Significance
Prolyl 3-hydroxylation is a crucial posttranslational modification of collagens. To investigate the role of prolyl 3-hydroxylase 2 (P3H2) and function of 3-hydroxylation in type IV collagen, we created a KO mouse. Prolyl 3-hydroxylation of type IV collagen is required to avoid an aberrant interaction with the platelet-specific glycoprotein VI (GPVI), resulting in platelet aggregation, thrombosis of the maternal blood, and death of the embryo. Homozygous P3H2-null embryos die before embryonic day 8.5. The lethal phenotype can be rescued by producing double mutants of P3H2 and GPVI. Double nulls are viable and fertile. Thus, 3-hydroxylation of type IV collagen is indispensable for embryonic development in mice. We assign a molecular function for prolyl 3-hydroxyl groups in type IV collagen.
Abstract
Collagens constitute nearly 30% of all proteins in our body. Type IV collagen is a major and crucial component of basement membranes. Collagen chains undergo several posttranslational modifications that are indispensable for proper collagen function. One of these modifications, prolyl 3-hydroxylation, is accomplished by a family of prolyl 3-hydroxylases (P3H1, P3H2, and P3H3). The present study shows that P3H2-null mice are embryonic-lethal by embryonic day 8.5. The mechanism of the unexpectedly early lethality involves the interaction of non–3-hydroxylated embryonic type IV collagen with the maternal platelet-specific glycoprotein VI (GPVI). This interaction results in maternal platelet aggregation, thrombosis of the maternal blood, and death of the embryo. The phenotype is completely rescued by producing double KOs of P3H2 and GPVI. Double nulls are viable and fertile. Under normal conditions, subendothelial collagens bear the GPVI-binding sites that initiate platelet aggregation upon blood exposure during injuries. In type IV collagen, these sites are normally 3-hydroxylated. Thus, prolyl 3-hydroxylation of type IV collagen has an important function preventing maternal platelet aggregation in response to the early developing embryo. A unique link between blood coagulation and the ECM is established. The newly described mechanism may elucidate some unexplained fetal losses in humans, where thrombosis is often observed at the maternal/fetal interface. Moreover, epigenetic silencing of P3H2 in breast cancers implies that the interaction between GPVI and non–3-hydroxylated type IV collagen might also play a role in the progression of malignant tumors and metastasis.
Collagens constitute nearly 30% of all proteins in our body (1). Among the 29 collagen types, type IV collagen is a major and crucial component of basement membranes (2). Different collagen types assemble into different structures. Examples of these structures are fibrils (collagen types I, II, and III) and networks (collagen types IV and VI) (1). Proper posttranslational modifications of collagen chains are critical for ultimate quaternary structure formation and function. Posttranslational modifications of collagen include prolyl 4-hydroxylation, lysyl hydroxylation and glycosylation, and prolyl 3-hydroxylation (3). The prolyl 3-hydroxylase family (P3H1, P3H2, and P3H3) is responsible for the process of prolyl 3-hydroxylation. These enzymes modify specific prolines in GlyProHyp [Hyp is 4(R)-hydroxyproline] sequences into 3(S)-hydroxyproline (3Hyp) (4). Although prolyl 4-hydroxylation occurs at almost every Yaa position proline in the Gly-Xaa-Yaa repeated sequence of collagen, prolyl 3-hydroxylation happens only at a few specific Xaa position prolines.
P3H1 is the main enzyme modifying type I collagen. Mutations in P3H1 have been found to cause severe osteogenesis imperfecta (OI) in both humans and mice (5, 6). The causative molecular mechanism of the OI phenotype remains speculative. Limited information is available about the substrate specificity of the P3H2 and P3H3 members of the family. Although cell culture studies have indicated that P3H2 exhibits some activity toward fibril-forming collagens (7), type IV collagen was suggested as the main substrate of this enzyme (8).
Type IV collagen has the highest number of 3-hydroxylations, about six to 16 3Hyps per 1,000 amino acids. However, only two sites have been previously identified in a specific sequence (9). This collagen appears very early in mouse embryonic development. It is already detected in the basement membrane at the time of implantation (10). The basement membrane-forming trophoblasts of the ectoplacental cone are infiltrated by the maternal blood. As a result, embryonic type IV collagen comes into direct contact with the maternal blood around embryonic day (E) 6 (11). Mice deficient in α1- and α2-chains of type IV collagen are embryonic-lethal between E10.5 and E11.5. They develop at the expected Mendelian ratio up to E9.5, indicating that type IV collagen is not essential during early development but is essential for basement membrane stability (12). Even mice lacking prolyl 4-hydroxylase develop up to E9.5, indicating that collagens are generally not required for development to this stage (13).
Interaction of subendothelial collagens (type I and type III) with platelets plays a central role in maintaining hemostasis by initiating thrombus formation upon vascular injury. Platelet-specific glycoprotein VI (GPVI) is a major factor in the initiation of platelet aggregation through binding to these collagens (14). It has been demonstrated that GPVI binds to repeated (GlyProHyp)n collagen sequences (15). Collagen-related peptides (CRPs) were also shown to interact with GPVI and initiate platelet aggregation (16). However, platelets do not react strongly with basement membranes (17). Despite the substantial presence of (GlyProHyp)n repeats within the type IV collagen sequence, this collagen type did not initiate significant platelet aggregation (17).
The role of P3H2 and function of the 3Hyps in type IV collagen have been studied here using a P3H2 KO mouse model.
