabolic pathways in recombinant S. cerevisiae (17–22), but less work has been focused on the first committed step of the process—xylose transport, an outstanding limitation in the efficient conversion of lignocellulosic sugars (23, 24).
In S. cerevisiae, monosaccharide uptake is mediated by transporters belonging to the major facilitator superfamily (MFS) (25, 26), a ubiquitous group of proteins found across species (27). The predominant transporters in yeast are members of the HXT family (28) and are marked by efficient hexose transport (29) with lower affinities to xylose thus contributing to diauxic growth and flux limitation when attempting pentose utilization in recombinant S. cerevisiae (30). Previous efforts have attempted to identify heterologous transporters with a higher affinity for xylose over glucose (31–36). However, the vast majority of these transporters are either nonfunctional, not efficient, or not xylose specific (24, 37). Furthermore, nearly all known wild-type transporters that enable growth on xylose in yeast confer higher growth rates on glucose than on xylose (24, 37). As an alternative to bioprospecting, we have previously reported that xylose affinity and exponential growth rates on xylose can be improved via directed evolution of Candida intermedia glucose-xylose symporter 1 (GXS1) and Scheffersomyces stipitis xylose uptake 3 (XUT3) (38). These results demonstrated that mutations at specific residues (e.g., Phe40 in C. intermedia GXS1) can have a significant impact on the carbohydrate selectivity of these MFS transporters. The fact that single amino acid substitutions can have such a significant impact on transport phenotype (38–40) indicates how simple homology based searches can be ineffective at identifying efficient xylose transporters (35, 36). However, evidence of natural xylose exclusivity is seen in the Escherichia coli xylE transporter that has recently been crystallized (41). The sequence-function flexibility of MFS transporters potentiates the capability to rewire hexose transporters from being glucose favoring, xylose permissive into being xylose-exclusive transporters.
In this work, we report on the discovery of a conserved Gly36-Gly37-Val38-Leu39-Phe40-Gly41 motif surrounding the previously identified Phe40 residue of C. intermedia GXS1 that controls transporter efficiency and selectivity. By evaluating 46 different heterologously expressed transporters, we find that this motif is conserved among functional transporters and highly enriched in transporters that confer growth on xylose, taking the general form G-G/F-XXX-G. We conduct saturation mutagenesis on Val38, Leu39, and Phe40 within the variable region of this motif in C. intermedia GXS1 to demonstrate control of sugar selectivity. Next, we combine xylose-favoring mutations to create a unique mutant version of gxs1 that transports xylose, but not glucose. Finally, we demonstrate the importance of this motif in the capacity to rewire the sugar preference of other hexose transporters including S. cerevisiae hexose transporter 7 (HXT7) and S. stipitis glucose transporter/sensor (RGT2, similar to S. cerevisiae RGT2). This work serves to increase our understanding of the structure–function relationships for molecular transporter engineering and demonstrates complete rewiring of hexose transporters into transporters that prefer xylose as a substrate.
Results
Identification of the G-G/F-XXX-G Motif That Controls Sugar Transport Preference.
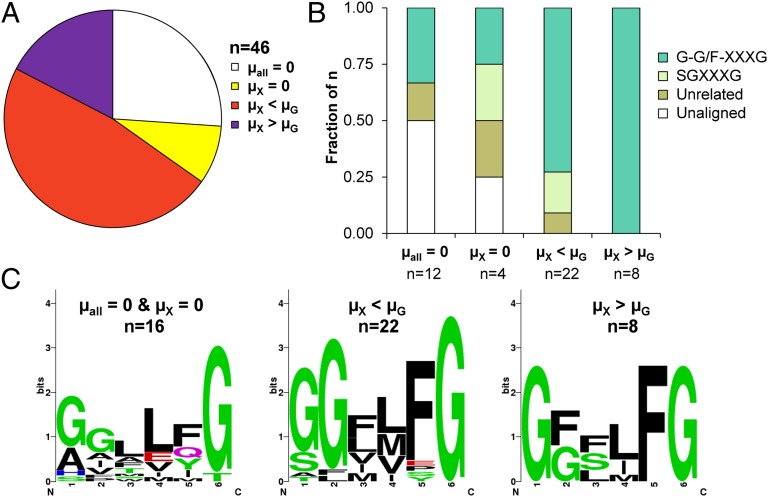
Previously, we reported on single amino acid substitutions at Phe40 in transmembrane span 1 (TMS1) that can alter monosaccharide transport profiles of C. intermedia GXS1 (38). The potency of this residue as well as its proximity to the outer pore of the transporter suggested it could be part of an important contact and recognition site for monosaccharides. A multiple sequence alignment of 26 previously cloned transporters (36) indicates that Phe40 was indeed part of a highly conserved glycine-rich motif of the form G-G/F-XXX-G, where X represents a variable, but usually nonpolar amino acid residue. In C. intermedia GXS1, the wild-type motif is Gly36Gly37Val38Leu39Phe40Gly41. The high conservation of this motif suggested it could be responsible for xylose uptake, transporter efficiency, and monosaccharide selectivity. To further corroborate this hypothesis, an additional 20 putative transporters were identified using a BLAST search seeded with transporters identified in our prior study and functionally characterized in S. cerevisiae EX.12, a recombinant strain lacking endogenous monosaccharide transporters (SI Appendix, Fig. S1 and Table S1) (26, 38). The vast majority of these transporters were functional and all possessed a similar motif. Among these transporters, Debaryomyces hansenii 2D01474 confers much faster growth on xylose than on glucose and S. stipitis RGT2 confers the fastest growth on xylose of all of the S. stipitis-derived transporters in this study.
