Significance
Th17 cells are a subset of T cells that secrete the cytokine IL-17 and play a role in mucosal immunity. LPS is a bacterial product that can influence the development of Th17 responses. We find that acyloxyacyl hydrolase (AOAH), a mammalian enzyme that inactivates LPS, is uniquely expressed in a subset of colonic dendritic cells and acts to maintain dendritic cell responsiveness to LPS expressed by commensal bacteria. This dendritic cell responsiveness is required to promote Th17 responses. These data identify the ability of AOAH to modulate microbial signals that influence mucosal T cell immunity, and suggest that host pathways to handle microbial products may be targeted to modulate Th17 responses in the context of inflammation or infection at mucosal surfaces.
Keywords: T cell polarization, gut immunity, endotoxin tolerance, oral immunization, Toll-like receptor
Abstract
Interleukin (IL) 17-secreting CD4+ helper T cells (Th17 cells) are essential for host defense at mucosal surfaces, and Th17 cell dysregulation can result in autoimmunity. Exposure to microbial products, such as bacterial LPS, can affect the ability of dendritic cells (DCs) to polarize Th17 cells. Acyloxyacyl hydrolase (AOAH) is a mammalian enzyme expressed by antigen (Ag)-presenting cells that deacylates and thereby inactivates LPS in host tissues. We hypothesized that inactivation of intestinal microbiota-derived LPS by AOAH influences the ability of DCs to polarize and generate Th17 effector cells. We found that LPS-containing Gram-negative microbiota augmented the differentiation of Ag-specific Th17 cells, and identified a colonic DC subset (CD103+CD11b+ALDH−) displaying a unique capacity to both express AOAH and polarize Th17 cells. Compared with WT, these Aoah−/− colonic DCs produce less IL-6, resulting in diminished Ag-specific Th17 polarization and increased regulatory T-cell induction in vitro. Oral administration of LPS led to reduced IL-6 production from CD103+CD11b+ALDH− colonic DCs in Aoah−/− mice compared with Aoah+/+ mice, resulting in an abrogated Ag-specific Th17 response in the colon after mucosal immunization that could be rescued by systemic delivery of recombinant IL-6. These data identify the ability of AOAH to modulate microbiota signals that drive Th17 polarization and influence mucosal T-cell immunity, and suggest that host pathways to handle microbiota-derived products may be targeted to modulate Th17 responses in the context of inflammatory disorders or infection at mucosal surfaces.
Regulation of CD4+ T cells is critically important for appropriate function of the vertebrate adaptive immune system. Soon after the initial description of polarization of naïve CD4+ T cells into Th1 and Th2 populations (1), the hygiene hypothesis was proposed (2) and has since evolved to suggest that modern increases in allergic and autoimmune diseases reflect decreased infectious exposure that results in dysregulated T-cell polarization (3). LPS, the prototypical microbial product derived from Gram-negative bacteria, has been implicated as a surrogate for infectious exposure and shown to influence T-cell polarization (4, 5). In recent years, additional T-cell subsets, including interleukin 17 (IL-17)-secreting CD4+ helper T cells (Th17 cells) and regulatory T (Treg) cells (6), and the role of the commensal microbiota in regulating their development (7, 8) have been identified.
A majority of T cells are found in the gastrointestinal tract, which also harbors commensal microbes that play key roles in regulating adaptive CD4+ T-cell mucosal immunity (7, 8). More than 70% of the human microbiota is estimated to inhabit the colon (9). The presence of luminal commensals is required to accumulate Th17 cells in the lamina propria (LP) of both the colon and small intestines, sites that are particularly enriched for this subset of T cells (10–12). Segmented filamentous bacteria (SFB), Gram-positive commensals associated with the small intestinal mucosa in mice, have been shown to promote the local development of Th17 cells (13). The regulation of Th17 cells in the colon, an area particularly susceptible to infection and inflammation, is less clear.