Results
Production of P3H2-Null Mice.
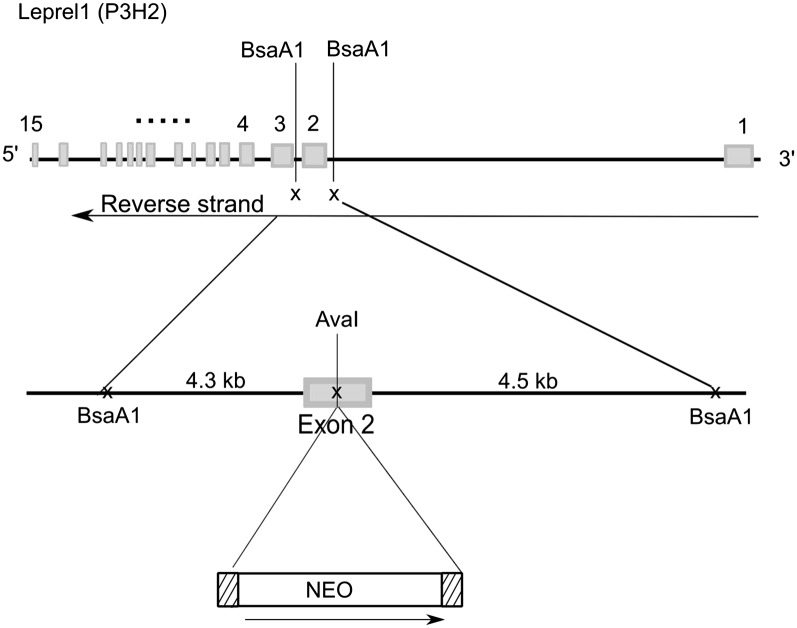
Fig. 1 shows the design of the targeted disruption of the Leprel1 gene for P3H2-null mouse production. Mice containing heterozygous Leprel1 loci on a mixed 129xC57BL background showed no obvious phenotype and bred normally. However, no null mice were born as a result of P3H2+/−/P3H2+/− breeding. To determine at what developmental age embryonic lethality occurs, pregnant heterozygous females were killed at various stages. At no stage studied was the expected Mendelian ratio of homozygous P3H2-null mice observed (Table 1). Therefore, the majority of the P3H2-null mice were embryonic-lethal before E8.5.
Fig. 1.
Targeted vector for P3H2-null production. The mouse Leprel1 gene spanning 15 exons is schematically presented. A targeted disruption containing a neocassette (NEO) was made in exon 2 of the gene. A 9-kb Bsa1 restriction fragment spanning exon 2, part of intron 1–2, and most of intron 2–3 was subcloned into a p26 vector, and the neocassette was cloned within the exon 2 sequence using the AvaI restriction site.
Table 1.
Death of P3H2 homozygous-null embryos
| Stage | No. of litters | No. of pups/embryos | P3H2+/+ | P3H2+/− | P3H2 −/− |
| P21 | 15 | 120 | 38 | 82 | 0 |
| E13.5 | 2 | 15 | 5 | 10 | 0 |
| E12.5 | 2 | 16 | 5 | 11 | 0 |
| E11.5 | 4 | 24 | 7 | 15 | 2* |
| E9.5 | 5 | 31 | 9 | 21 | 1† |
| E8.5 | 4 | 27 | 10 | 16 | 1† |
P, postnatal day.
These embryos appeared morphologically normal. Immunofluorescent light microscopy and EM demonstrated greatly reduced type IV collagen in these embryos. The cause of the reduced amount of type IV collagen is not known. However, this likely allowed the embryos to avoid early lethality.
These embryos appeared morphologically normal. No further analysis was performed.
The result was very surprising considering that when both α-chains of type IV collagen were ablated, the embryos still survived to at least E9.5 (12). Although P3H2-deficient embryos presumably lack the 3Hyps in their type IV collagen sequence, the protein remains in place. Why would the absence of a protein posttranslational modification cause a more dramatic effect than the absence of the protein itself?
Maternal Platelets Aggregate in Response to Early Developing P3H2−/− Embryos.
