Significance
Mammalian nonmuscle myosin II, a major player in cell migration and polarity, is regulated through heavy chain phosphorylation. The signaling mechanisms remain largely unknown. We implicate here coronin in this pathway. Coronin has a Cdc42- and Rac-interactive binding (CRIB) motif with which it binds preferentially to the GDP-loaded form of Rho GTPases. Loss of coronin in Dictyostelium amoebae leads to elevated levels of activated Rac, resulting in increased myosin II assembly through activation of p21-activated kinase a (PAKa). PAKa inactivates myosin heavy chain kinases that no longer can phosphorylate myosin II and prevent filament formation. By including myosin heavy chain kinase–deficient cells we were able to further map the signaling cascade regulating myosin II.
Keywords: Rac GTPases, protein–protein interaction, cytoskeleton, cell signaling
Abstract
The Cdc42- and Rac-interactive binding motif (CRIB) of coronin binds to Rho GTPases with a preference for GDP-loaded Rac. Mutation of the Cdc42- and Rac-interactive binding motif abrogates Rac binding. This results in increased 1evels of activated Rac in coronin-deficient Dictyostelium cells (corA−), which impacts myosin II assembly. corA− cells show increased accumulation of myosin II in the cortex of growth-phase cells. Myosin II assembly is regulated by myosin heavy chain kinase–mediated phosphorylation of its tail. Kinase activity depends on the activation state of the p21-activated kinase a. The myosin II defect of corA− mutant is alleviated by dominant-negative p21-activated kinase a. It is rescued by wild-type coronin, whereas coronin carrying a mutated Cdc42- and Rac-interactive binding motif failed to rescue the myosin defect in corA− mutant cells. Ectopically expressed myosin heavy chain kinases affinity purified from corA− cells show reduced kinase activity. We propose that coronin through its affinity for GDP–Rac regulates the availability of GTP–Rac for activation of downstream effectors.
Coronins are a highly conserved family of proteins that are important regulators of actin-dependent processes. The founding member of this family, coronin from Dictyostelium discoideum, localizes to actin-rich crown-like structures at the dorsal site of the cells and is present in leading edges and pseudopodia (1). In vivo analysis revealed a dynamic behavior and showed coronin accumulation also at sites where F-actin disassembles. During phagocytosis, coronin associated with the phagosome after the assembly of actin filaments supporting the proposal that coronin promotes the disassembly of actin filaments. For mammalian coronins, an interaction with the Arp2/3 complex has been shown that inhibited the actin-nucleating activity of this complex (2, 3). In yeast a dual mode of the impact on Arp2/3 has been reported. At low concentrations coronin enhanced filament binding by the Arp2/3 complex, whereas at high concentrations binding of the Arp2/3 complex to actin filaments was inhibited (4). For Dictyostelium coronin, an interaction with Arp2/3 has not been reported, however in analogy the mechanism to promote the disassembly of actin filaments might be similar (5, 6).
Analysis of Dictyostelium mutants lacking coronin revealed its roles in cell motility, phagocytosis, and cytokinesis. Cell motility was reduced to less than half compared with the parent strain, the phagocytosis rate was only ∼30% that of wild type, and ∼50% of the cells were multinucleated (7, 8).
The structural characteristic of coronins is a tryptophan-aspartic acid (WD) repeat domain containing seven repeats that form a seven-bladed β-propeller (9). In addition to this domain, coronins have N- and C-terminal extensions. At the C terminus, a unique region is followed by a coiled coil that mediates oligomerization. A further feature is a recently discovered Cdc42- and Rac-interactive binding (CRIB) motif located at the surface of the β-propeller between blades 2 and 3. It was initially identified in Xenopus coronin. When expressed in Swiss 3T3 fibroblasts, the location of Xcoronin was affected by active Rac. It relocalized to the cell periphery when coexpressed with RacV12 and was found in lamellipodia that were induced by active Rac. Rac-induced lamellipodia formation was inhibited by a truncated coronin (residues 64–299). An analysis of this region led to the identification of a sequence related to the CRIB motif (10), which in turn was proposed to interact with and recruit Rac (11). The CRIB motif was also identified in several mammalian coronins like human Coronin1A, 1B, 1C, and in the Dictyostelium coronins (12). D. discoideum harbors two coronins, coronin encoded by the corA gene and coronin7 encoded by the corB gene. Coronin7 has two WD repeat domains separated by a proline–serine–threonine-rich domain (13).
D. discoideum has several members of the Rho family of small GTPases that mostly belong to the Rac subfamily (14, 15). Although it has no clear homolog of Cdc42, RacB has been proposed as a functional equivalent (16). Rho GTPases are key regulators of the actomyosin cytoskeleton in the cell and regulate cellular processes like phagocytosis, cytokinesis, and chemotaxis. Among their effectors are Wiskott–Aldrich syndrome protein (WASP), an activator of the ubiquitous actin nucleation factor Arp2/3 complex, the IQ motif containing GTPase-activating proteins (IQGAPs), and the p21-activated kinases (PAKs) (17). PAKs contain an N-terminal regulatory domain that harbors a CRIB domain and a C-terminal Ser/Thr kinase domain. Dictyostelium PAKa localizes to the cell cortex and accumulates specifically in the posterior part during chemotaxis where myosin II filaments assemble. PAKa is activated in response to cAMP presumably by binding of Rac GTPases to its CRIB domain whereupon it translocates to the cytoskeleton and negatively regulates myosin heavy chain kinases (MHCKs). This then leads to myosin II assembly in the posterior cortex (18, 19).
Here we study the CRIB domain in D. discoideum coronin, analyze the potential to interact with Rac proteins in vitro, and probe the significance in vivo. We propose that coronin through its CRIB motif can regulate the availability of Rac for activation. This has an impact on Rac’s downstream activities like the activation of PAKa and inhibition of MHCK activity.
Results
The CRIB Motif of Coronin Binds to Rac Proteins from D. discoideum.
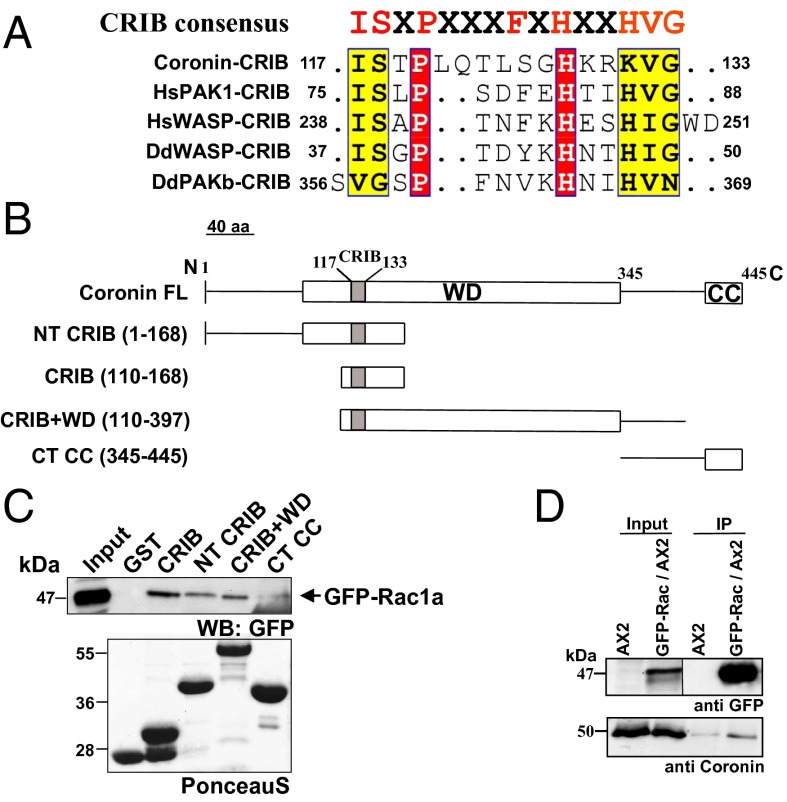
The minimal CRIB motif encompasses ∼15 amino acids (10). The binding motif is divided into two halves that are separated by a region of variable length. It contains eight core amino acids (ISXPXXXXFXHXXHVG) and tolerates one to two substitutions (20). This motif has been identified in coronins from all species (Fig. S1). In D. discoidem coronin it is located at position 117–133. The homology to CRIB motifs from other proteins is high (Fig. 1A).
Fig. 1.