Th17 cells are important for mucosal host defense, secreting signature cytokines, such as IL-17 (also known as IL-17A), IL-17F, and IL-22 to elicit neutrophil recruitment and antimicrobial peptide production (14). Polarization of Th17 cells from naïve CD4+ T cells is controlled by the local cytokine milieu and is critically dependent on TGF-β1 and IL-6 (15–17). In the absence of IL-6, TGF-β1 mediates generation of immunosuppressive Treg cells. This reciprocal balance of Treg and Th17 cells is critical for immune homeostasis, and if not properly regulated, Th17 cells can drive pathogenic inflammation (18).
Intestinal dendritic cells (DCs) are professional antigen (Ag)-presenting cells, characterized by cell-surface expression of CD11c and MHC II molecules, strategically positioned in the LP to interact with bacteria and subsequently migrate to mesenteric lymph nodes (mLN) and to present bacterial antigens to naïve CD4+ T cells (19). Characterization of LP DC subsets has included subdivision based on differential expression of the CD11b and CD103 integrin molecules (20, 21). CD103+ LP DCs have been proposed to migrate to mLN and initiate adaptive immune responses (22). A subset of CD103+ LP DCs expresses aldehyde dehydrogenase (ALDH) activity, which can inhibit Th17 and promote Treg cell development through the generation of retinoic acid from vitamin A (23, 24). Toll-like receptor (TLR) signaling, including TLR4 signaling by LPS, has been shown to promote Th17 polarization in vitro and expansion in the LP (5, 25, 26). Persistent exposure of DCs to LPS can lead to a period of “tolerance” to subsequent LPS signaling, characterized by down-regulation of costimulatory molecules and of antigen processing and presentation mechanisms required for activation of T cells (27–30), raising the possibility that the timing and chronicity of LPS exposure may influence the ability of DCs to drive T-cell polarization.
Acyloxyacyl hydrolase (AOAH) is a host enzyme that inactivates LPS, deacylating secondary fatty acyl chains on the lipid A moiety of LPS (31, 32). Deacylated LPS does not activate TLR4 and competitively inhibits signaling by biologically active LPS (33). Owing to accumulation of bioactive LPS in the absence of AOAH-mediated deacylation, LPS exposure induces prolonged innate immune tolerance in Aoah−/− mice (34–36). We hypothesized that AOAH, by decreasing the exposure of DCs to LPS, would influence the ability of gut DCs to drive mucosal CD4+ T-cell polarization.