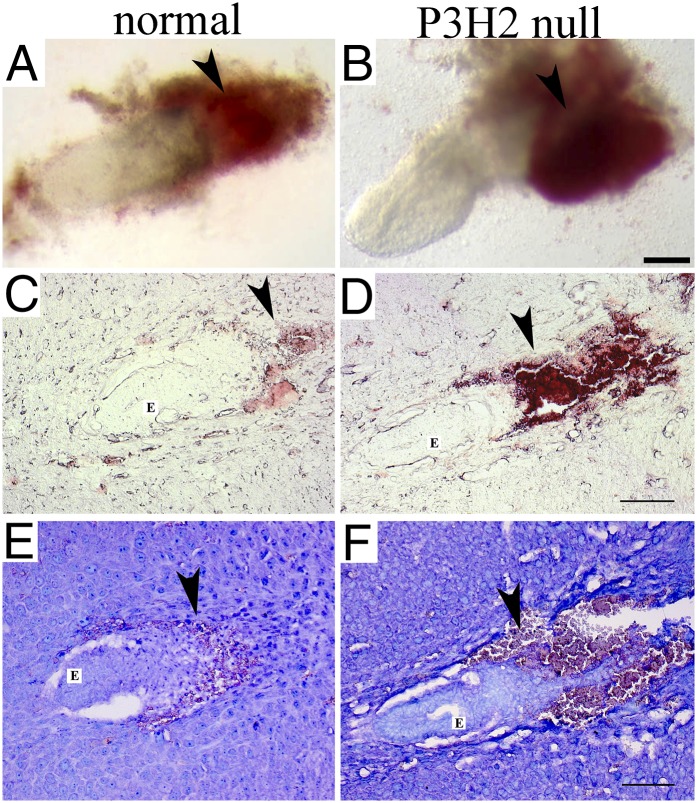
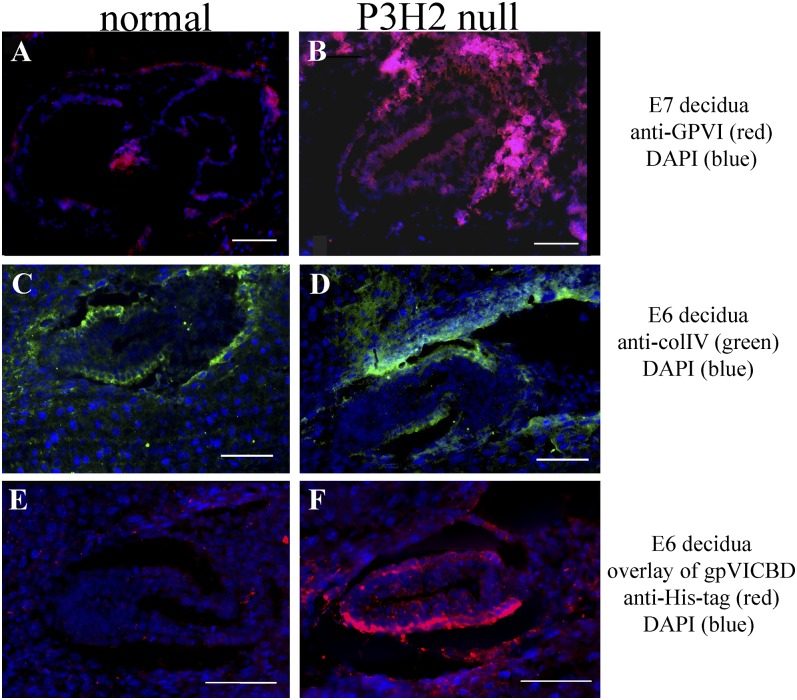
At 6.5 d postconception, blood clots were present in close proximity to some embryos (Fig. 2B), indicating abnormal coagulation of the maternal blood in response to these embryos. It is possible that maternal blood thrombus formation was initiated by altered embryonic type IV collagen. To investigate this, the whole deciduae of three litters that resulted from heterozygous crosses were sectioned and analyzed. Representative unstained decidua sections are shown in Fig. 2 C and D. Thrombus formation is evident in the maternal tissue surrounding the P3H2 KO embryo (Fig. 2D). Wright’s stain was used to visualize maternal platelets that were found aggregated around the ectoplacental cone of the null embryo (Fig. 2F). In addition, immunofluorescent staining against platelet-specific GPVI was performed and confirmed maternal platelet aggregation linked to the mutant embryos. Thus, in each analyzed litter, morphologically normal P3H2−/− embryos surrounded by maternal platelet aggregates were found (Figs. 2 D and F and 3B). No platelet aggregation was present in the deciduae carrying WT or heterozygous embryos (Figs. 2 C and E and 3A). The presence of type IV collagen in normal embryo and mutant embryo is demonstrated in Fig. 3 C and D. Therefore, P3H2−/− embryos were destroyed through maternal blood thrombosis around E6.5, the time when maternal blood infiltrates the embryo (11).
Fig. 2.
P3H2 KOs and normal littermates at 6.5 d postconception. (A and B) Isolated embryos at E6.5. Both embryos appear morphologically normal. Arrowheads are pointing to the maternal blood neighboring embryos. There is a blood clot in close proximity to the embryo seen in B. Representative sections of normal and P3H2-null E6 deciduae are shown unstained (C and D) and stained with the Wright’s stain (E and F). Arrowheads indicate maternal blood invading the ectoplacental cone. Thrombus formation next to the mutant embryo (E) is observed in D and F. Wright’s stain visualizes maternal platelets in purple (F, arrowhead). Note that erythrocytes appear orange to pink and are difficult to differentiate from the purple platelets. (Scale bars: A and B, 200 μm; C–F, 100 μm.)
Fig. 3.
Immunofluorescence analysis of early embryos. Maternal platelets aggregated around a P3H2-null embryo in the 7-d postconception decidua section (B) in comparison to no platelet aggregation around a normal embryo (A). In both images, maternal platelets are immunolabeled with anti- GPVI antibody in red. (C and D) Presence of type IV collagen in both P3H2-null and control embryos at E6 (green) is shown. colIV, type IV collagen. (E and F) Results of a tissue overlay experiment. Recombinant GPVI (gpVICBD) was incubated with E6 decidua sections and then detected with anti–His-tag antibody. Only the recombinant protein bound to the P3H2-null embryonic type IV collagen containing the basement membrane is seen in F (red staining). Normal embryonic type IV collagen does not interact with GPVI, which results in no staining of the basement membrane in E. Nuclei are visualized with DAPI stain (blue). (Scale bars: 100 μm.)
Identification of 3Hyps in Type IV Collagen.