The CRIB motif in coronins. (A) Sequence alignment of the CRIB domain in coronin with similar regions of selected CRIB-containing proteins. (B) Schematic diagram showing the different GST fusion polypeptides of coronin. The position of the amino acids is indicated. The modular structure of coronin with the location of the CRIB domain is shown. CC, coiled coil; WD, WD40 repeat domain. (C) Coronin polypeptides encompassing the CRIB motif interact with Rac proteins. Glutathione Sepharose beads coated with GST, GST–CRIB, GST–NT CRIB, GST–CRIB+WD, and GST–CT CC were incubated with lysates of AX2 cells expressing GFP–Rac1a (arrow). The pull-down eluates were immunoblotted with GFP-specific mAb K3-184-2 (Western blot: GFP). The molecular weights are indicated in kDa. The Lower panel shows PonceauS staining of GST fusion proteins. (D) Coronin and Rac interact in vivo. GFP–Rac1a was immunoprecipitated from wild-type AX2 cells and probed for the presence of endogenous coronin with mAb 176-3-6.
To probe its significance for Rac binding, we expressed parts of coronin with and without the CRIB motif as GST fusion proteins in Escherichia coli (Fig. 1B and Fig. S2A). The proteins were bound to Glutathione Sepharose beads and used to precipitate GFP-tagged Rac proteins from wild-type strain AX2. In these experiments, the CRIB motif containing coronin fusion proteins could precipitate GFP–Rac1a from cell lysates (Fig. 1C). The interaction appeared to be direct, as bacterially expressed Rac1a fused to GST and also GST–RacC precipitated NT CRIB, a polypeptide encompassing residues 1–168 of coronin (Fig. S2 B and C). As the CRIB motif primarily interacts with Rac proteins in complex with GTP, we examined whether the nucleotide state is important for binding and used GDP and GTPγS-loaded Rac1a and RacC GST fusions and quantified the precipitated CRIB polypeptide. We found that NT CRIB interacted with both forms nearly equally well and did not observe a preference for the nucleotide state of the Rac protein (Fig. S2 B and C). When we expressed the CRIB motif as GFP fusion (GFP–cor CRIB) in AX2 cells, it was distributed throughout the cytosol and relocated to cellular extensions (Fig. S2D). This behavior resembled the one of the isolated CRIB motifs expressed as GFP fusions such as the one of PAK1 that localized to leading edges of migrating cells and was used as a probe to monitor the distribution of active Rac (21, 22). For comparison, GFP–cor WT was also present in the cytosol and strongly accumulated in new extensions (Fig. S2D).
Endogenous Coronin Interacts Primarily with GDP Bound Rac.
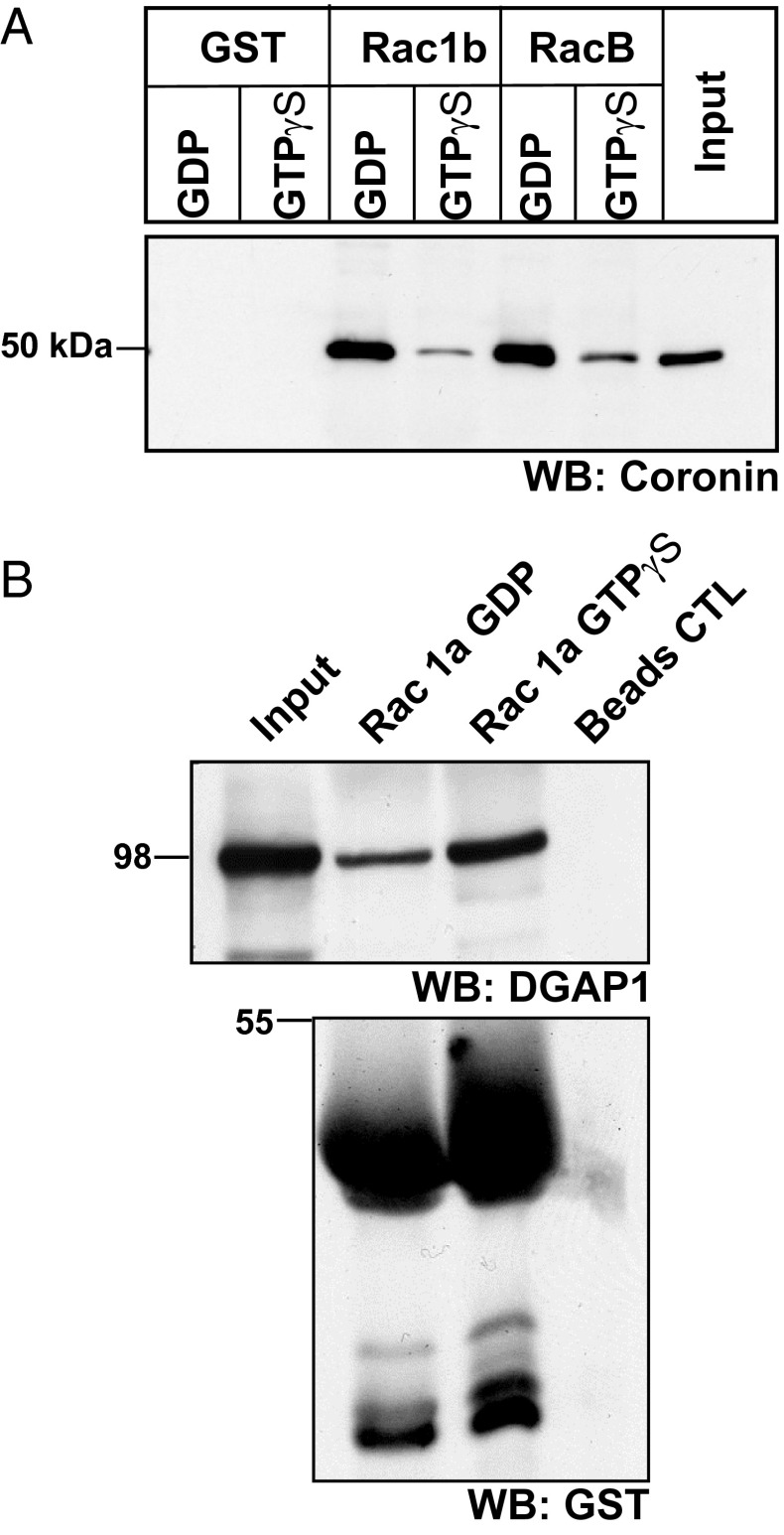
To investigate the interaction of full-length coronin with Rac proteins, we carried out coimmunoprecipitation experiments using wild-type cells expressing GFP–Rac1a. In our coimmunoprecipitation experiments, we found that GFP–Rac1a could precipitate endogenous coronin, suggesting complex formation in vivo (Fig. 1D). We further investigated the nucleotide specificity of the interaction using pull-down assays. Here, we used GST fusions of Rac1b and RacB preloaded with GDP or GTPγS to pull down endogenous coronin from AX2 cell lysates and probed the presence of coronin with mAb 176-3-6 (1). We detected coronin in the precipitates of GST–Rac1b and GST–RacB. For both Rac proteins the interaction with coronin was more efficient in the case of the GDP loaded form (Fig. 2A). In a control experiment we analyzed the interaction of Rac1a with Dictyostelium IQGAP-related protein 1 (DGAP1), an IQGAP-related protein that was previously shown to interact preferentially with the activated GTP-bound form of Rac1a (22). Consistent with previous data, DGAP1 was precipitated by GDP and GTP-charged Rac1a GST fusions with higher enrichment in the pellet of GTP-loaded Rac1a (Fig. 2B). We also probed the interaction of GST-fused RacA, RacC, and RacE loaded with GDP or GTPγS with coronin and found that primarily GDP-loaded RacC could significantly precipitate coronin in this experiment, whereas the other Rac proteins were less efficient (Fig. S3).
Fig. 2.
Coronin interacts primarily with GDP-bound Rac. (A) GST, GST–Rac1b, and GST–RacB bound to Glutathione Sepharose beads were loaded with GDP or GTPγS and incubated with AX2 cell lysates. After repeated washing of the beads, the bound proteins were immunoblotted with anticoronin antibody mAb 176-3-6 (WB: Coronin). (B) In vitro Rho GTPase interaction assay. GST–Rac1a bound to Glutathione Sepharose beads was loaded with GDP or GTPγS and incubated with AX2 cell lysates, and the bound proteins were separated on a SDS-polyacrylamide gel (10% acrylamide), transferred to nitrocellulose membrane, and probed for DGAP1 using mAb 216-394-1 (WB: DGAP1). The GST fusion proteins were detected with polyclonal GST antibodies (WB: GST). Beads were used for control (Beads CTL). The molecular weights are indicated in kDa on the left.