Following the functional characterization, we correlated motif sequence and the resulting transporter carbon source profile. Four major phenotypic classifications were made: (i) transporters that failed to function heterologously (µall = 0), (ii) transporters that conferred growth on a hexose but not xylose (µX = 0), (iii) transporters that conferred growth on xylose but not as fast as glucose (µX < µG), and (iv) transporters that conferred a higher growth rate on xylose than on glucose (µX > µG). Fig. 1A displays the relative proportions of each of these classifications in the group of 46 transporters studied. To characterize the sequence, four major motif classifications were made: (i) a full G-G/F-XXXG motif, (ii) a related S-G-XXXG motif, (iii) a motif unrelated to the glycine-rich motif, and (iv) the lack of homology to other transporters at both the motif and surrounding residues. Fig. 1B depicts the distribution of the four sequence motif classifications within the four phenotypic classifications. Strikingly, there is a clear enrichment of the G-G/F-XXXG motif among the functional transporters that enable high xylose transport rates. In fact, this motif is exclusively seen in phenotype class iv where µX > µG. The enrichment and convergence of the variable residues within the motif is displayed in Fig. 1C. It should be noted that the consensus sequence from this analysis appears to be G-G/F-XX-F-G. However, variations at the consensus F residue led to the discovery of the motif, therefore this position was considered variable. Fig. 1C highlights the strong correlation between sequence motif and xylose transport function and suggests an important role of TMS1 on sugar recognition.
Fig. 1.
Sequence categorization and phenotypic classification of native and heterologous transporters. (A) The distribution of phenotypic classes for all 46 transporters. (B) The distribution of each sequence category present in each phenotypic class. Transporters containing the conserved motif are enriched in the phenotypic classes that confer growth on xylose. (C) Weblogos of the phenotypic classes illustrate enrichment of the G-G/F-XXXG motif in TMS1. µall = 0 represents no growth in the five carbon sources tested; µX = 0 represents growth on hexoses but not xylose, µX < µG represents growth on xylose is less than that on glucose, and µX > µG represents growth on xylose is greater than that on glucose.
Identification of Potentiating Variable Residues Within the G-G/F-X-X-X-G Motif.
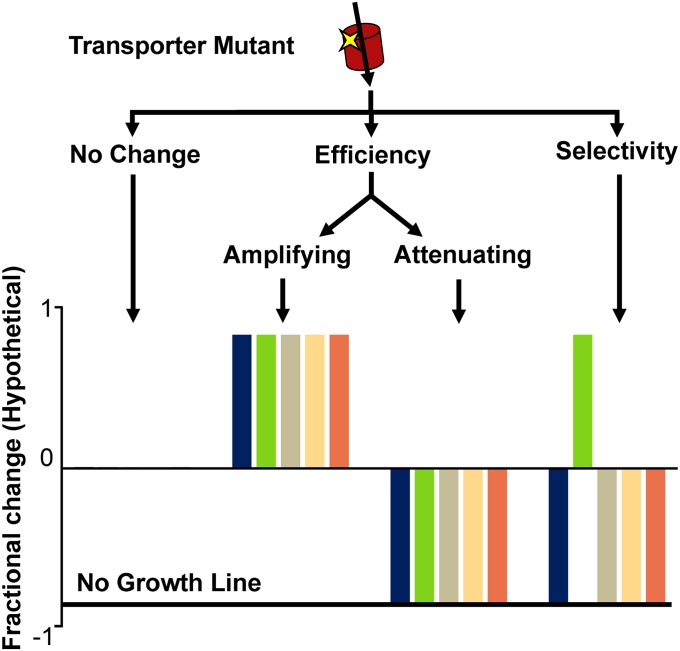
To examine the role of the variable region, we performed complete saturation mutagenesis for each of the three residues (Val38, Leu39, and Phe40) in C. intermedia GXS1 and evaluated the impact on carbon source growth profile as measured by growth rate. Our previous studies demonstrate that growth rate in this test strain is a good surrogate for transporter kinetics (36, 38). Specifically, the fractional change in the growth rate of S. cerevisiae EX.12 on glucose, xylose, galactose, fructose, or mannose as the sole carbon source was evaluated compared with the wild-type transporter. The impact of each residue can be classified as having no change, altered efficiency, altered selectivity, or a combination of the three (Fig. 2). For creating xylose-specific transporters, the goal is to identify mutations that attenuate hexose growth while either amplifying or maintaining xylose growth.
Fig. 2.
Classification tree of fractional change in carbon source growth profile. This figure depicts hypothetical fractional change data to demonstrate how these phenotypes were classified. Little fractional change across all sugars indicates that the substitution does not control efficiency or selectivity in this background. Amplification or attenuation of growth rates across all carbon sources indicates an efficiency substitution. Amplification of growth on one sugar, ideally xylose, and attenuation of all others indicates a selectivity substitution.