To determine the extent of prolyl 3-hydroxylation in type IV collagen, cyanogen bromide (CNBr) peptides of the α1-chain of bovine lens capsule type IV collagen were sequenced and the sequence positions of six 3Hyp residues, in addition to two previously known sites, were identified. Interestingly, two clusters of repetitive 3Hyp-containing tripeptide units were found, something not observed in subendothelial collagens (Fig. 4). Therefore, repetitive 3-hydroxylations of the prolines in (GlyProHyp)n sequences in type IV collagen distinguish it from fibrillar collagens.
Fig. 4.
Identification of the 3-Hyps in type IV collagen. The amino acid sequence of the CNBr peptides of the α1-chain of bovine lens capsule type IV collagen is represented. Underlined sequences correspond to the proteolytic peptides of CNBr peptides sequenced by N-terminal (Edman) sequencing. The boldfaced P indicates previously published 3-Hyps that were also sequenced by us. The red boldfaced P indicates previously unknown 3-Hyps identified by sequencing. Curly brackets indicate 3-Hyps that were confirmed by MS in addition to sequencing. Regular brackets indicate 3- or 4-hydroxyprolines identified by MS only.
Hypothesis.
Type IV collagen does not initiate significant platelet aggregation, whereas fibrillar collagen types do (18). Is the repetitive 3-hydroxylation of the (GlyProHyp)n sequences in type IV collagen required to avoid the binding of GPVI and subsequent platelet aggregation in response to type IV collagen? If so, P3H2−/− embryos, containing no 3Hyps in type IV collagen, will initiate aggregation of the maternal platelets.
3-Hydroxylation Ablates Binding of GPVI and Prevents Platelet Aggregation.
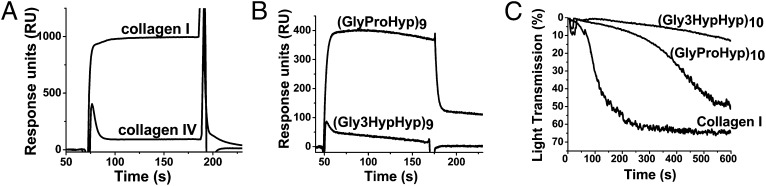
The interaction of the monomeric collagen-binding domain of GPVI (gpVICBD) with type I and type IV collagens was compared (Fig. 5A). Previous studies have found only weak affinity of GPVI to type IV collagen, which was ascribed to the contamination with type I collagen (18). Bovine lens capsule contains no type I collagen; therefore, this tissue was used to purify type IV collagen for these experiments. Fig. 5A shows surface plasmon resonance (SPR) experiments, which demonstrate that gpVICBD binds to type I collagen but not to type IV collagen. This indicates that GPVI would not interact with type IV collagen under normal circumstances.
Fig. 5.
Binding of gpVICBD to collagen types I and IV and CRPs as demonstrated by platelet aggregation assays. (A) Interaction of 0.1 mg/mL gpVICBD with type I and IV collagens immobilized on the surface of the Biacore chip. A low-affinity interaction is detected for type I collagen, but no binding is observed for type IV collagen. RU, response units. (B) Interaction of gpVICBD with the surface immobilized (GlyProHyp)9 and (Gly3HypHyp)9 peptides. No binding is observed for the 3-hydroxylated peptide. (C) Human platelet-rich plasma aggregates in response to different agonists. The turbidity of the solution was monitored. The following agonists were used: type I collagen at a concentration of 50 μg/mL (control) and collagen-like peptides (GlyProHyp)10 and (Gly3HypHyp)10 at a concentration of 200 μg/mL each. The 3-hydroxylated peptide does not initiate platelet aggregation.
To determine if the binding of GPVI to the (GlyProHyp)n peptides is affected by modifications of Pro to 3Hyp, corresponding Biacore (Biacore Life Sciences, GE Healthcare) binding curves were measured. Fig. 5B shows binding of gpVICBD to the (GlyProHyp)9 peptide. No binding to the (Gly3HypHyp)9 peptide was detected.
Platelet aggregation assays (Fig. 5C) demonstrated that the (Gly3HypHyp)10 peptide was unable to induce detectable platelet aggregation, whereas (GlyProHyp)10 worked reasonably well. Thus, 3-hydroxylation prevents binding of the GPVI to CRPs and abolishes platelet aggregation.
A tissue overlay assay was used to demonstrate that GPVI binds to non–3-hydroxylated type IV collagen in the P3H2-null embryos. The recombinant GPVI (the same as used for Biacore measurements and the platelet aggregation assay) was incubated with tissue sections, followed by the standard immunofluorescence protocol. Fig. 3 E and F represents the results of this experiment. The staining pattern outlines embryonic basement membrane in case of the P3H-null embryo (red, Fig. 3F), whereas no staining is observed in the control (Fig. 3E).
P3H2/GPVI Double-KO Mice Are Viable.
The hypothesis was strongly supported by biochemical and histological data. To obtain in vivo proof of concept, P3H2/GPVI double-KO mice were generated. Mice deficient in GPVI were previously characterized and demonstrated the absence of collagen-induced platelet aggregation but otherwise exhibited no phenotype (19). To determine if P3H2/GPVI double-KO mice would survive longer than P3H2-nulls, mice that are heterozygous in P3H2 and homozygous in GPVI were bred. Dramatically, the P3H2/GPVI double-KOs were born with the expected Mendelian ratio, survived to adulthood, and were capable of reproduction (Table 2). No gross phenotype for the P3H2−/−/GPVI−/− mice was observed.