Impact of Mutations in the CRIB Motif on Rac Binding and in Vivo Functions of Coronin.
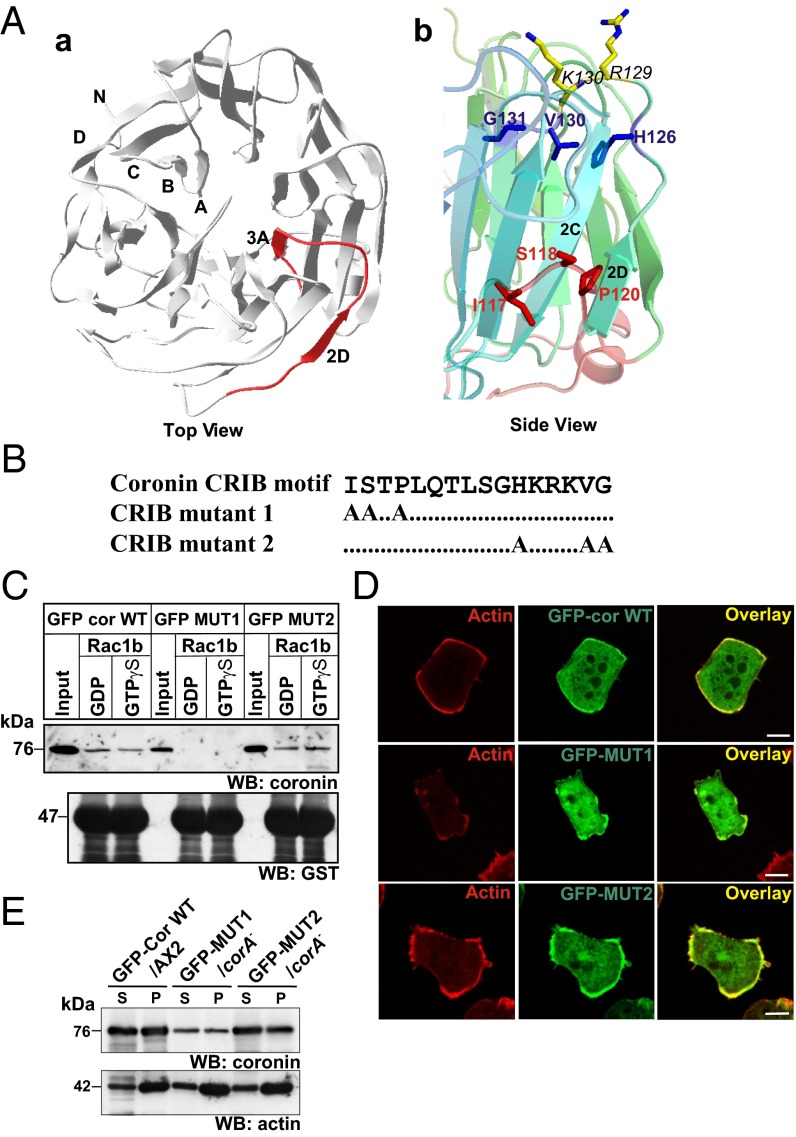
To gain further insight into the structure and position of the CRIB motif in coronin, we modeled the structure of D. discoideum coronin using coronin1A [Protein Data Bank (PDB) ID code 2AQ5] structural coordinates as a template. The CRIB motif is located between blades 2 and 3 of the beta propeller domain. Based on the location of the CRIB motif, at least the first half of the motif is clearly surface accessible, whereas the second one is more embedded in the protein (Fig. 3 A, a and A, b). To assess the significance of the CRIB motif in a protein, deletions of the whole motif are commonly generated, whereas point mutations are less frequently used (23, 24). Here, we changed the conserved residues ISXP and HXXXVG in the N- and C-terminal half of the CRIB domain to alanines by site-directed mutagenesis in a plasmid encoding GFP–cor WT, thereby generating GFP–mutant 1 (GFP-MUT1) and GFP–mutant 2 (GFP-MUT2), respectively (Fig. 3B). GFP–cor WT, GFP–MUT1, and GFP–MUT2 proteins were expressed in AX2 and the corA− strain for analysis of the Rac binding activity and the significance of the CRIB motif for coronin function. The expression levels of the GFP fusion proteins were determined by Western blotting with anticoronin antibody 176-3-6. We found that they were comparable to that of endogenous coronin in AX2 cells (Fig. S4A). Next, the Rac binding activity of the mutant proteins was probed by immunoprecipitation experiments using GFP-specific antibodies. The wild-type protein showed a preference for GDP-bound Rac1b; in GFP–MUT1 Rac binding was completely abolished, whereas GFP–MUT2 still bound Rac1b (Fig. 3C).
Fig. 3.
Expression of coronin CRIB mutant protein. (A, a) Structure of the D. discoideum coronin CRIB domain. The 3D structure of D. discoideum coronin was predicted using the SWISS-MODEL program using murine coronin 1 (PDB ID code 2AQ5) as a template. The top view of the modeled D. discoideum coronin is shown. The CRIB domain is highlighted in red, and the corresponding strands are labeled. The figure was generated using Jmol (Jmol, www.jmol.org). (A, b) Side view of the coronin CRIB motif. The mutated residues are highlighted in a ball and stick model (red and blue). Only side chains are shown for clarity. The positively charged residues adjacent to the CRIB domain (R129 and K130) are also shown. (B) Coronin CRIB mutant constructs used in the study. Conserved residues in the N-terminal and C-terminal part of the CRIB domain were mutated to alanine. (C) Interaction of CRIB mutants with Rac GTPases. GST, and GST–Rac1b bound to Glutathione Sepharose beads was loaded with GDP or GTPγS and incubated with cell lysates from GFP–cor WT/AX2, GFP–MUT1/corA−, and GFP–MUT2/corA− cells. After repeated washing of beads, the bound proteins were immunoblotted with anticoronin antibody mAb 176-3-6 (WB: coronin). The membrane was stripped and reprobed with polyclonal antibodies against GST (WB: GST). (D) Cellular localization of coronin CRIB mutants. corA− cells expressing GFP–CRIB mutants were fixed and stained for actin with mAb act1. The overlay images show colocalization of coronin and actin in the cortex. (Scale bar, 5 μm.) (E) Association of coronin CRIB mutants with the detergent insoluble cytoskeleton. Equivalent amount of GFP–cor WT/AX2, GFP–MUT1/corA−, and GFP–MUT2/corA− cells were lysed and separated into soluble and cytoskeletal fractions and immunoblotted with anticoronin antibody mAb 176-3-6 (WB: coronin). The membrane was stripped and reprobed with antiactin mAb act1 (WB: actin).
To investigate if mutation in the CRIB motif alters the localization of coronin and its oligomerization potential, we performed immunofluorescence and coimmunoprecipitation experiments. Both the wild-type and CRIB mutant GFP fusion proteins showed a similar distribution in growth phase cells being present in the cytosol and enriched at the cell cortex where actin accumulated (Fig. 3D). The cytoskeleton association of the GFP fusion proteins was further confirmed when we fractionated cell lysates into detergent-soluble and -insoluble fractions, where coronin was found in both fractions (Fig. 3E). In an experiment designed to probe the oligomerization potential of the GFP-tagged proteins, GFP–cor WT and GFP–MUT2 coprecipitated endogenous coronin from AX2 cells (Fig. S4B). Together, these data suggest that the basic properties of the coronin protein such as localization and oligomerization potential are unaffected by the CRIB mutation.
Loss of coronin from D. discoideum cells results in a cytokinesis and phagocytosis defect (7, 8). We assayed these properties in corA− cells expressing GFP–MUT1 and GFP–MUT2. Both mutant proteins could rescue the phagocytosis and multinucleation defect of the corA− strain. In a typical phagocytosis experiment, 30% of AX2 cells had ingested yeast after 15 min; in corA− this was reduced to 8%; and in corA− expressing GFP–MUT1 ∼23% and for GFP–MUT2 17% of the cells had ingested yeast (Fig. S4C). Similarly, only a few cells of the rescue strains were multinucleated. The majority of the cells (∼90%) were mono- and binucleated as opposed to the corA− strain, where more than 50% of the cells had three and more nuclei (Fig. S4D). The observation that the wild-type phenotype was not completely rescued is presumably due to the fact that the levels of expression varied from cell to cell.
Coronin Regulates Myosin II Assembly.