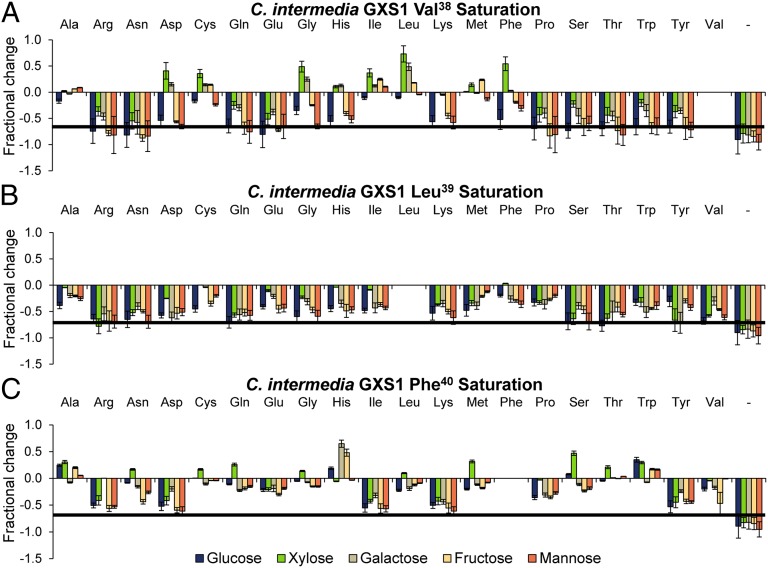
Members of the C. intermedia gxs1 Val38 saturation library (Fig. 3A) display differential exponential growth rates with the most significant one being the Phe38 substitution. This mutant confers a selectivity phenotype that almost completely attenuates glucose exponential growth rate while amplifying exponential xylose growth rate by 50%. Other substitutions that confer desirable selectivity phenotypes are Asp38, Cys38, Gly38, and His38. All of these affect the growth profile in different patterns, but none as significantly as Phe38. Three substitutions, Ile38, Leu38, and Met38, differentially amplify growth on multiple sugars whereas glucose growth remains unchanged. The Leu38 substitution in particular increases the exponential xylose growth rate by 73% without altering glucose exponential growth rate significantly. Ala38 attenuates growth on glucose only. Nearly all of the remaining substitutions attenuate growth, yet many preferentially attenuate growth on hexoses. In this subset, Lys38 severely attenuates growth on glucose, fructose, and mannose without affecting the growth rate on xylose. The high frequency of selectivity and differentially attenuating phenotypes arising at this residue indicates that position 38 predominately influences monosaccharide selectivity.
Fig. 3.
Fractional change of saturation mutagenesis libraries of C. intermedia GXS1. Fractional change in growth by substitutions at positions 38 (A), 39 (B), and 40 (C). The solid line is the confidence line for no growth based on the negative control sample. (A–C) Error bars were calculated using the sum of least squares method.
Nearly all members of the Leu39 saturation library (Fig. 3B) display uniform attenuation patterns across sugars. Thus, it is clear that residue 39 greatly controls transporter efficiency. Nevertheless, several of these substitutions differentially attenuate growth. Specifically, Asp39, Cys39, Gly39, His39, Ile39, and Phe39 reduce exponential growth on hexoses without drastically altering xylose growth rate. Of these, His39 and Ile39 establish the greatest difference between the hexose and pentose growth rates.
Members of the Phe40 library (Fig. 3C) display differential carbon source selectivity similar to Val38 and have the greatest frequency of selectivity substitutions. Specifically, amino acid substitutions that confer a selectivity phenotype for xylose over glucose are Asn40, Cys40, Gly40, Leu40, Met40, Ser40, and Thr40. Of these, Ser40 and Met40 are the most significant. There are several attenuating substitutions that can be seen at residue 40 including Arg40, Asp40, Glu40, Ile40, Lys40, Pro40, and Tyr40. Of these, Pro40 is the only one that does not attenuate growth on xylose. Finally, Ala40, His40, and Trp40 conferred increased growth on most of the monosaccharides tested. In summary, residues 38 and 40 appear to play a significant role in transporter selectivity whereas residue 39 appears essential for controlling net transporter efficiency. In general, hydrophobic residues of moderate to large size were beneficial for xylose growth, whereas charged residues were not (also seen with the evaluated transporters in Fig. 1C). These motif design rules may be used to reprogram transporter function.
Rewiring C. intermedia GXS1 into a Xylose-Specific Transporter.