Table 2.
Distribution of P3H2 genotypes on the GPVI-null background
| Developmental stage | No. of litters | No. of mice | WTs | P3H2 heterozygous mice | P3H2 homozygous mice |
| P21 | 7 | 51 | 14 | 26 | 11 |
GPVI Binds to Non–3-Hydroxylated Type IV Collagen Directly in Tissue.
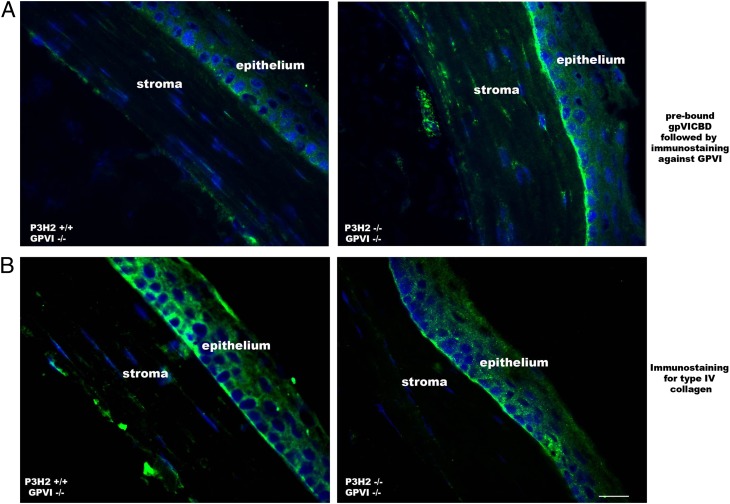
As shown in Fig. 3F, gpVICBD binds to altered embryonic type IV collagen. To demonstrate this interaction in adult tissue, eye sections of double nulls and controls were preincubated with gpVICBD, followed by a standard immunofluorescent labeling protocol (Fig. 6). Because mice used in this experiment were homozygous nulls in GPVI, the only antibody recognition sites were represented by exogenous prebound gpVICBD. Fig. 6 demonstrates binding of the recombinant GPVI to the P3H2−/−/GPVI−/− corneal basement membrane, whereas no such binding is seen in the P3H2+/+/GPVI−/− control section. Therefore, repetitive prolyl 3-hydroxylation prevents binding of GPVI to collagen both in vitro and in vivo.
Fig. 6.
Recombinant GPVI binds to the basement membrane in the cornea of the P3H2/GPVI double-null mouse eye. (A) Immunofluorescent staining of mouse eye sections with anti-GPVI antibody. Before a standard immunostaining protocol was applied, sections were preincubated with recombinant GPVI (gpVICBD). Note specific staining of the basement membrane in the case of a double mutant. No staining specific to the basement membrane is seen in the control. (B) Immunostaining of similar sections against type IV collagen. All images were acquired under the same settings. (Scale bar: 20 μm.)
Fate of GPVI-Positive Embryos in the Absence of Maternal GPVI.
Platelets first appear by E10.5 in normal mouse development (20). If maternal, but not fetal, platelets are deficient in GPVI, it is reasonable to expect that internal embryonic thrombosis will occur at later developmental stages when fetuses produce their own blood cells. Double-P3H2/GPVI-null female mice were mated with P3H2 heterozygous males carrying WT GPVI. The resulting offspring are heterozygous in GPVI, and half of them are expected to be null in P3H2. In two analyzed litters, no P3H2-null mice were found at birth, indicating that the P3H2−/−/GPVI+/− phenotype is embryonic-lethal. Two pregnant females were killed at E8.5. As expected, approximately half of the embryos in these litters (four of eight embryos and three of seven embryos) were P3H2−/−/GPVI+/−. The P3H2-null embryos were morphologically and developmentally normal. Thus, if maternal GPVI is not present, P3H2-null embryos carrying heterozygous GPVI survive to at least E8.5 but do not survive until birth. Further detailed analysis of embryos at later developmental stages is needed to identify the reason and time point of this embryonic lethality.
Discussion
To summarize, the deficiency in P3H2 leads to early (around E6.5) embryonic lethality in mice. P3H2-null embryos are destroyed through maternal blood thrombosis in response to non–3-hydroxylated type IV collagen. Two receptors are known to be responsible for the initiation of collagen-induced platelet aggregation: GPVI and integrin α2β1. Although integrin α2β1 could potentially be involved in the lethality of the P3H2-null embryos, its known binding sites do not involve Gly-Pro-Hyp sequences and the binding sequences are distant from the 3-hydroxyprolines in type IV collagen (21). The data represented in this paper, and rescue of double-P3H2/GPVI KOs in particular, clearly indicate that thrombus formation is initiated by interaction of unmodified type IV collagen (GlyProHyp)n sequences with platelet-specific GPVI. This previously recognized interaction plays a central role for blood coagulation in response to subendothelial collagens (15). Subendothelial collagens are normally not exposed to the blood unless injury occurs. The story is different for type IV collagen. Basement membranes are present in virtually every tissue. Embryonic basement membrane meets the maternal blood very early in the course of development. Prolyl 3-hydroxylation in type IV collagen protects it from the interaction with GPVI, and consequently ablates the ability of this collagen type to initiate platelet aggregation. This identifies a direct molecular function and important biological role for prolyl 3-hydroxyl groups in collagen.