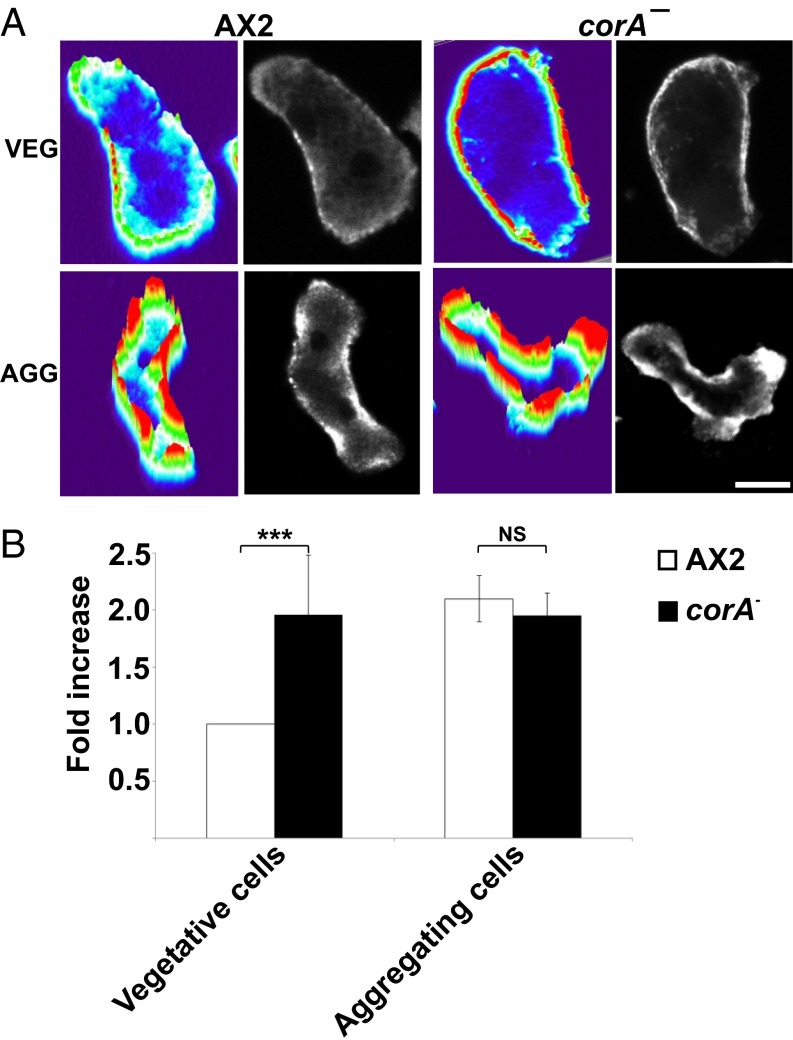
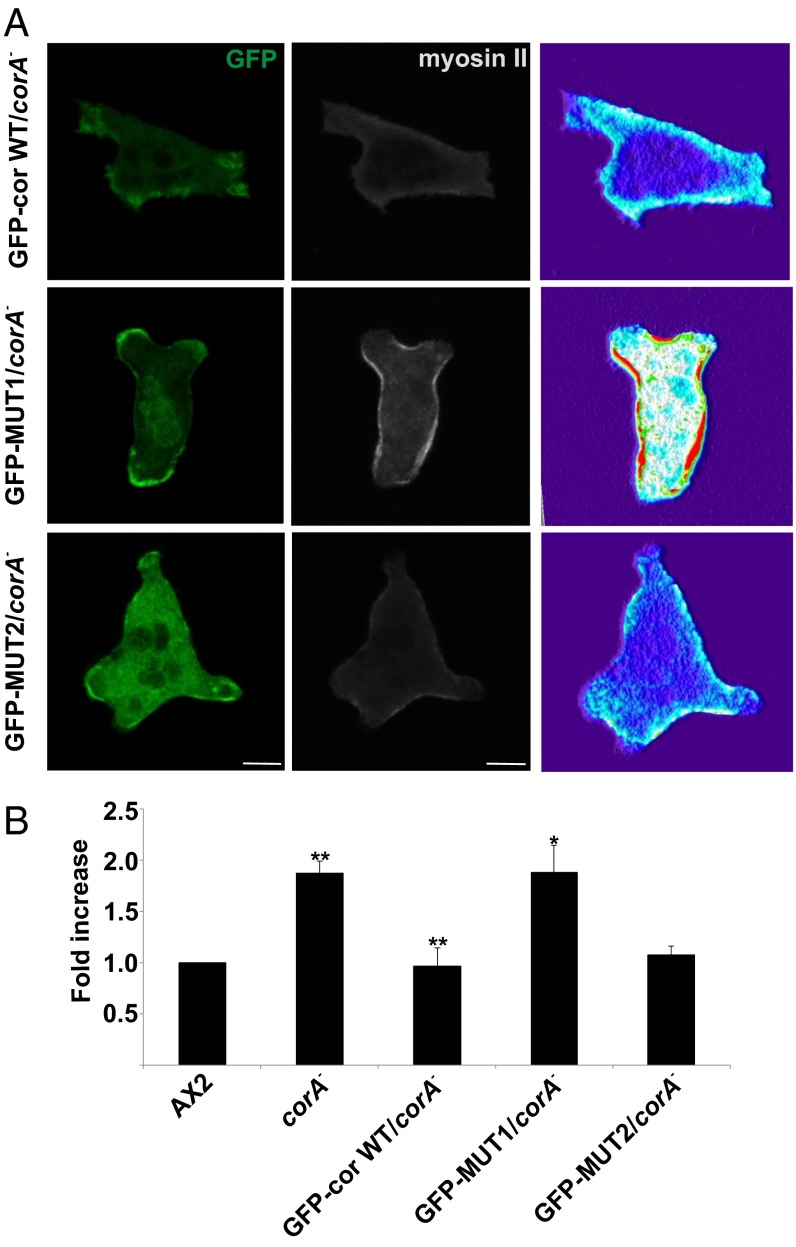
We next focused on the chemotactic behavior of the coronin mutant. Recently, we reported that in addition to the strong motility and chemotaxis defect, the corA− cells extended pseudopods in all directions during migration and were not polarized like the wild type (13). Here we focused on myosin II assembly and distribution. In aggregation-competent wild-type cells, the cytoskeleton-associated myosin II nearly doubles in amount and the protein translocates to the posterior cortex of the cells. This is achieved by a series of regulatory events that end in phosphorylation and dephosphorylation of myosin heavy chain. We stained vegetative and aggregation-competent corA− cells for myosin II and observed strong myosin II accumulation in the cell cortex in both stages. By contrast, in AX2 wild-type cells, myosin II staining in the cortex was low in vegetative cells and increased in aggregation-competent cells (Fig. 4A). A quantitative assessment showed a twofold increase of myosin II in the cytoskeletons in aggregation-competent compared with growth phase cells. Notably, in growing corA− cells, the levels of cytoskeletal myosin II were significantly higher and paralleled those of aggregation-competent AX2 cells. This level did not increase further at the aggregation stage (Fig. 4B). Upon expression of GFP–MUT1 in corA−, the myosin II levels in the cytoskeleton of vegetative cells remained high, whereas upon expression of GFP–cor WT and GFP–MUT2, it returned to the wild-type level. This was observed by myosin II immunofluorescence analysis and by quantitative determination of the cytoskeletal myosin II content during the growth phase (Fig. 5 A and B).
Fig. 4.
Dictyostelium coronin regulates myosin II assembly. (A) Vegetative (veg) and aggregation-competent (agg) AX2 and corA− cells were fixed and stained for myosin II with mAb 56-396-5 (38). Images were taken with a confocal microscope. The confocal stacks were processed with imageJ using the pseudo-3D projection plug-in. The z axis represents the intensity of myosin II over the scanned area. (Scale bar, 5 μm.) (B) Myosin II assembly levels in cytoskeletal fractions from vegetative and aggregation competent cells. The bar represents the mean and SD of six independent experiments for vegetative and two for aggregating cells (**P < 0.01; *P < 0.05; NS, not significant).
Fig. 5.
Coronin CRIB mutant fails to rescue the myosin II defect and prevents myosin II overassembly. (A) corA− expressing GFP–cor WT, GFP–MUT1, and GFP–MUT2 was fixed and stained for myosin II. (Scale bar, 5 μm.) (B) Myosin II assembly levels in cytoskeletal preparations from vegetative cells. The bar represents the mean and SD of three independent experiments. The experiments were carried out with surface-grown cells (**P < 0.01; *P < 0.05; NS, not significant).
PAKa Kinase as Downstream Effector of Coronin.