In support of this hypothesis, we report here that AOAH is preferentially expressed in a unique population of colonic DCs (CD103+CD11b+ALDH−), where it drives Th17 immunity by preventing impaired IL-6 production that would otherwise occur owing to persistent exposure to commensal LPS. This effect of host LPS inactivation on T-cell polarization suggests a potential impact of AOAH function on conditions influenced by the balance of Th17 and Treg cells, including host defense and immune-mediated inflammatory diseases.
Results
Colonic LP DCs Preferentially Express AOAH.
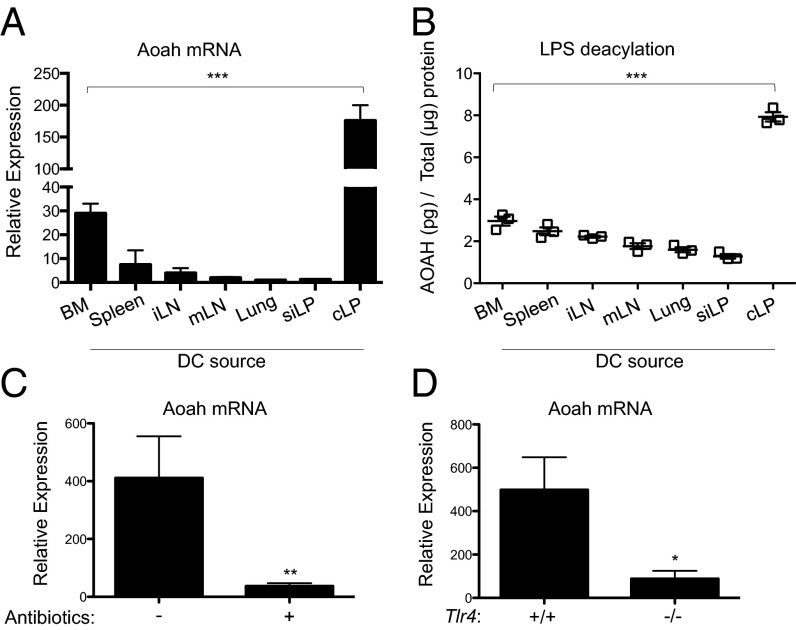
To evaluate the effect of AOAH on DC function, we first examined AOAH mRNA abundance and enzymatic activity in tissue-specific DC subsets. To extend the previously reported detection of AOAH in DCs derived from bone marrow precursors (BMDCs) and DC cell lines (37), we isolated DC-containing cell subsets expressing CD45, CD11c, and I-Ab (MHC class II) from secondary lymphoid organs and mucosal tissues (Fig. S1). AOAH mRNA abundance (Fig. 1A) and enzymatic activity (Fig. 1B) were much greater in freshly isolated DCs from the colonic LP (cLP) than in DCs from other mucosal areas, including the small intestinal LP (siLP) and lungs, or secondary lymphoid organs. Consistent with the known regulation of AOAH by LPS, ex vivo culture of cLP DCs with LPS increased the abundance of AOAH mRNA (Fig. S2A). Although LPS stimulation triggered IL-6 secretion in all DC subsets (Fig. S2B), it did not universally increase AOAH mRNA (Fig. S2A), implicating additional factors in the tissue-specific regulation of AOAH. Depletion of the Gram-negative microbiota with antibiotics (Fig. 1C) or deletion of host Tlr4 (Fig. 1D) reduced AOAH mRNA in cLP DCs by ∼90%, suggesting that commensal LPS signaling is required to drive AOAH mRNA expression in cLP. These results suggest that only specific DC subsets are programmed with the ability to express AOAH, and that cLP DCs uniquely express high levels of AOAH under homeostatic conditions through a process driven by commensal LPS signaling.