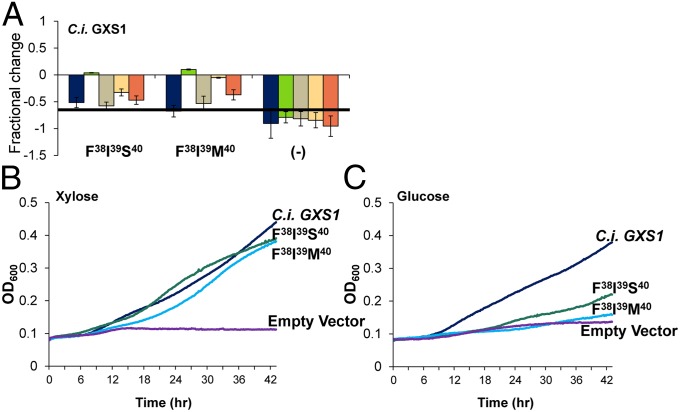
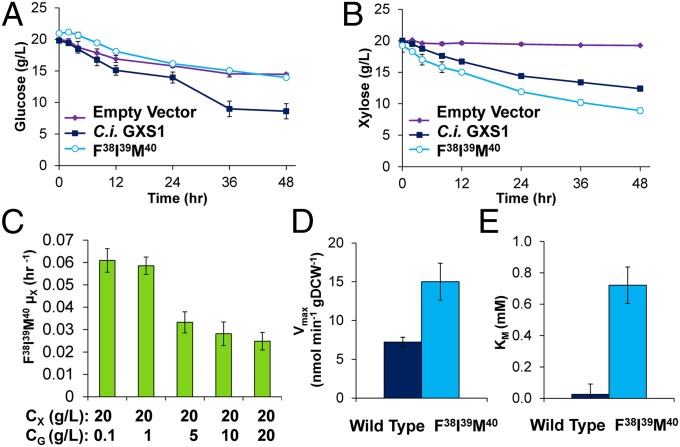
Using the design rules discovered above, triple mutants were constructed to investigate the synergy between xylose favoring substitutions (in particular Phe38, Ile39, and Ser40/Met40). Both Phe38Ile39Ser40 and Phe38Ile39Met40 attenuate glucose exponential growth while maintaining or slightly increasing xylose exponential growth (Fig. 4A), with the Phe38 Ile39 Met40 triple mutant attenuating glucose growth to the same level as the negative control. Average growth curves on xylose and glucose (Fig. 4 B and C) highlight that both triple mutants maintain wild-type xylose growth profile while severely attenuate glucose growth. We next performed further characterization of the best mutant, gxs1 Phe38Ile39Met40. First, to assay transport capacity, high cell density fermentations with xylose and glucose were performed (Fig. 5 A and B). The Phe38 Ile39 Met40 triple mutant displayed no appreciable glucose uptake whereas xylose uptake has become more efficient compared with the wild-type GXS1. These results display a rewiring of the sugar uptake ratio. However, despite eradicating glucose transport capacity, glucose at levels of 5 g/L still inhibit xylose growth (Fig. 5C). This finding is corroborated by high cell density cofermentations (SI Appendix, Figs. S2 and S3).
Fig. 4.
Growth characterization of C. intermedia gxs1 triple mutants. (A) Fractional change from wild type for the two triple mutants and an empty vector control. A bar chart of the exponential growth rates from which fractional change was calculated can be found in SI Appendix. Error bars were calculated using the sum of least squares method. (B) Average growth curves on xylose based on OD600. (C) Average growth curves on glucose based on OD600. Calculated exponential growth rates are shown in SI Appendix, Fig. S7.
Fig. 5.
Further characterization of C. intermedia gxs1 Phe38Ile39Met40 triple mutant. (A) Glucose uptake at high cell density for S. cerevisiae EX.12 expressing wild type, Phe38Ile39Met40, and empty vector. (B) Xylose uptake at high cell density for S. cerevisiae EX.12 expressing wild type, Phe38Ile39Met40, and empty vector. (C) Inhibition of the growth rate on xylose with increasing glucose concentration. (D) Vmax of both the wild type and the mutant. (E) KM of both the wild type and triple mutant. (A–E) Error is based on SD of biological replicates.
Second, radiolabeled xylose uptake experiments were performed to quantify the improvement of transport kinetics in the Phe38Ile39Met40 triple mutant. The improvements in xylose utilization observed at high cell density culturing were mainly due to a doubling in Vmax (Fig. 5D). An increased KM was observed as well (Fig. 5E), a phenotype observed in previous efforts to engineer this transporter (38). Nevertheless, the binding affinity is still quite high for practical culturing at a value corresponding to around 0.1 g/L (SI Appendix, Table S2). These kinetics experiments were also performed in the presence of glucose and no radiolabeled xylose uptake was detected indicating that although glucose cannot pass through the transporter, it can still bind and inhibit xylose uptake. Hence, binding must occur at a different residue.
The G-G/F-XXXG Motif Can Be Used to Rewire Other Transporters.
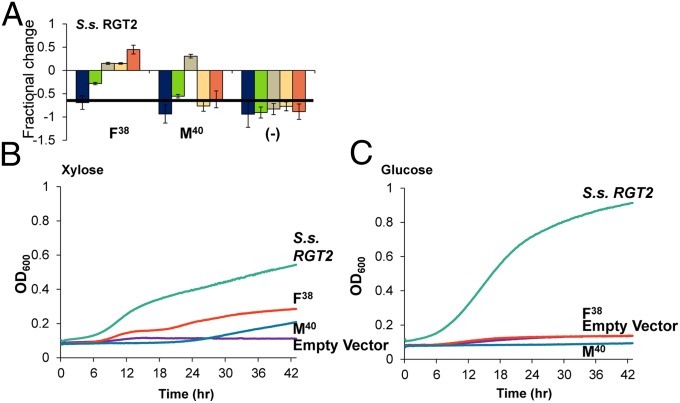
Finally, we used the conserved G-G/F-XXXG motif to rewire the sugar preference of other predominately hexose transporters. Specifically, two transporters, S. stipitis RGT2 and S. cerevisiae HXT7, were selected based on evolutionary distance from GXS1. S. stipitis RGT2 is closely related to C. intermedia GXS1, whereas the native HXT transporters are more distant (SI Appendix, Figs. S4 and S5). First, we investigated the impact of rewiring the closely related transporter, S. stipitis RGT2. This transporter contains a G36G37I38L39F40G41 motif and we characterized two separate point mutations, Phe38 and Met40. In both cases, glucose growth has been completely attenuated (Fig. 6). Most striking is the Met40 mutation, which eliminates growth on all carbon sources but xylose and galactose. By modifying the motif in RGT2, we generated two additional mutant proteins that transport xylose but not glucose.
