Abstract
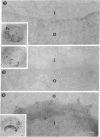
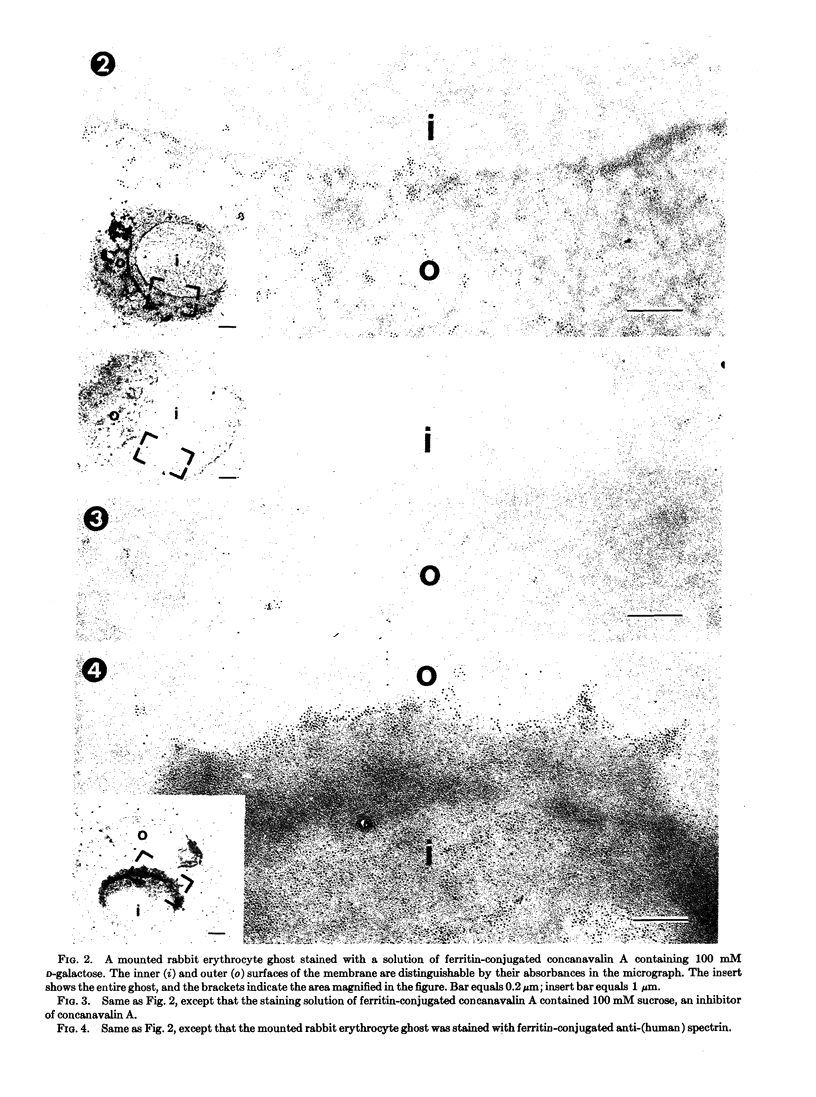
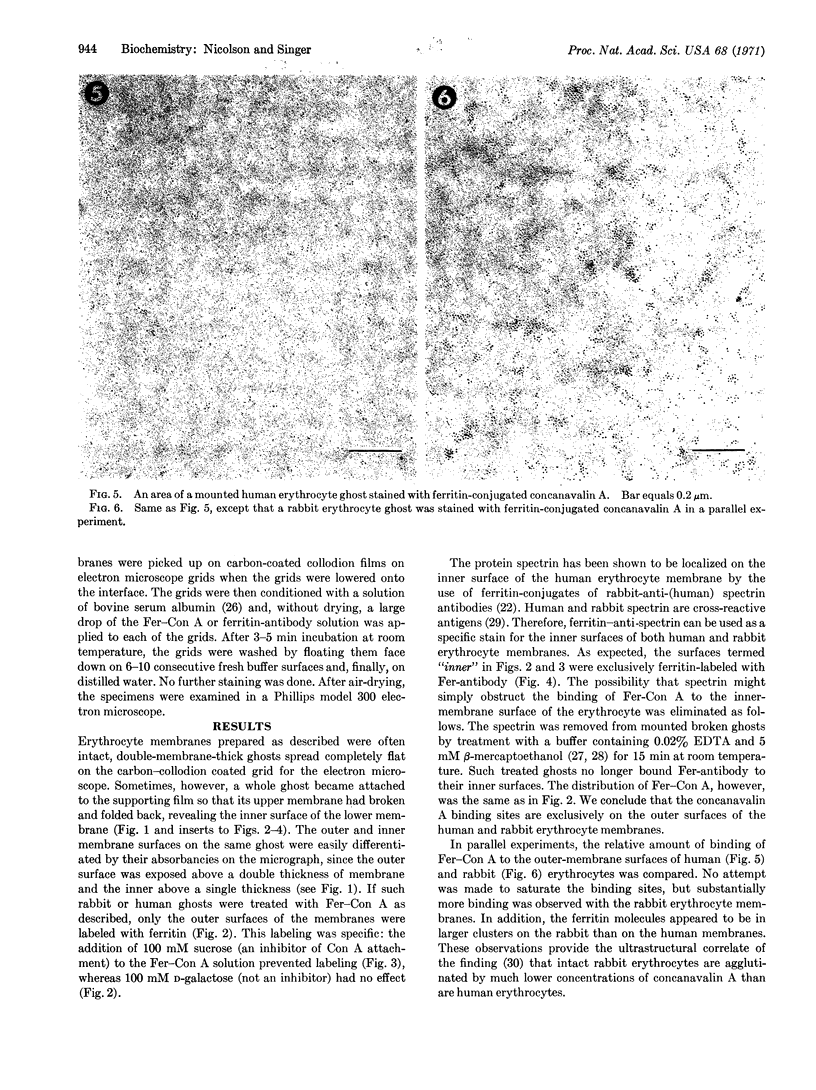
An electron microscopic stain for specific saccharides was prepared by the conjugation of ferritin to concanavalin A, a plant agglutinin that specifically binds to oligosaccharides containing terminal d-glucose, d-mannose, or sterically related sugar residues. A technique was developed to allow topological visualization of erythrocyte and other membranes by means of transmission electron microscopy, and the distribution of the binding sites for ferritin-concanavalin A on such membrane preparations was determined. The conjugate was found to bind specifically to the outer, but not the inner, surface of erythrocyte membranes. The number of conjugate molecules bound per unit area of the membrane was larger for rabbit than for human erythrocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Emmelot P. Studies on plasma membranes. IV. The ultrastructural localization and content of sialic acid in plasma membranes isolated from rat liver and hepatoma. J Cell Sci. 1967 Dec;2(4):499–512. doi: 10.1242/jcs.2.4.499. [DOI] [PubMed] [Google Scholar]
- Drysdale R. G., Herrick P. R., Franks D. The specificity of the haemagglutinin of the Castor bean, Ricinus communis. Vox Sang. 1968;15(3):194–202. doi: 10.1111/j.1423-0410.1968.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Sheppard J. R., Burger M. M. Cyclic membrane changes in animal cells: transformed cells permanently display a surface architecture detected in normal cells only during mitosis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):244–247. doi: 10.1073/pnas.68.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASIC G., BERWICK L. HALE STAIN FOR SIALIC ACID-CONTAINING MUCINS. ADAPTATION TO ELECTRON MICROSCOPY. J Cell Biol. 1963 Oct;19:223–228. doi: 10.1083/jcb.19.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASIC G., GASIC T. REMOVAL OF PAS POSITIVE SURFACE SUGARS IN TUMOR CELLS BY GLYCOSIDASES. Proc Soc Exp Biol Med. 1963 Dec;114:660–663. doi: 10.3181/00379727-114-28762. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Berwick L., Sorrentino M. Positive and negative colloidal iron as cell surface electron stains. Lab Invest. 1968 Jan;18(1):63–71. [PubMed] [Google Scholar]