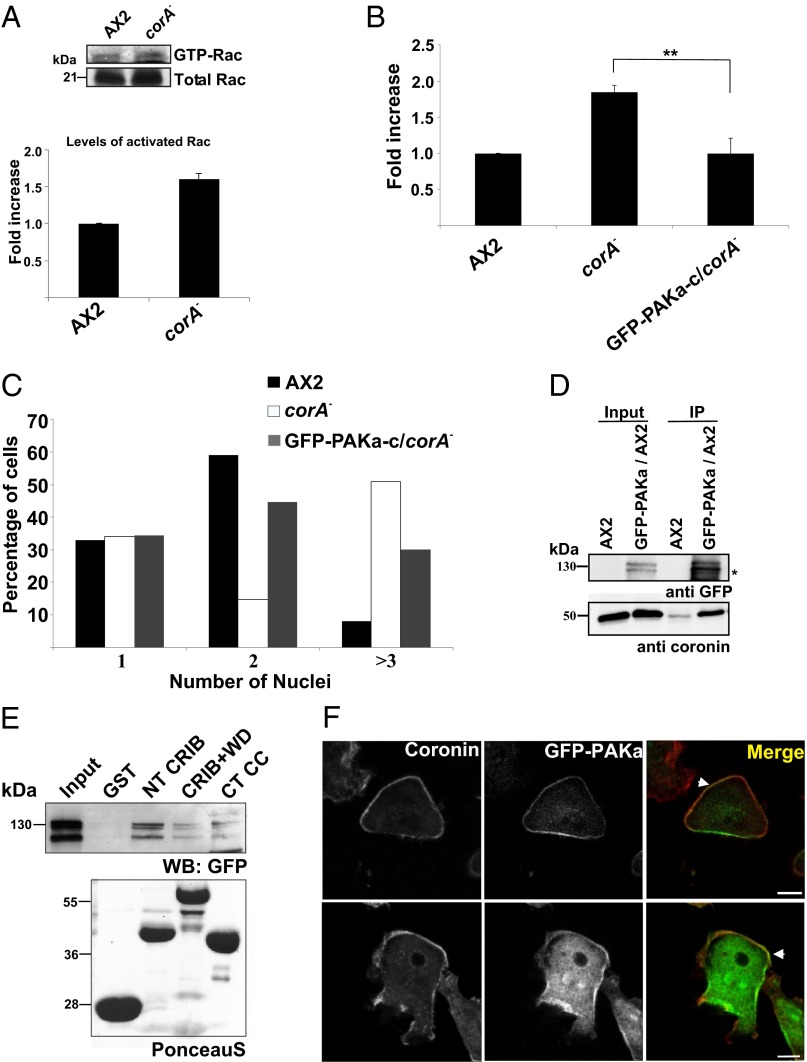
We have shown above that coronin can bind to Rac GTPases primarily in their GDP-bound form and that a mutation in the CRIB motif abolishes this activity. As coronin is quite an abundant protein, its loss could lead to an enhanced level of GTP-loaded Rac. We probed this directly in vegetative corA− cells by carrying out a pull-down assay with the GST-tagged p21 binding domain of PAK (GST–PBD—i.e., the CRIB domain of PAK) from rat that interacts with activated Racs in cell lysates (21). Precipitated Rac was revealed by probing with polyclonal Rac antibodies. We found that the levels of activated Rac were 1.6-fold higher in the pull-downs from unstimulated corA− cells compared with control AX2 cells. The precipitated amounts were compared with the amounts of total Rac protein in the cell lysate (Fig. 6A).
Fig. 6.
Expression of GFP–PAKa–c rescues the defects in corA− cells. (A) corA− cells show increased levels of GTP–Rac. Equivalent numbers of AX2 and corA− cells were lysed and incubated with GST–PBD. After repeated washing, bound proteins were immunoblotted with anti-Rac1 polyclonal antibodies. The bar chart shows the fold increase in activated Rac. n = 3. (B) Myosin II assembly levels in cytoskeletal preparations from vegetative cells. The bar represents the mean and SD of three independent experiments. The values for corA− were taken from Fig. 5B (**P < 0.01; *P < 0.05; NS, not significant). (C) Quantification of nuclei. Cells were collected from Petri dishes and fixed with methanol, and nuclei were stained with DAPI. Nuclei in more than 500 cells of each strain were quantified. (D) Coronin interacts with PAKa in vivo. Equivalent amounts of AX2 and GFP–PAKa–expressing AX2 cells were lysed and incubated with GFP–TRAP beads and probed for coprecipitated coronin with mAb 176-3-6. The star indicates a proteolytic breakdown product. (E) Coronin interacts with PAKa in vitro. Equivalent amounts of GST, GST–NT CRIB, GST–CRIB+WD, and GST–CT CC bound to Glutathione Sepharose beads were incubated with cells expressing GFP–PAKa. Bound proteins were immunoblotted with anti-GFP mAb K3-184-2 (WB: GFP). In the Lower panel, the PonceauS stained GST fusion proteins are shown. The molecular weights are indicated. (F) Coronin colocalizes with PAKa in the cortex. Cells expressing GFP–PAKa were fixed and stained for coronin with anticoronin mAb 176-3-6. The regions of colocalization are shown with arrowhead. (Scale bar, 5 μm.)
PAK kinases are one of the major effectors for Rac GTPase in the cell. In Dictyostelium, there are three PAK kinase homologs present, two of which, PAKa and PAKc, have been linked to myosin II regulation (18, 19). Additionally, expression of a constitutively active PAKa version containing only the kinase domain caused elevated cytoskeletal myosin II levels, whereas a truncated dominant-negative PAKa protein (PAKa–c) composed of the CRIB and kinase domain led to reduced phagocytosis and decreased migration velocity (18, 19).
We hypothesized that the myosin II phenotype in the corA− mutant cells might be caused by an altered MHCK activity due to an overactive PAKa. Therefore, we expressed PAKa–c as GFP fusion protein (GFP–PAKa–c) in corA− cells and analyzed its effect with respect to myosin II regulation and cytokinesis. GFP–PAKa–c reduced the myosin II assembly levels in the cytoskeletal preparations of growing corA− cells to the one of the wild-type AX2 (Fig. 6B). Moreover, it also partially rescued the cytokinesis defect. Whereas ∼50% of corA− cells contained three or more nuclei, this number was reduced to ∼30% in GFP–PAKa–c–expressing cells (Fig. 6C). We also tested whether coronin interacted with PAKa in vivo by coimmunoprecipitation experiments and found that GFP full-length PAKa could precipitate endogenous coronin (Fig. 6D). To map the coronin-binding region for PAKa, we used the GST fusion peptides of coronin in a pull-down assay with wild-type AX2 cells expressing GFP–PAKa and observed that GST–NT CRIB efficiently precipitated the GFP–PAKa protein (Fig. 6E). PAKa and coronin also partially colocalized, as revealed by staining of AX2 expressing GFP–PAKa with coronin antibodies (Fig. 6F).
Reduced Kinase Activity of MHCKs in the Coronin Mutant.
In Dictyostelium, analysis of a PAKa mutant pointed toward an involvement of this protein in the regulation of myosin II assembly presumably by inhibition of MHCK (25, 26). Dictyostelium encodes four MHCK homologs (MHCK A–D). MHCK A has been shown to enhance myosin II filament disassembly through phosphorylation of threonine residues in the tail region of myosin II. MHCK B and MHCK C might act in a similar fashion to disassemble myosin II, as the expression of these kinases in wild-type background reduced the myosin II levels in the cortex (27).