Fig. 6.
Growth characterization of S. stipitis RGT2 and mutants. (A) Fractional change from wild type for the two single mutants and an empty vector control. Error bars were calculated using the sum of least squares method. A bar chart of the exponential growth rates from which fractional change was calculated can be found in SI Appendix. (B) Average growth curves on xylose based on OD600. (C) Average growth curves on glucose based on OD600. Calculated maximum exponential growth rates are shown in SI Appendix, Fig. S7.
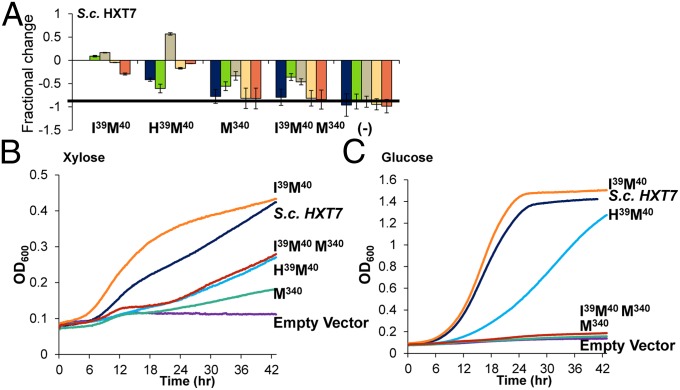
Second, we evaluated the potential to rewire S. cerevisiae HXT7, a more distantly related protein yet able to efficiently transport hexoses and xylose in yeast (32, 42). Given the proficiency of hexose transport by this protein, rewiring to attenuate growth on hexoses presents a greater challenge. The native motif within S. cerevisiae HXT7 is G36G37F38V39F40G41. We initially evaluated two double mutations to this motif: Ile39Met40 and His39Met40. Fig. 7 demonstrates that the Ile39Met40 double mutant amplified xylose exponential growth and attenuated growth on all hexoses save glucose whereas the His39Met40 double mutant attenuated glucose growth yet severely attenuated xylose exponential growth. Previous studies have indicated that mutations at Asp340 can eliminate glucose transport (39) in HXT7 and we verify here that transport of nearly all monosaccharides is severely attenuated with this mutation (Fig. 7). Coupling the Met340 mutation with the Ile39Met40 double mutant resulted in robust growth on xylose while maintaining the inability to transport glucose. With this triple mutant, a robust hexose transporter was converted to a xylose transporter unable to support growth on glucose.
Fig. 7.
Growth characterization of S. cerevisiae HXT7 and mutants. (A) Fractional change from wild type for the mutants and an empty vector control. Error bars were calculated using the sum of least squares method. A bar chart of the exponential growth rates from which fractional change was calculated can be found in SI Appendix. (B) Average growth curves on xylose based on OD600. (C) Average growth curves on glucose based on OD600. Calculated maximum exponential growth rates are shown in SI Appendix, Fig. S7.
Discussion
We identified a short, six-residue motif of the form G-G/F-XXXG in TMS1 that exerts control over selectivity and efficiency of monosaccharide transport of MFS family transporters. This motif is conserved among functional transporters and highly enriched in transporters that confer growth on xylose. Altering the composition of the variable region changes the sugar uptake profiles of these transporters and can thus be used to rewire transporter function. Altering the residues in this domain can eliminate glucose transport while retaining xylose transport, a major step forward for molecular transporter engineering. In doing so, we create several transporter mutants that support the transport of xylose and not glucose.
Hydrophobic, nonpolar, and moderate-to-large-size residues often attenuated glucose compared with xylose. Amino acids such as Phe, Ile, Ser, and Met were among the most effective substitutions that differentially amplified xylose growth rate. Although many of these residues are found naturally in wild-type motif sequences (Fig. 2), the most effective combinations found here (particularly Phe38Ile39Met40) are not found naturally (SI Appendix, Table S3). Hypotheses concerning transporter substrate recognition and transport mechanism may be formed based on these results. The advantage of large and nonpolar residues suggests that glucose growth attenuation is due to steric exclusion. The larger side chains would physically restrict the size of the pore, allowing the smaller xylose molecule to bind and traverse more efficiently than larger hexoses. A similar hypothesis has been proposed to explain an observed correlation between amino acid size and transporter function for glucose (43). This hypothesis is supported by the crystal structure of a related MFS transporter, E. coli xylE (41). Based on the structure, E. coli xylE Phe24, an analogous residue to C. intermedia gxs1 Phe40, appears to interact with sugars as they pass through the pore. Unfortunately, E. coli xylE is too dissimilar from yeast MFS transporters to enable structure prediction, yet this evidence suggests that this residue appears to play a role in all MFS sugar transporters. Further crystal structures of more closely related transporters will greatly enhance the understanding of the yeast MFS sugar transporter mechanism.
Before this study, prior evidence has pointed to the possibility of xylose exclusivity. Transporters from Neurospora crassa and S. stipitis were found to be exclusive for xylose in uptake assays (35), but are unable to support robust growth of recombinant S. cerevisiae on xylose. The E. coli xylE transporter is xylose specific when expressed in its native host (44), but is inhibited by glucose and remains nonfunctional in S. cerevisiae despite attempts at directed evolution. This work has demonstrated a defined transporter engineering approach that is able to effectively eliminate glucose transport while amplifying xylose transport and supporting robust xylose growth. The mutants generated in this study demonstrate this desirable phenotype and provide evidence that the G-G/F-XXXG motif controls the transport phenotype in a large number of MFS transport proteins.