Implying the similarity of mouse and human embryonic development, 3-hydroxylation may also be a requirement for type IV collagen in humans. However, an inactivating mutation in P3H2 has recently been identified in a large consanguineous Israeli Bedouin kindred and found to cause a high degree of myopia (22). Apparently, these individuals survived to adulthood and escaped the mechanism of embryonic lethality described in this paper. There is no easy explanation for this discrepancy between mice and humans, but the current knowledge about GPVI polymorphisms allows some speculation. A number of polymorphic variations of GPVI in healthy individuals have been identified (23–25). There are two common alleles of GPVI (“a” and “b”), which differ by five amino acids and occur at population frequencies of 0.85 and 0.13, respectively (26). Individuals homozygous for the b allele have a lower GPVI density on their platelet surface and reduced thrombogenicity in response to collagen (26, 27). It is thus possible that the above-mentioned family with a mutation in P3H2 carries the low-frequency b allele of GPVI, which allowed them to escape the maternal platelet aggregation in response to non–3-hydroxylated type IV collagen.
Other GPVI polymorphisms have recently been associated with platelet hyperaggregability in certain cases of fetal loss (28). Thrombosis is often observed at the maternal/fetal interface in recurrent pregnancy losses of unexplained etiology (29). Incomplete prolyl 3-hydroxylation of embryonic type IV collagen might contribute to the pathogenesis of this condition.
The aberrant interaction between type IV collagen and GPVI seems to be important for certain cancers. Expression of P3H2 is down-regulated in breast cancer cells by epigenetic inactivation (30). It is likely that metastasizing tumor cells that express type IV collagen in the absence of P3H2 will facilitate the interaction with platelets in the vascular system, which will protect these circulating tumor cells from shear stress and natural killer cells (31, 32). Such a mechanism is also supported by a 50% reduction in experimental tumor lung metastasis found in GPVI-null mice (33).
Thus, the link between blood coagulation and the ECM elaborated upon in the present study opens up unique venues in the areas of pregnancy loss and cancer research.
Materials and Methods
Generation of P3H2-Null and P3H2/GPVI Double-Null Mice.
All procedures involving animals were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. The mouse Leprel1 gene spanning 15 exons is schematically presented in Fig. 1. A targeted disruption containing a neocassette was made in exon 2 of the gene. A 9-kb Bsa1 restriction fragment spanning exon 2, part of intron 1–2, and most of intron 2–3 was subcloned into a p26 vector, and the neocassette was cloned within the exon 2 sequence using the AvaI restriction site. The positive ES cells were microinjected into 129/SvJ mouse blastocysts and implanted into pseudopregnant females. Chimeric mice were bred to C57BL/6J mice to test for germ-line transmission. Two chimeric mice from different ES cells produced germ-line offspring as assessed by coat color transmission. Heterozygous mice were crossed.
To produce P3H2/GPVI double nulls, P3H2 heterozygous (P3H2het) mice were first bred on a GPVI-null (GPVInull) background (19). The P3H2het/GPVInull mice were crossed to produce all three P3H2 genotypes (Table 2). The resulting genetic background for the P3H2/GPVI double-null mice was mixed (129/SvJ × C57BL/6J × Black Swiss).
Enzymatic Cleavage and Edman Sequencing of Type IV Collagen.
Bovine lens capsule type IV collagen was digested by CNBr overnight at room temperature in 70% (vol/vol) formic acid. The digested solution was lyophilized and subjected to sieve chromatography on tandem Superose12 columns using the Akta FPLC system (Amersham Biosciences) to separate fragments in 0.1 M sodium acetate buffer (pH 4.5). Individual CNBr fragments were digested with trypsin, and the resulting peptides were separated on a Vydac C18 (218TP52) column (Grace). Additional cleavages of the tryptic peptides were carried out as needed to release individual 3Hyp-containing peptides using thermolysin (Calbiochem), Asp-N (Princeton Separations Systems), or both. Reverse phase fractions were lyophilized, resuspended in 50 μL of 0.1 M ammonium bicarbonate (pH 7.4) with 0.5–1.0 μg of enzyme, and allowed to digest overnight at room temperature. The digest mixture was separated, and fractions were sequenced on an ABI Procise Protein Sequencer (Applied Biosystems).
MS Analysis for 3-Hydroxyproline Identification in Bovine Lens Capsule Collagen Type IV.
In-gel digestion with trypsin was performed on 1D SDS/PAGE bands using a protocol similar to that described (33). Identification of peptides containing 3-hydroxyproline was performed on a Q-TOF micro mass spectrometer (Waters) equipped with an electrospray ionization source. Data were collected with MassLynx (version 4.1; Waters) data acquisition software and processed using Mascot Distiller (Matrix Software). HPLC was performed with a nanoAcquity system (Waters) using a 75 μm × 100 mm 3-μm Atlantis dC18 analytical column. Tryptic peptides were identified from all MS/MS spectra by a Mascot search against the National Center for Biotechnology Information nonredundant database (taxonomy: Mammalia). Oxidation on proline and lysine was specified as variable modifications, and the search tolerance was 1.0 Da for both the MS and MS/MS scans. Peptides identified as containing a hydroxyproline in the X-position were flagged as containing a potential 3-hydroxyproline and were verified by visual examination of the MS/MS data.
Monomeric Recombinant Collagen-Binding Domain of Human GPVI.