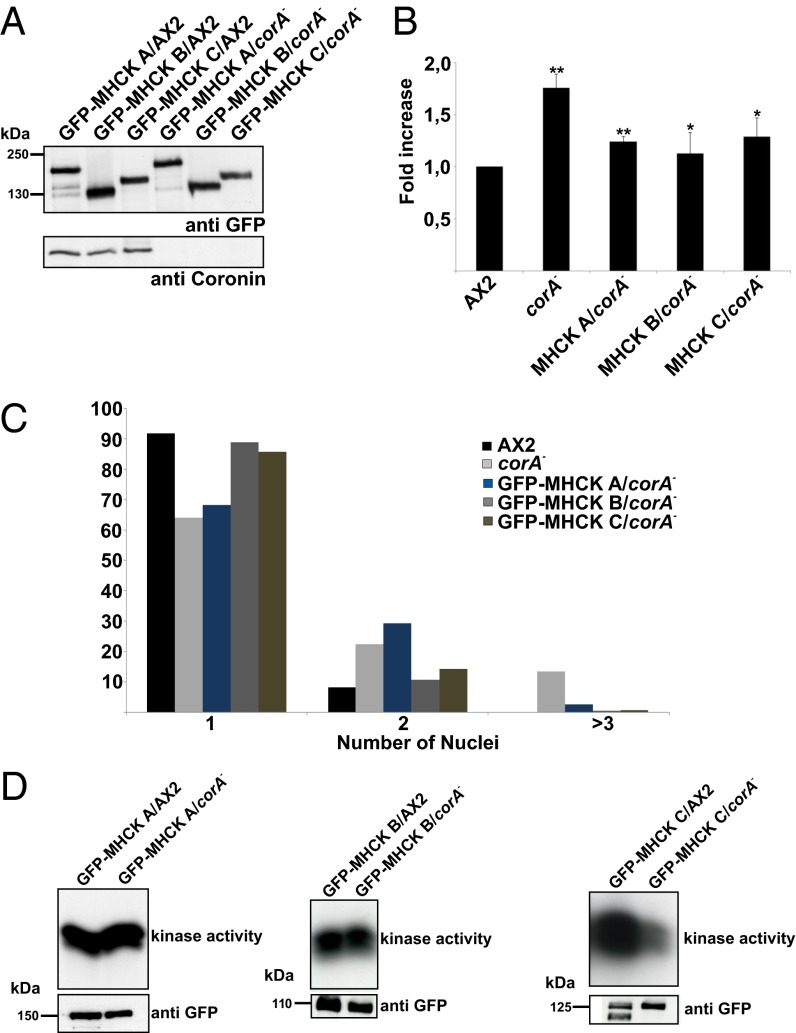
Here, we speculated that the MHCK activity is reduced in the corA− mutant, which then leads to increased myosin II assembly levels in the cortex. So we first asked if the expression of particular MHCKs could compensate for the loss of MHCK activity and rescue the myosin II phenotype in the corA− cells. To this end, we expressed GFP–MHCK A, GFP–MHCK B, and GFP–MHCK C in AX2 and corA− cells (Fig. 7A). A Western blot of the cell lysates with anti-GFP antibody showed similar expression levels of the GFP–MHCK fusion proteins in AX2 and corA− cells. We next investigated the localization of the GFP–MHCK fusion proteins in growth phase cells. In AX2, both GFP–MHCK A and GFP–MHCK C were enriched in the cortex, whereas GFP–MHCK B showed a cytosolic localization as previously reported (27). A similar cellular distribution of GFP–MHCKs was observed in the corA− strain (Fig. S5A). Myosin II assembly levels were then quantified in the corA− cells expressing GFP–MHCKs (Fig. 7B). Expression of all of the three GFP–MHCKs reduced the levels of myosin II assembly in growth phase cells, suggesting the involvement of one or more kinases in the regulation of myosin II levels in the corA− mutant.
Fig. 7.
Reduced MHCK kinase activity in coronin mutant cells. (A) GFP–MHCK expression levels were analyzed in a Western blot with mAb K3-184-2 against GFP. The molecular weights are indicated. (B) Expression of GFP–MHCKs in corA− mutant cells rescued the myosin II phenotype. Myosin II assembly levels in cytoskeletal preparations from vegetative cells are displayed. The bar represents the mean and SD of three independent experiments (**P < 0.01; *P < 0.05; NS, not significant). (C) Expression of GFP–MHCKs in corA− mutant cells rescued the multinucleation defect. Cells were fixed with methanol, and nuclei were stained with DAPI. Nuclei in more than 500 cells of each strain were quantified. (D) Kinase assay. GFP–MHCK A, GFP–MHCK B, and GFP–MHCK C were immunoprecipitated from growth phase AX2 and corA− cells. The kinase activities of the purified GFP–MHCKs were then measured after 10 min incubation with H2B substrate using autoradiography.
As Dictyostelium MHCKs are also involved in the regulation of cytokinesis (27), we determined the number of nuclei in corA− cells expressing GFP–MHCKs. Expression of GFP–MHCK B and GFP–MHCK C completely rescued the multinuclearity defect of corA− (14% in corA−, 0.5% in GFP–MHCK B/corA−, and GFP–MHCK C/corA−), whereas expression of GFP–MHCK A in the corA− strain showed a less efficient rescue (2.5%) (Fig. 7C).
As MHCK B and C rescued the myosin II defect and multinuclearity, we further performed kinase assays to investigate if the increased myosin II assembly levels in corA− are directly due to reduced activity of these kinases. We immunoprecipitated GFP–MHCK A, GFP–MHCK B, and GFP–MHCK C from vegetative AX2 and corA− cells using GFP antibodies bound to agarose beads. The precipitated GFP–MHCKs were further incubated with histone H2B as a substrate, and the basal kinase activity was determined after a 10 min incubation time. Both GFP–MHCK A and GFP–MHCK B showed similar basal kinase activity when immunoprecipitated from AX2 and corA− cells. In contrast, GFP–MHCK C immunoprecipitated from corA− showed a reduced basal kinase activity (Fig. 7D).
To find out which one of the MHCKs is regulated by PAKa, we expressed GFP–PAKa–c in MHCK knockout cells (mhck A−, mhck C−, mhck D−) and investigated the levels of myosin II assembly. We found increased myosin II assembly levels in growth phase cells of mhck A− and mhck C− as reported before (27). Upon ectopic expression of GFP–PAKa–c, both mhck C− and mhck D− mutant cells showed a higher sensitivity to the expression of GFP–PAKa–c with increased myosin II assembly, whereas the mhck A− mutant cells showed no difference (Fig. S5B). This suggests that MHCK A and MHCK C are possibly targets of PAKa in myosin heavy chain regulation acting downstream to coronin (Discussion).
Discussion
A link between Rac GTPases and coronins has been suggested by earlier work. We reported that in Swiss 3T3 cells the localization of human Coronin 1C (Coronin3, CRN2) was strongly influenced by expression of constitutively active or inactive Rac1. This was thought to result from changes in the actin cytoskeleton caused by the different states of Rac proteins (28). Furthermore, a truncated Coronin 1C containing only the core region, which is composed of the WD repeats and lacking nearly all regions implied in F-actin colocalization, failed to localize to membranes and affected the shape of the cells. The cells exhibited an impaired spreading and adhesion to solid supports, whereas cell–cell adhesion was unaffected, leading to a rounded or spindle-like cell shape and suppressed neurite formation in neuronal cell lines (29). Similarly, truncated Xenopus coronin led to impaired Rac-mediated spreading and lamellipodia formation. On this basis, Mishima and Nishida (11) had suggested that the coronin core might directly interact with a Rac GTPase and might block signal transmission to downstream effectors.
We investigate here the putative CRIB motif in coronin and show in our in vitro studies that it binds to various Rac proteins from D. discoideum, revealing a higher affinity for the GDP-loaded form compared with the GTP-loaded Rac. Furthermore, our studies show that coronin presumably acts as a regulator of PAK by sequestering GDP–Rac, preventing it from becoming activated and activating its downstream target PAK. This report demonstrates a role for CRIB motifs in coronin functions.
The CRIB motif has been identified in a subset of Cdc42 and Rac effectors. It is comprised of a short sequence of ∼15–16 residues, which makes contact with the switch I and switch II regions of the GTPase. In general, six positions in the CRIB motif are well conserved, among them two histidines located in the C-terminal half. These residues and an adjacent alpha-helix appear to mediate sensitivity to the nucleotide switch (20). The CRIB motif binds preferentially to the GTP-loaded GTPase and exhibits reduced binding activity when the GDP form is used. In coronin, the CRIB motif, although being well conserved, is not followed by an alpha-helix as the CRIB motifs of WASP, activated Cdc42 kinases (ACK), or PAK65 (20); instead, it is embedded into beta sheets. This and the location in the molecule might explain the observed preference for GDP-bound Rac.
There have also been reports that both forms are accepted by a particular CRIB. For example, the CRIB domain in a plant Rop-GTPases activating protein (RopGAP) interacts with GTP- and GDP-bound Rop1. The authors argued that this domain binds to the transitional state of the GTPase and supported this by creating a transitional state from GDP-bound Rop1 using aluminum tetrafluoride, which interacted with its target (24). The degree of conservation among the CRIB motifs is also more variable than previously thought. In a recent study, two CRIB motifs have been discovered in the phospholipase D2, which overlaps with the Pleckstrin homology (PH) domain. Although they are highly divergent, both of them appear to be active and bind Rac2. The preference for active Rac2 is somewhat higher compared with GDP-bound Rac2 (30). Another example is plenty of SH3s protein 2 (POSH2), a really interesting new gene (RING) finger E3 ligase, which binds Rac1 through a partial CRIB domain in which only the highly conserved residues ISXP in the N-terminal half are present. Mutation of all three residues or of individual residues into other amino acids reduced the Rac1 binding but did not abolish it. POSH2 is involved in c-Jun N-terminal kinases (JNK) activation presumably by serving as a scaffold for a multiprotein complex that transduces signals from GTP-loaded Rac1 to JNK. This activity was only moderately reduced by a mutant POSH2 with a mutation in the half CRIB domain (31).