It is also important to note that altering this motif in C. intermedia GXS1 not only had an impact on glucose uptake, but also had an impact on the kinetics of xylose uptake. Specifically, the Km for xylose was significantly increased compared with wild type, indicating that exclusion of glucose was obtained at the expense of a reduced affinity for xylose. Nevertheless, the affinity for xylose remains sufficiently high for nearly all fermentation conditions (KM = 0.721 ± 0.116 mM, or ∼0.1 g/L), and was partially compensated by a doubling in Vmax (Fig. 5). This result suggests a complex set of interactions between the transporter and sugar substrate, and is similar to other mutants of C. intermedia GXS1 (38).
In the course of identifying and validating this motif, several previously uncharacterized native and heterologous transporters were identified and shown to possess previously unreported phenotypes (SI Appendix, Fig. S6). The transporter D. hansenii 2D01474 can natively support high exponential growth on xylose compared with glucose. The transporter S. stipitis RGT2 confers the fastest growth rate on xylose over any ORF cloned from S. stipitis and thus may be a member of the long sought-after xylose transporter suite in the efficient xylose-fermenting organism S. stipitis. Both of these transporters are closely related to C. intermedia GXS1 (SI Appendix, Fig. S5) and may present a unique class of related transporters that make excellent starting scaffolds for engineering exclusive xylose uptake. Of the remaining ORFs studied here, one group (D. hansenii 2E01166, D. hansenii 2B05060, S. cerevisiae STL1, and S. stipitis AUT1) confers much higher exponential growth rates on galactose than any other sugar tested. This hexose transport profile is indicative of the potential for l-arabinose transport, as the galactose transporter S. cerevisiae GAL2 is one of the few transporters able to facilitate l-arabinose (45). This correlation is likely due to the similar stereochemistry between l-arabinose and galactose.
In summary, this work describes the discovery of a conserved G-G/F-XXXG motif and an engineering approach to modify this motif. This motif allowed for the rewiring of several transporters and yielded the mutant transporters C. intermedia gxs1 Phe38Ile39Met40, S. stipitis rgt2 Phe38 and Met40, and S. cerevisiae hxt7 Ile39Met40Met340 that do not transport glucose yet support S. cerevisiae EX.12 growth on xylose. These MFS transporters are channels and thus a substrate molecule interacts with many residues during transport, yet the few residues in this motif appear to have a great deal of influence over glucose transport attenuation and xylose transport amplification. Thus, this study provides further insight into the residues responsible for monosaccharide transport in MFS proteins while establishing a platform for engineering a specific, efficient xylose transporter.
Materials and Methods
Strains, Media, and Plasmids.
Molecular cloning and standard culturing techniques with E. coli DH10B were performed according to Sambrook (46). S. cerevisiae EX.12 was used for all yeast experiments and was constructed as previously described (38). All transporters were cloned into p414-TEF, a standard yeast shuttle vector created by Mumberg et al. (47). Yeast synthetic complete media was used for culture and experimental growth media. Complete supplemented media without tryptophan was used when S. cerevisiae EX.12 was carrying a transporter. Carbon sources were provided at 20 g/L.
Transporter Cloning.
Potential xylose transporters were identified from the literature and BLAST search. To obtain this list of 46, we combined 26 transporters from our previous survey of transporters (36) along with 20 additional transporters identified through homology search using C. intermedia GXS1 and S. cerevisiae STL1 as a template. Details on cloning and transporter libraries are described in SI Appendix. Primers for cloning, saturation mutagenesis, and point mutations are listed in SI Appendix, Tables S4, S5, and S6, respectively.
Growth Rate Measurements.
All exponential growth rates were measured and calculated according to the method previously described using a Bioscreen C (Growth Curves USA) and a MATLAB script (36, 38). Details of the measurements are provided in SI Appendix.
Fractional Change.
Fractional change in the growth rate from wild type was calculated by taking the difference between the growth rates of the mutant and wild type over the growth rate of the wild type for each individual carbon source. Error was propagated using the least squares method based on the SD in exponential growth rates of the mutant and the wild type.
High Cell Density Fermentation.
High cell density experiments were conducted as previously described (38). Yeast cultures were suspended at OD600 of 20 in 20 g/L glucose, 10 g/L glucose and 10 g/L xylose, or 20 g/L xylose. The supernatant concentration of xylose and/or glucose was measured using a YSI Life Sciences Bioanalyzer 7100MBS.
Radiolabeled Xylose Uptake.
Uptake of 14C-labeled xylose was used to determine the Michaelis–Menten parameters for C. intermedia GXS1 and the Phe38Ile39Met40 triple mutant. The method was performed as previously described (38).
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation (NSF) Grant CBET-1067506 and E. Young is supported by an NSF Graduate Research Fellowship. We acknowledge Dr. Marvin Whiteley for providing laboratory space and equipment for the radiolabeled xylose uptake experiments and thank Dr. Eckhard Boles for providing S. cerevisiae EBY.VW4000.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311970111/-/DCSupplemental.