Fig. 1.
cLP DCs preferentially express AOAH. (A and B) Relative AOAH mRNA expression (A) and LPS deacylation activity (B) in DCs from bone marrow (BM) precursors, spleen, inguinal LN (iLN), mLN, lung, or siLP or cLP. (C) Relative AOAH mRNA expression in cLP DCs from WT mice on normal or antibiotic-treated water. (D) Relative AOAH mRNA expression in cLP DCs from Tlr4−/− or Tlr4+/+ mice. All DCs were obtained by sorting viable CD45+CD11c+I-Ab+ cells. Data are mean ± SD of triplicate samples and are representative of at least three independent experiments.
CD103+CD11b+ALDH− cLP DCs Uniquely Express High Levels of AOAH.
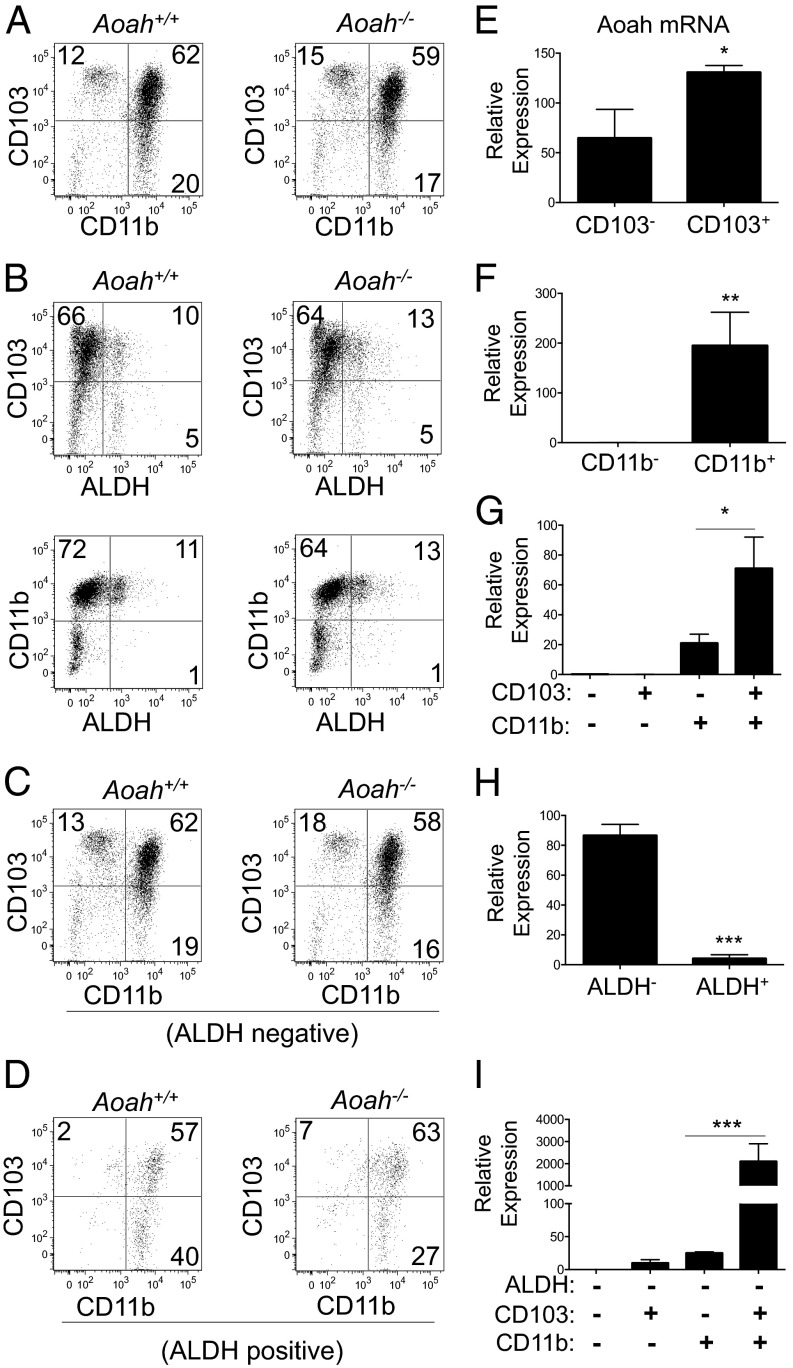
We further delineated AOAH expression within cLP DC subsets based on cell surface CD11b and CD103 integrin expression and intracellular ALDH activity. The cLP DCs (CD45+CD11c+I-Ab+) consisted of CD103+CD11b− (12%), CD103−CD11b+ (20%), and CD103+CD11b+ (62%) populations (Fig. 2A, Left). The majority (80–90%) of cLP DCs did not exhibit ALDH activity (Fig. 2B, Left), and both ALDH− (Fig. 2C, Left) and ALDH+ (Fig. 2D, Left) subsets consisted mainly of CD103+CD11b+ and CD103−CD11b+ populations. AOAH deficiency did not significantly alter the frequency of cLP DC subsets (Fig. 2 A–D, Right and Fig. S3). AOAH mRNA was enriched in sorted cLP DC populations that displayed either CD103 or CD11b (Fig. 2 E–G) or lacked ALDH activity (Fig. 2H), but was most notably associated with the CD103+CD11b+ALDH− cLP DC population (∼2,000-fold increase) (Fig. 2I).
Fig. 2.
CD103+CD11b+ALDH− cLP DCs highly express AOAH. (A–D) CD103 and CD11b expression and ALDH activity in cLP DCs from Aoah+/+ mice (Left) and Aoah−/− mice (Right) gated on viable CD45+CD11c+I-Ab+ cells (A and B) that were negative (C) or positive (D) for ALDH activity. (E–I) AOAH mRNA expression in cLP DC subsets sorted based on ALDH activity and CD103 and CD11b expression. Data are shown as flow cytometry plots or mean ± SD of triplicate samples and are representative of at least three independent experiments.
AOAH Deficiency Impairs Th17 Polarization by cLP DCs.