- Imada I., Watanabe M., Matsumoto N., Morimoto H. Metabolism of ubiquinone-7. Biochemistry. 1970 Jul 7;9(14):2870–2878. doi: 10.1021/bi00816a018. [DOI] [PubMed] [Google Scholar]
- Inbar M., Rabinowitz Z., Sachs L. The formation of variants with a reversion of properties of transformed cells. 3. Reversion of the structure of the cell surface membrane. Int J Cancer. 1969 Sep 15;4(5):690–696. doi: 10.1002/ijc.2910040515. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- MARINOZZI V. Silver impregnation of ultrathin sections for electron microscopy. J Biophys Biochem Cytol. 1961 Jan;9:121–133. doi: 10.1083/jcb.9.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z. Silver impregnation methods for electron microscopy. Am J Clin Pathol. 1961 Jun;35:528–537. doi: 10.1093/ajcp/35.6.528. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Steers E., Marchesi V. T., Tillack T. W. Physical and chemical properties of a protein isolated from red cell membranes. Biochemistry. 1970 Jan 6;9(1):50–57. doi: 10.1021/bi00803a007. [DOI] [PubMed] [Google Scholar]
- McLean J. D., Singer S. J. A general method for the specific staining of intracellular antigens with ferritin-antibody conjugates. Proc Natl Acad Sci U S A. 1970 Jan;65(1):122–128. doi: 10.1073/pnas.65.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. O., Liener I. E. The association and dissociation of concanavalin A, the phytohemagglutinin of the jack bean. Biochemistry. 1967 Dec;6(12):3801–3808. doi: 10.1021/bi00864a025. [DOI] [PubMed] [Google Scholar]
- PAHLKE G. Elektronenmikroskopische Untersuchungen an der Interzellularsubstanz des menschlichen Sehnengewebes. Z Zellforsch Mikrosk Anat. 1954;39(4):421–430. [PubMed] [Google Scholar]
- Rambourg A., Leblond C. P. Electron microscope observations on the carbohydrate-rich cell coat present at the surface of cells in the rat. J Cell Biol. 1967 Jan;32(1):27–53. doi: 10.1083/jcb.32.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER S. J. Preparation of an electron-dense antibody conjugate. Nature. 1959 May 30;183(4674):1523–1524. doi: 10.1038/1831523a0. [DOI] [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHEYNINGEN H. E. CORRELATED LIGHT AND ELECTRON MICROSCOPE OBSERVATIONS ON GLYCOPROTEIN-CONTAINING GLOBULES IN THE FOLLICULAR CELLS OF THE THYROID GLAND OF THE RAT. J Histochem Cytochem. 1965 Apr;13:286–295. doi: 10.1177/13.4.286. [DOI] [PubMed] [Google Scholar]