To facilitate expression and purification of the extracellular collagen-binding domain of GPVI, its cDNA sequence was cloned as a part of a fusion molecule with a His-tagged thioredoxin and a thrombin cleavage site on the amino terminus. The resulting sequence and detailed information on expression, refolding, and purification is given in SI Materials and Methods.
Platelet Aggregation Assay.
Platelet-rich plasma or washed platelets were used in platelet aggregometer studies. Twenty milliliters of donor blood was drawn into standard acid citrate dextrose anticoagulant and centrifuged at room temperature for 10 min at 1,000 × g to pellet RBCs and obtain platelet-rich plasma. An aliquot of the platelet-rich plasma supernatant was centrifuged for 10 min at 10,000 × g to obtain platelet-poor plasma, which was used to blank the aggregometer (Bio/Data). Platelet-rich plasma (0.45 mL) was activated with either 50 μg/mL type I collagen, 200 μg/mL (GlyProHyp)10 peptide, or (Gly3HypHyp)10 peptide to induce aggregation, and aggregation was monitored for 6 min at 37 °C.
SPR.
Binding analyses were performed using BIAcoreX (Biacore Life Sciences, GE Healthcare). Type I collagen (extracted from mouse skin), type IV collagen (extracted from bovine lens capsule), or synthetic peptides were covalently coupled to a CM5 sensor chip (research grade) according to the manufacturer’s instructions (Biacore Life Sciences, GE Healthcare). gpVICBD was diluted in HBS buffer [20 mM Hepes buffer (pH 7.4), containing 0.15 mM NaCl] and injected at several concentrations and different flow rates over the immobilized ligands. Binding responses due to analyte interaction with the surface-coupled ligand were normalized by subtraction of background binding to plain control flow cells. Binding assays were performed at 25 °C.
Mouse tissue histology and immunofluorescence.
Sections were produced using a Leica CM 1850 UV cryostat. Wright’s stain was purchased from Fisher Scientific and used according to the manufacturer’s instructions. Genotyping of E6.5 embryos was done directly from slides using a laser light capture microscope (PIXCELL II; Arcturus Engineering).
Freshly cut tissue sections were fixed in cold acetone for 10 min and blocked with 5% goat serum for 1 h at room temperature. The following antibodies were used for immunofluorescence experiments: rabbit polyclonal anti-GPVI antibody (Thermo Scientific), rabbit polyclonal anti-type IV collagen antibody (made in-house against type IV collagen purified from HR9 cells), and rabbit polyclonal anti-6His antibody (Abcam). Generally, a primary antibody was diluted 1:1,000 in PBS buffer containing 0.1% Tween and 0.5% goat serum and was then incubated with sections overnight in the cold room. After three 15-min washes with PBS, secondary antibody was applied and incubated with sections for 1.5 h at room temperature, followed by several washing steps. Goat anti-rabbit IgGs were conjugated with rhodamine (EMD Millipore), and Alexa Fluor 568 or Alexa Fluor 488 (Molecular Probes) was chosen as a secondary antibody.
Tissue overlay assay.
Deciduae or eye sections were initially incubated with gpVICBD overnight in the cold room and extensively washed. The protocol described above for standard immunostaining was then applied, with the exception of the fixation step.
Collagen extraction from tissue.
Type I collagen used for SPR experiments was extracted from mouse skin as previously described (6). Type IV collagen extracted from bovine lens capsule (a gift from Shunji Hattori) was used for identification of 3-hydroxyprolines and binding experiments.
Supplementary Material
Acknowledgments
We thank Dr. Lynn Boshkov for giving us an opportunity to use her laboratory equipment to measure the platelet aggregation assay. We thank Dr. Shunji Hattori (Nippi Research Institute), who supplied us with bovine lens capsule type IV collagen. We also thank Douglas Keen and Sara Tufa for EM images that were not included in this paper but supported our data. This work was supported by a grant from the Shriners Hospital for Children (to H.P.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307597111/-/DCSupplemental.