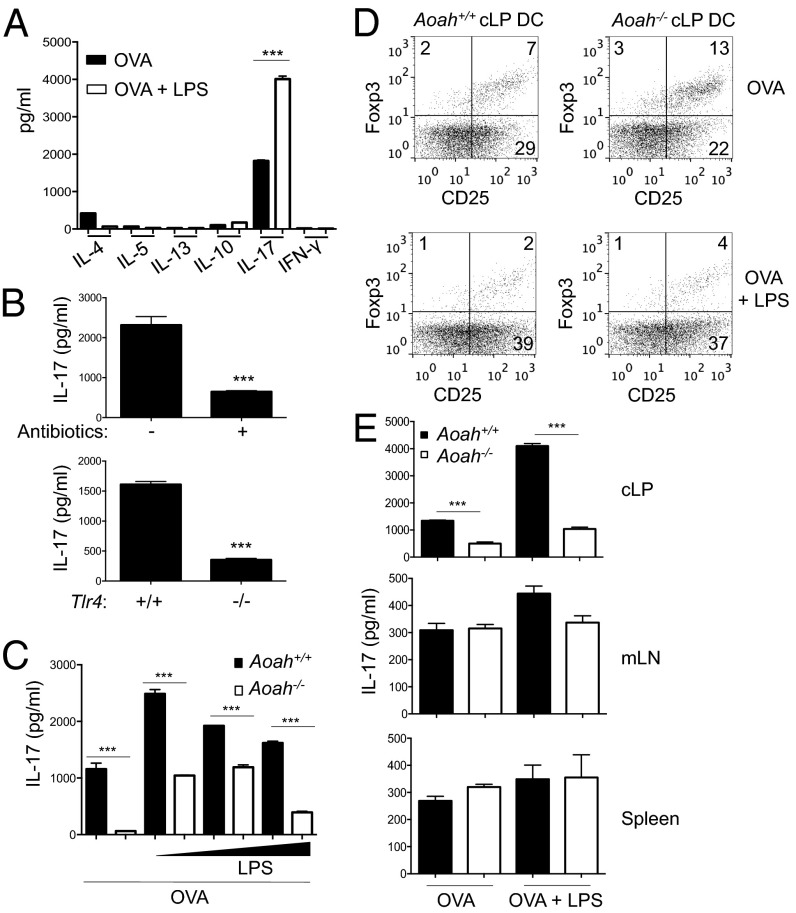
To assess the ability of cLP DCs to polarize naïve CD4+ T cells, we incubated ovalbumin (OVA)-loaded cLP DCs with naïve CD4+ T cells from OT-II mice, which express a transgenic T-cell receptor that recognizes I-Ab–restricted OVA. cLP DCs favored polarization of Th17 cells, characterized by IL-17 secretion (∼1,800 pg/mL), over Th1 (IFN-γ, undetectable) or Th2 (IL-4, 400 pg/mL) cells (Fig. 3A, black bars). LPS stimulation of OVA-loaded cLP DCs resulted in further amplification of Th17 polarization and abrogation of Th2 cytokines (Fig. 3A, white bars). Depletion of Gram-negative microbiota or Tlr4 deficiency inhibited the ability of cLP DCs to elicit Th17 polarization (Fig. 3B), but had only modest effects on Treg polarization (Fig. S4). cLP DCs from Aoah−/− mice exhibited a reduced ability to polarize OVA-specific Th17 cells compared with Aoah+/+ littermates (Fig. 3C). Aoah−/− cLP DCs were able to augment Th17 induction after LPS exposure, but not to the same extent as Aoah+/+ cLP DCs (Fig. 3C). In accordance with the reciprocal regulation of Th17 and Treg cells, Aoah−/− cLP DCs increased the induction of OVA-specific CD4+CD25+Foxp3+ Treg cells (Fig. 3D). Consistent with the expression of AOAH uniquely in cLP DCs, the effect of AOAH deficiency on Th17 polarization was not observed in DCs from other sites (Fig. 3E). Collectively, these results demonstrate that cLP DCs respond to commensal LPS exposure and subsequently drive Th17 polarization, and that AOAH-deficiency impairs this Th17 polarization ability while modestly enhancing Treg induction.
Fig. 3.