References
- 1.Reijenga KA, et al. Control of glycolytic dynamics by hexose transport in Saccharomyces cerevisiae. Biophys J. 2001;80(2):626–634. doi: 10.1016/S0006-3495(01)76043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gárdonyi M, Jeppsson M, Lidén G, Gorwa-Grauslund MF, Hahn-Hägerdal B. Control of xylose consumption by xylose transport in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng. 2003;82(7):818–824. doi: 10.1002/bit.10631. [DOI] [PubMed] [Google Scholar]
- 3.Elbing K, et al. Role of hexose transport in control of glycolytic flux in Saccharomyces cerevisiae. Appl Environ Microbiol. 2004;70(9):5323–5330. doi: 10.1128/AEM.70.9.5323-5330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahlbom CF, Cordero Otero RR, van Zyl WH, Hahn-Hägerdal B, Jönsson LJ. Molecular analysis of a Saccharomyces cerevisiae mutant with improved ability to utilize xylose shows enhanced expression of proteins involved in transport, initial xylose metabolism, and the pentose phosphate pathway. Appl Environ Microbiol. 2003;69(2):740–746. doi: 10.1128/AEM.69.2.740-746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengtsson O, et al. Identification of common traits in improved xylose-growing Saccharomyces cerevisiae for inverse metabolic engineering. Yeast. 2008;25(11):835–847. doi: 10.1002/yea.1638. [DOI] [PubMed] [Google Scholar]
- 6.Jeffries TW, Jin YS. Metabolic engineering for improved fermentation of pentoses by yeasts. Appl Microbiol Biotechnol. 2004;63(5):495–509. doi: 10.1007/s00253-003-1450-0. [DOI] [PubMed] [Google Scholar]
- 7.Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF. Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol. 2007;74(5):937–953. doi: 10.1007/s00253-006-0827-2. [DOI] [PubMed] [Google Scholar]
- 8.Martin CH, Nielsen DR, Solomon KV, Prather KLJ. Synthetic metabolism: Engineering biology at the protein and pathway scales. Chem Biol. 2009;16(3):277–286. doi: 10.1016/j.chembiol.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Tyo KEJ, Kocharin K, Nielsen J. Toward design-based engineering of industrial microbes. Curr Opin Microbiol. 2010;13(3):255–262. doi: 10.1016/j.mib.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran KA, Alper HS. Expanding the chemical palate of cells by combining systems biology and metabolic engineering. Metab Eng. 2012;14(4):289–297. doi: 10.1016/j.ymben.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Hahn-Hägerdal B, Galbe M, Gorwa-Grauslund MF, Lidén G, Zacchi G. Bio-ethanol—the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006;24(12):549–556. doi: 10.1016/j.tibtech.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Almeida JR, et al. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol. 2007;82(4):340–349. [Google Scholar]
- 13.Van Vleet JH, Jeffries TW. Yeast metabolic engineering for hemicellulosic ethanol production. Curr Opin Biotechnol. 2009;20(3):300–306. doi: 10.1016/j.copbio.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F, Rodriguez S, Keasling JD. Metabolic engineering of microbial pathways for advanced biofuels production. Curr Opin Biotechnol. 2011;22(6):775–783. doi: 10.1016/j.copbio.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Redden H, Alper HS. Frontiers of yeast metabolic engineering: Diversifying beyond ethanol and Saccharomyces. Curr Opin Biotechnol. 2013;24(6):1023–1030. doi: 10.1016/j.copbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Hong KK, Nielsen J. Metabolic engineering of Saccharomyces cerevisiae: A key cell factory platform for future biorefineries. Cell Mol Life Sci. 2012;69(16):2671–2690. doi: 10.1007/s00018-012-0945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae JY, Laplaza J, Jeffries TW. Effects of gene orientation and use of multiple promoters on the expression of XYL1 and XYL2 in Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2008;145(1-3):69–78. doi: 10.1007/s12010-007-8076-0. [DOI] [PubMed] [Google Scholar]
- 18.Karhumaa K, Påhlman AK, Hahn-Hägerdal B, Levander F, Gorwa-Grauslund MF. Proteome analysis of the xylose-fermenting mutant yeast strain TMB 3400. Yeast. 2009;26(7):371–382. doi: 10.1002/yea.1673. [DOI] [PubMed] [Google Scholar]
- 19.Runquist D, Hahn-Hägerdal B, Bettiga M. Increased ethanol productivity in xylose-utilizing Saccharomyces cerevisiae via a randomly mutagenized xylose reductase. Appl Environ Microbiol. 2010;76(23):7796–7802. doi: 10.1128/AEM.01505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krahulec S, Klimacek M, Nidetzky B. Analysis and prediction of the physiological effects of altered coenzyme specificity in xylose reductase and xylitol dehydrogenase during xylose fermentation by Saccharomyces cerevisiae. J Biotechnol. 2012;158(4):192–202. doi: 10.1016/j.jbiotec.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SM, Jellison T, Alper HS. Directed evolution of xylose isomerase for improved xylose catabolism and fermentation in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 2012;78(16):5708–5716. doi: 10.1128/AEM.01419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scalcinati G, et al. Evolutionary engineering of Saccharomyces cerevisiae for efficient aerobic xylose consumption. FEMS Yeast Res. 2012;12(5):582–597. doi: 10.1111/j.1567-1364.2012.00808.x. [DOI] [PubMed] [Google Scholar]
- 23.Jojima T, Omumasaba CA, Inui M, Yukawa H. Sugar transporters in efficient utilization of mixed sugar substrates: Current knowledge and outlook. Appl Microbiol Biotechnol. 2010;85(3):471–480. doi: 10.1007/s00253-009-2292-1. [DOI] [PubMed] [Google Scholar]