In our analysis of the coronin CRIB domain, the surface-exposed N-terminal half appears to be more relevant for the Rac interaction as its mutation leads to a loss of binding activity. The mutated coronin proteins also revealed the importance of the domain for a particular function of coronin, namely its involvement in the regulation of the myosin II cytoskeleton.
The role of CRIB motifs has been well studied in the PAK serine/threonine kinases and in the WASP family proteins. Activation of PAK signals to the cytoskeleton, where the CRIB motif links the membrane receptor signaling to the actin cytoskeleton, which finally regulates cell polarity. When WASP, a regulator of the actin cytoskeleton, is released from its autoinhibited form by, for example, binding of GTP–Cdc42, it interacts with the Arp2/3 complex to initiate the polymerization of actin filaments (32).
Because we identified a myosin II phenotype in corA− cells, we focused here on PAKa as an effector of Rac. In mammalian cells, a downstream effector of PAK is the myosin II light chain kinase whose activity is down-regulated after phosphorylation by PAK1. This results in reduced myosin II activity (33). Like mammalian cells, myosin II assembly in D. discoideum is also regulated by phosphorylation of its heavy chain (34). MHCKs phosphorylate the protein in the tail region, which leads to disassembly of the myosin II filaments and release of myosin II from the cell cortex. The activity of the MHCKs is subject to regulation by various mechanisms. In particular, Chung and Firtel (18) suggested that PAKa affects myosin II assembly in response to cAMP signaling, whereby PAKa does not phosphorylate myosin II directly but regulates it in a negative fashion through regulation of MHCK. In PAKa-null cells the level of cytoskeletal myosin II was reduced to ∼65% of the wild-type level, and constitutively active PAKa led to enhanced cytoskeletal myosin II already in growth phase cells. PAKs are activated by GTP-bound Rac proteins, and for PAKa, binding to several Racs including Rac1a and Rac1b has been shown. For this interaction, the CRIB domain was responsible (16, 18, 19).
In our work we found up-regulated cortical myosin II assembly levels already in corA− growth phase cells, which exhibited a strong cortical staining for myosin II that did not increase further during development. Enhanced myosin II content in the cortex suggested that MHCK is not active and cannot phosphorylate myosin II heavy chain and release it from the cortex. MHCK inactivation might be achieved by an overactive PAKa. PAKa, like myosin II, resides in the cortex during aggregation. Its activity is regulated by active Rac GTPase, and we propose that in the corA− the balance between GDP-bound and GTP-bound Rac is altered due to the loss of coronin. In fact, the Rac–GTP levels are increased as demonstrated by pull-downs with the PBD of rat PAK1, which binds only activated Rac (21). This leads to an overactive PAKa in the cytoskeleton, which inhibits MHCK, resulting in overassembly of myosin II in the cortex (Fig. 8). This hypothesis was further tested by expressing in corA− cells a dominant-negative version of PAKa, PAKa–c, consisting of the CRIB domain and the kinase domain. In contrast to full-length PAKa, PAKa–c is located in the cytosol (19). This results in normal myosin II assembly levels in the mutant during growth phase. As PAKa is not known to form (homo-)oligomers, it is unlikely that the PAKa protein in the cortex is influenced by PAKa–c in its activity. PAKa–c rather rescues the phenotype because it sequesters active Rac to a large extent in the cytoplasm, restoring the wild-type phenotype of PAKa activation in the cortex and proper regulation of the MHCK. Alternatively it could directly sequester PAKa substrates. In addition to this, coronin might also inhibit the activity of PAKa through its direct interaction.
Fig. 8.
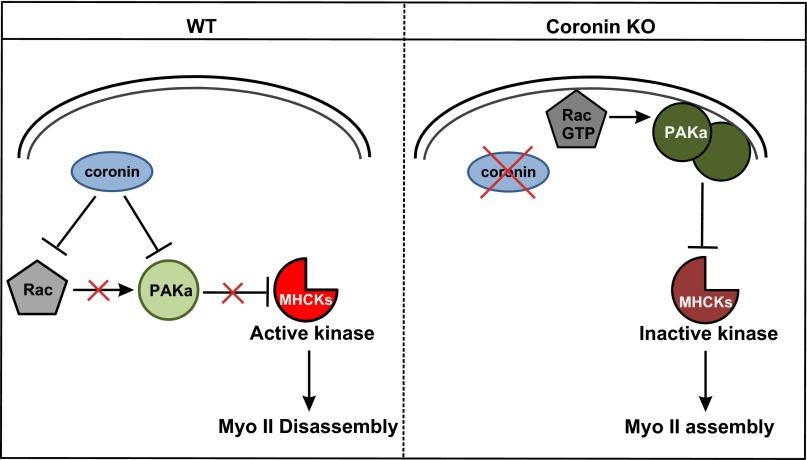
Model of coronin interaction with Rac proteins in the regulation of the myosin II cytoskeleton. In the normal wild-type situation, the coronin–Rac–GDP interaction maintains the GTPase cycle and prevents spurious activation of Rac and thereby myosin II assembly. In the knockout situation (coronin KO), the disruption of the coronin–Rac–GDP interaction leads to higher availability of Rac for activation. Rac–GTP then activates PAKa, leading to higher kinase activity, which in turn inhibits MHCK. Accumulation of unphosphorylated myosin II leads to increased myosin II assembly in the cortex.
Our model is further supported by the finding that expression of all of the three D. discoideum MHCK homologs rescued the myosin II assembly levels and cytokinesis defect in coronin mutant cells. Furthermore, we observed a reduced basal kinase activity of MHCK C immunoprecipitated from the corA− mutant. In addition, we found that expression of dominant-negative PAKa–c in mhck C− and mhck D− mutants resulted in increased myosin II assembly levels. This result is in contrast to our signaling model, in which inhibition of PAKa activity might relieve the constraint on MHCKs, leading to increased kinase activity of MHCK and myosin II disassembly. We therefore speculate that the increased myosin II assembly levels observed in mhckC− and mhckD− mutants is presumably due to the sequesteration of MHCK A by dominant-negative PAKa. Together we propose that PAKa might regulate both the stability and assembly of myosin II filaments. PAKa inhibits the activities of MHCKs (MHCK B, MHCK C, and MHCK D) and stabilizes the myosin II filaments. On the other hand, it enhances myosin II assembly through an additional signaling mechanism directly dependent on MHCK A (Fig. S6). It should be noted that both of these signaling cascades regulated by PAKa might be dependent on coronin.
Taken together, coronin can exert its functions by two ways. It can act like a Rho protein GDP dissociation inhibitor (RhoGDI) that binds GDP-bound Rho GTPases and prevents them from becoming available for activation of their downstream targets like PAK (35), or it can interact with PAKs directly to regulate their activity.
Materials and Methods
Detailed protocols of cloning, protein purification, kinase assays, immunoprecipitation, pull-down assays, and cytoskeletal fractionation are available in SI Materials and Methods.
Dictyostelium Strains.
D. discoideum strain AX2 was used as a wild-type strain. Generation of corA knockout cells has been described previously (1). All strains were grown and maintained as described (13).
Mutant Analysis.
Immunofluorescence was performed as described (13, 36). Briefly, cells were collected from Petri dishes and fixed by ice-cold methanol (5 min, –20 °C). Actin was recognized by mAb act-1 (37). For myosin II staining, aggregation-competent cells were fixed using ice-cold methanol and stained for myosin II using mAb 56-396-2 (38). Fixed cells were imaged using confocal laser scanning microscopy with a 100× objective (TCS SP5 Leica). Image processing was done with Leica LAS AF Lite Software. For surface plot rendering (Fig. 6A), scanning parameters for AX2 were used to image corA− cells, and the image stacks were processed with ImageJ plug-in for pseudo-3D representation, in which the z axis represents intensity. Phagocytosis assay for corA− and rescue cells were performed as described (13). Briefly, cells were collected from Petri dishes, an equivalent amount of TRITC labeled yeast was added to the cells, the cells were fixed after a 15 min incubation time, and the number of ingested yeast counted.