References
- 1.Bächinger HP, Mizuno K, Vranka J, Boudko S. Collagen formation and structure. In: Mander L, Liu HW, editors. Comprehensive Natural Products II: Chemistry and Biology. Oxford: Elsevier; 2010. pp. 469–530. [Google Scholar]
- 2.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71(5):357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myllyharju J. Intracellular post-translational modifications of collagens. In: Brinckmann J, Notbohm H, Muller PK, editors. Collagen. Primer in Structure, Processing and Assembly. Vol 247. Berlin, Heidelberg: Springer; 2005. pp. 115–147. [Google Scholar]
- 4.Vranka JA, Sakai LY, Bächinger HP. Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J Biol Chem. 2004;279(22):23615–23621. doi: 10.1074/jbc.M312807200. [DOI] [PubMed] [Google Scholar]
- 5.Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res. 2010;339(1):59–70. doi: 10.1007/s00441-009-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vranka JA, et al. Prolyl 3-hydroxylase 1 null mice display abnormalities in fibrillar collagen-rich tissues such as tendons, skin, and bones. J Biol Chem. 2010;285(22):17253–17262. doi: 10.1074/jbc.M110.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes RJ, Farnand AW, Traeger GR, Weis MA, Eyre DR. A role for prolyl 3-hydroxylase 2 in post-translational modification of fibril-forming collagens. J Biol Chem. 2011;286(35):30662–30669. doi: 10.1074/jbc.M111.267906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiainen P, Pasanen A, Sormunen R, Myllyharju J. Characterization of recombinant human prolyl 3-hydroxylase isoenzyme 2, an enzyme modifying the basement membrane collagen IV. J Biol Chem. 2008;283(28):19432–19439. doi: 10.1074/jbc.M802973200. [DOI] [PubMed] [Google Scholar]
- 9.Schuppan D, Glanville RW, Timpl R, Dixit SN, Kang AH. Sequence comparison of pepsin-resistant segments of basement-membrane collagen alpha 1(IV) chains from bovine lens capsule and mouse tumour. Biochem J. 1984;220(1):227–233. doi: 10.1042/bj2200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leivo I, Wartiovaara J. Basement membrane matrices in mouse embryogenesis, teratocarcinoma differentiation and in neuromuscular maturation. Int J Dev Biol. 1989;33(1):81–89. [PubMed] [Google Scholar]
- 11.Theiler K. The House Mouse: Atlas of Embryonic Development. New York: Springler; 1989. [Google Scholar]
- 12.Pöschl E, et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131(7):1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- 13.Holster T, et al. Loss of assembly of the main basement membrane collagen, type IV, but not fibril-forming collagens and embryonic death in collagen prolyl 4-hydroxylase I null mice. J Biol Chem. 2007;282(4):2512–2519. doi: 10.1074/jbc.M606608200. [DOI] [PubMed] [Google Scholar]
- 14.Moroi M, Jung SM. Platelet glycoprotein VI: Its structure and function. Thromb Res. 2004;114(4):221–233. doi: 10.1016/j.thromres.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Knight CG, et al. Collagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagen. Cardiovasc Res. 1999;41(2):450–457. doi: 10.1016/s0008-6363(98)00306-x. [DOI] [PubMed] [Google Scholar]
- 16.Asselin J, Knight CG, Farndale RW, Barnes MJ, Watson SP. Monomeric (glycine-proline-hydroxyproline)10 repeat sequence is a partial agonist of the platelet collagen receptor glycoprotein VI. Biochem J. 1999;339(Pt 2):413–418. [PMC free article] [PubMed] [Google Scholar]
- 17.Packham MA, Mustard JF. Platelet adhesion. Prog Hemost Thromb. 1984;7:211–288. [PubMed] [Google Scholar]
- 18.Jung SM, et al. Collagen-type specificity of glycoprotein VI as a determinant of platelet adhesion. Platelets. 2008;19(1):32–42. doi: 10.1080/09537100701609027. [DOI] [PubMed] [Google Scholar]
- 19.Kato K, et al. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102(5):1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 20.Tober J, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109(4):1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkin JD, et al. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum Mutat. 2011;32(2):127–143. doi: 10.1002/humu.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mordechai S, et al. High myopia caused by a mutation in LEPREL1, encoding prolyl 3-hydroxylase 2. Am J Hum Genet. 2011;89(3):438–445. doi: 10.1016/j.ajhg.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croft SA, et al. Novel platelet membrane glycoprotein VI dimorphism is a risk factor for myocardial infarction. Circulation. 2001;104(13):1459–1463. doi: 10.1161/hc3801.096397. [DOI] [PubMed] [Google Scholar]
- 24.Takagi S, et al. A GPVI polymorphism is a risk factor for myocardial infarction in Japanese. Atherosclerosis. 2002;165(2):397–398. doi: 10.1016/s0021-9150(02)00241-1. [DOI] [PubMed] [Google Scholar]
- 25.Cole VJ, et al. Collagen platelet receptor polymorphisms integrin alpha2beta1 C807T and GPVI Q317L and risk of ischemic stroke. J Thromb Haemost. 2003;1(5):963–970. doi: 10.1046/j.1538-7836.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 26.Joutsi-Korhonen L, et al. The low-frequency allele of the platelet collagen signaling receptor glycoprotein VI is associated with reduced functional responses and expression. Blood. 2003;101(11):4372–4379. doi: 10.1182/blood-2002-08-2591. [DOI] [PubMed] [Google Scholar]
- 27.Best D, et al. GPVI levels in platelets: Relationship to platelet function at high shear. Blood. 2003;102(8):2811–2818. doi: 10.1182/blood-2003-01-0231. [DOI] [PubMed] [Google Scholar]
- 28.Sokol J, et al. Platelet aggregation abnormalities in patients with fetal losses: The GP6 gene polymorphism. Fertil Steril. 2012;98(5):1170–1174. doi: 10.1016/j.fertnstert.2012.07.1108. [DOI] [PubMed] [Google Scholar]
- 29.Kwak-Kim J, Yang KM, Gilman-Sachs A. Recurrent pregnancy loss: A disease of inflammation and coagulation. J Obstet Gynaecol Res. 2009;35(4):609–622. doi: 10.1111/j.1447-0756.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- 30.Shah R, et al. The prolyl 3-hydroxylases P3H2 and P3H3 are novel targets for epigenetic silencing in breast cancer. Br J Cancer. 2009;100(10):1687–1696. doi: 10.1038/sj.bjc.6605042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labelle M, Hynes RO. The initial hours of metastasis: The importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 33.Jain S, Russell S, Ware J. Platelet glycoprotein VI facilitates experimental lung metastasis in syngenic mouse models. J Thromb Haemost. 2009;7(10):1713–1717. doi: 10.1111/j.1538-7836.2009.03559.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.