- 24.Young E, Lee SM, Alper H. Optimizing pentose utilization in yeast: The need for novel tools and approaches. Biotechnol Biofuels. 2010;3(24):24. doi: 10.1186/1754-6834-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boles E, Hollenberg CP. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev. 1997;21(1):85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 26.Wieczorke R, et al. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999;464(3):123–128. doi: 10.1016/s0014-5793(99)01698-1. [DOI] [PubMed] [Google Scholar]
- 27.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62(1):1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozcan S, Johnston M. Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev. 1999;63(3):554–569. doi: 10.1128/mmbr.63.3.554-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedlak M, Ho NWY. Characterization of the effectiveness of hexose transporters for transporting xylose during glucose and xylose co-fermentation by a recombinant Saccharomyces yeast. Yeast. 2004;21(8):671–684. doi: 10.1002/yea.1060. [DOI] [PubMed] [Google Scholar]
- 30.Subtil T, Boles E. Competition between pentoses and glucose during uptake and catabolism in recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2012;5:14. doi: 10.1186/1754-6834-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leandro MJ, Gonçalves P, Spencer-Martins I. Two glucose/xylose transporter genes from the yeast Candida intermedia: First molecular characterization of a yeast xylose-H+ symporter. Biochem J. 2006;395(3):543–549. doi: 10.1042/BJ20051465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saloheimo A, et al. Xylose transport studies with xylose-utilizing Saccharomyces cerevisiae strains expressing heterologous and homologous permeases. Appl Microbiol Biotechnol. 2007;74(5):1041–1052. doi: 10.1007/s00253-006-0747-1. [DOI] [PubMed] [Google Scholar]
- 33.Hector RE, Qureshi N, Hughes SR, Cotta MA. Expression of a heterologous xylose transporter in a Saccharomyces cerevisiae strain engineered to utilize xylose improves aerobic xylose consumption. Appl Microbiol Biotechnol. 2008;80(4):675–684. doi: 10.1007/s00253-008-1583-2. [DOI] [PubMed] [Google Scholar]
- 34.Katahira S, et al. Improvement of ethanol productivity during xylose and glucose co-fermentation by xylose-assimilating S. cerevisiae via expression of glucose transporter Sut1. Enzyme Microb Technol. 2008;43(2):115–119. [Google Scholar]
- 35.Du J, Li SJ, Zhao HM. Discovery and characterization of novel d-xylose-specific transporters from Neurospora crassa and Pichia stipitis. Mol Biosyst. 2010;6(11):2150–2156. doi: 10.1039/c0mb00007h. [DOI] [PubMed] [Google Scholar]
- 36.Young E, Poucher A, Comer A, Bailey A, Alper H. Functional survey for heterologous sugar transport proteins, using Saccharomyces cerevisiae as a host. Appl Environ Microbiol. 2011;77(10):3311–3319. doi: 10.1128/AEM.02651-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leandro MJ, Fonseca C, Gonçalves P. Hexose and pentose transport in ascomycetous yeasts: an overview. FEMS Yeast Res. 2009;9(4):511–525. doi: 10.1111/j.1567-1364.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 38.Young EM, Comer AD, Huang HS, Alper HS. A molecular transporter engineering approach to improving xylose catabolism in Saccharomyces cerevisiae. Metab Eng. 2012;14(4):401–411. doi: 10.1016/j.ymben.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Kasahara T, Kasahara M. Identification of a key residue determining substrate affinity in the yeast glucose transporter Hxt7: A two-dimensional comprehensive study. J Biol Chem. 2010;285(34):26263–26268. doi: 10.1074/jbc.M110.149716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ha SJ, et al. Single amino acid substitutions in HXT2.4 from Scheffersomyces stipitis lead to improved cellobiose fermentation by engineered Saccharomyces cerevisiae. Appl Environ Microbiol. 2013;79(5):1500–1507. doi: 10.1128/AEM.03253-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun L, et al. Crystal structure of a bacterial homologue of glucose transporters GLUT1-4. Nature. 2012;490(7420):361–366. doi: 10.1038/nature11524. [DOI] [PubMed] [Google Scholar]
- 42.Hamacher T, Becker J, Gárdonyi M, Hahn-Hägerdal B, Boles E. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology. 2002;148(Pt 9):2783–2788. doi: 10.1099/00221287-148-9-2783. [DOI] [PubMed] [Google Scholar]
- 43.Kasahara T, Shimogawara K, Kasahara M. Crucial effects of amino acid side chain length in transmembrane segment 5 on substrate affinity in yeast glucose transporter Hxt7. Biochemistry. 2011;50(40):8674–8681. doi: 10.1021/bi200958s. [DOI] [PubMed] [Google Scholar]
- 44.Davis EO, Henderson PJF. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coli K12. J Biol Chem. 1987;262(29):13928–13932. [PubMed] [Google Scholar]
- 45.Subtil T, Boles E. Improving L-arabinose utilization of pentose fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol Biofuels. 2011;4:38. doi: 10.1186/1754-6834-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J. In: Molecular Cloning: A Laboratory Manual. Russell DW, editor. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 47.Mumberg D, Müller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156(1):119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.