Supplementary Material
Acknowledgments
We thank Rolf Müller and Berthold Gaßen for help during the course of the experiments and Dr. Vivek Peche for helpful suggestions. We thank dictyBase for providing strains and plasmids. This work was supported by Deutsche Forschungsgemeinschaft (DFG), Sonderforschungsbereich 670 (SFB 670) and Köln Fortune (to A.A.N.). A.M.-T. acknowledges support by the SFB 914, and J.F. acknowledges support by Grant FA330/6-1 within the framework of the DFG priority programme “Principles and Evolution of Actin Nucleator Complexes” (SPP1464). Work in F.R. lab is supported by grants from the Hull York Medical School.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315368111/-/DCSupplemental.
References
- 1.de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991;10(13):4097–4104. doi: 10.1002/j.1460-2075.1991.tb04986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128(5):915–929. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L, Makhov AM, Schafer DA, Bear JE. Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell. 2008;134(5):828–842. doi: 10.1016/j.cell.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu SL, Needham KM, May JR, Nolen BJ. Mechanism of a concentration-dependent switch between activation and inhibition of Arp2/3 complex by coronin. J Biol Chem. 2011;286(19):17039–17046. doi: 10.1074/jbc.M111.219964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa-Ankerhold HC, Gerisch G, Müller-Taubenberger A. Genetic evidence for concerted control of actin dynamics in cytokinesis, endocytic traffic, and cell motility by coronin and Aip1. Cytoskeleton (Hoboken) 2010;67(7):442–455. doi: 10.1002/cm.20456. [DOI] [PubMed] [Google Scholar]
- 6.Bretschneider T, et al. The three-dimensional dynamics of actin waves, a model of cytoskeletal self-organization. Biophys J. 2009;96(7):2888–2900. doi: 10.1016/j.bpj.2008.12.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Hostos EL, et al. Dictyostelium mutants lacking the cytoskeletal protein coronin are defective in cytokinesis and cell motility. J Cell Biol. 1993;120(1):163–173. doi: 10.1083/jcb.120.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G. Coronin involved in phagocytosis: Dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell. 1995;83(6):915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- 9.Appleton BA, Wu P, Wiesmann C. The crystal structure of murine coronin-1: A regulator of actin cytoskeletal dynamics in lymphocytes. Structure. 2006;14(1):87–96. doi: 10.1016/j.str.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270(49):29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 11.Mishima M, Nishida E. Coronin localizes to leading edges and is involved in cell spreading and lamellipodium extension in vertebrate cells. J Cell Sci. 1999;112(Pt 17):2833–2842. doi: 10.1242/jcs.112.17.2833. [DOI] [PubMed] [Google Scholar]
- 12.Xavier CP, Eichinger L, Fernandez MP, Morgan RO, Clemen CS. Evolutionary and functional diversity of coronin proteins. Subcell Biochem. 2008;48:98–109. doi: 10.1007/978-0-387-09595-0_9. [DOI] [PubMed] [Google Scholar]
- 13.Shina MC, et al. Redundant and unique roles of coronin proteins in Dictyostelium. Cell Mol Life Sci. 2011;68(2):303–313. doi: 10.1007/s00018-010-0455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivero F, Dislich H, Glöckner G, Noegel AA. The Dictyostelium discoideum family of Rho-related proteins. Nucleic Acids Res. 2001;29(5):1068–1079. doi: 10.1093/nar/29.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlahou G, Rivero F. Rho GTPase signaling in Dictyostelium discoideum: Insights from the genome. Eur J Cell Biol. 2006;85(9-10):947–959. doi: 10.1016/j.ejcb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Park KC, et al. Rac regulation of chemotaxis and morphogenesis in Dictyostelium. EMBO J. 2004;23(21):4177–4189. doi: 10.1038/sj.emboj.7600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivero F, Somesh BP. Signal transduction pathways regulated by Rho GTPases in Dictyostelium. J Muscle Res Cell Motil. 2002;23(7-8):737–749. doi: 10.1023/a:1024423611223. [DOI] [PubMed] [Google Scholar]
- 18.Chung CY, Firtel RA. PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J Cell Biol. 1999;147(3):559–576. doi: 10.1083/jcb.147.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller-Taubenberger A, et al. Differential localization of the Dictyostelium kinase DPAKa during cytokinesis and cell migration. J Muscle Res Cell Motil. 2002;23(7-8):751–763. doi: 10.1023/a:1024475628061. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Manan N, et al. Structure of Cdc42 in complex with the GTPase-binding domain of the ‘Wiskott-Aldrich syndrome’ protein. Nature. 1999;399(6734):379–383. doi: 10.1038/20726. [DOI] [PubMed] [Google Scholar]
- 21.Filić V, Marinović M, Faix J, Weber I. A dual role for Rac1 GTPases in the regulation of cell motility. J Cell Sci. 2012;125(Pt 2):387–398. doi: 10.1242/jcs.089680. [DOI] [PubMed] [Google Scholar]
- 22.Faix J, et al. The IQGAP-related protein DGAP1 interacts with Rac and is involved in the modulation of the F-actin cytoskeleton and control of cell motility. J Cell Sci. 1998;111(Pt 20):3059–3071. doi: 10.1242/jcs.111.20.3059. [DOI] [PubMed] [Google Scholar]
- 23.Moskow JJ, Gladfelter AS, Lamson RE, Pryciak PM, Lew DJ. Role of Cdc42p in pheromone-stimulated signal transduction in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20(20):7559–7571. doi: 10.1128/mcb.20.20.7559-7571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu G, Li H, Yang Z. Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant Physiol. 2000;124(4):1625–1636. doi: 10.1104/pp.124.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De la Roche MA, Smith JL, Betapudi V, Egelhoff TT, Côté GP. Signaling pathways regulating Dictyostelium myosin II. J Muscle Res Cell Motil. 2002;23(7-8):703–718. doi: 10.1023/a:1024467426244. [DOI] [PubMed] [Google Scholar]
- 26.Bosgraaf L, van Haastert PJ. The regulation of myosin II in Dictyostelium. Eur J Cell Biol. 2006;85(9-10):969–979. doi: 10.1016/j.ejcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Liang W, Licate L, Warrick H, Spudich J, Egelhoff T. Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biol. 2002;3:19. doi: 10.1186/1471-2121-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spoerl Z, Stumpf M, Noegel AA, Hasse A. Oligomerization, F-actin interaction, and membrane association of the ubiquitous mammalian coronin 3 are mediated by its carboxyl terminus. J Biol Chem. 2002;277(50):48858–48867. doi: 10.1074/jbc.M205136200. [DOI] [PubMed] [Google Scholar]
- 29.Hasse A, et al. Coronin 3 and its role in murine brain morphogenesis. Eur J Neurosci. 2005;21(5):1155–1168. doi: 10.1111/j.1460-9568.2005.03917.x. [DOI] [PubMed] [Google Scholar]
- 30.Peng HJ, Henkels KM, Mahankali M, Dinauer MC, Gomez-Cambronero J. Evidence for two CRIB domains in phospholipase D2 (PLD2) that the enzyme uses to specifically bind to the small GTPase Rac2. J Biol Chem. 2011;286(18):16308–16320. doi: 10.1074/jbc.M110.206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kärkkäinen S, van der Linden M, Renkema GH. POSH2 is a RING finger E3 ligase with Rac1 binding activity through a partial CRIB domain. FEBS Lett. 2010;584(18):3867–3872. doi: 10.1016/j.febslet.2010.07.060. [DOI] [PubMed] [Google Scholar]
- 32.Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol. 2000;150(6):1299–1310. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283(5410):2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 34.Breckenridge MT, Dulyaninova NG, Egelhoff TT. Multiple regulatory steps control mammalian nonmuscle myosin II assembly in live cells. Mol Biol Cell. 2009;20(1):338–347. doi: 10.1091/mbc.E08-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: Regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12(8):493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faix J, Dittrich W. DGAP1, a homologue of rasGTPase activating proteins that controls growth, cytokinesis, and development in Dictyostelium discoideum. FEBS Lett. 1996;394(3):251–257. doi: 10.1016/0014-5793(96)00963-5. [DOI] [PubMed] [Google Scholar]
- 37.Simpson PA, Spudich JA, Parham P. Monoclonal antibodies prepared against Dictyostelium actin: Characterization and interactions with actin. J Cell Biol. 1984;99(1 Pt 1):287–295. doi: 10.1083/jcb.99.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagh K, Gerisch G. Monoclonal antibodies binding to the tail of Dictyostelium discoideum myosin: Their effects on antiparallel and parallel assembly and actin-activated ATPase activity. J Cell Biol. 1986;103(4):1527–1538. doi: 10.1083/jcb.103.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.