Impaired Th17 polarization capacity of AOAH-deficient cLP DCs. (A) Supernatant cytokine levels after coculturing of OT-II naïve CD4+ T cells with cLP DCs loaded with OVA (1 mg/mL) in the absence (dark bars) or presence (white bars) of LPS (400 ng/mL). (B) Supernatant IL-17 levels from OT-II cell cultures with OVA-loaded cLP DCs from WT mice receiving normal or antibiotic-treated water (Upper) or Tlr4+/+ and Tlr4−/− mice (Lower). (C) Supernatant IL-17 levels from OT-II cell cultures with Aoah+/+ (dark bars) and Aoah−/− (white bars) cLP DCs loaded with OVA and LPS (0, 4, 40 and 400 ng/mL). (D) CD4+ Treg (CD25+Foxp3+) induction from OT-II naïve CD4+ T cells after coculture with cLP DCs loaded with OVA with or without LPS (400 ng/mL), gated on viable CD4+ T cells. (E) Supernatant IL-17 levels from OT-II cell cocultures with Aoah+/+ (dark bars) and Aoah−/− (white bars) cLP, mLN, or spleen DCs loaded with OVA with or without LPS (400 ng/mL). No exogenous cytokines were added to these cocultures. Data are mean ± SD of triplicate samples or flow cytometry plots and are representative of at least three independent experiments.
CD103+CD11b+ALDH− cLP DCs Elicit Th17 Immunity That Is Dependent on AOAH.
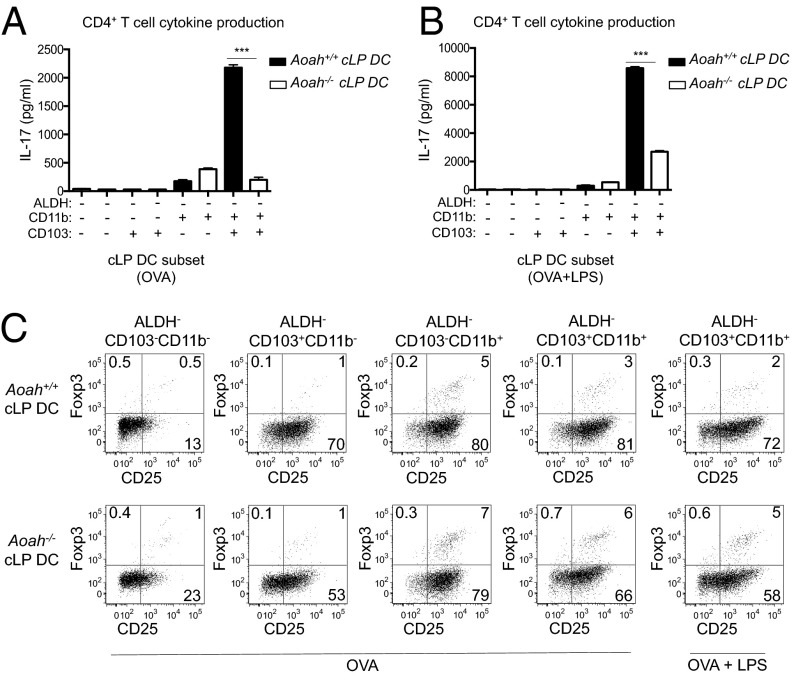
To further investigate the correlation of AOAH expression and Th17-polarizing capacity, we isolated cLP DC subsets from Aoah+/+ and Aoah−/− mice and assessed their ability to polarize naïve OT-II CD4+ T cells toward OVA-specific Th17 and Treg responses. CD103+CD11b+ALDH− cLP DCs, the subset with highest AOAH expression (Fig. 2I), were unique in their ability to polarize Th17 cells (Fig. 4A, dark bars). This same subset from Aoah−/− littermates demonstrated a diminished ability to induce IL-17 secretion (Fig. 4A, white bars). LPS stimulation augmented the ability of this DC subset from both Aoah+/+ and Aoah−/− mice to polarize Th17 cells, but the difference between AOAH-sufficient and -deficient DCs remained (Fig. 4B). The diminished ability of CD103+CD11b+ALDH− DCs to drive Th17 polarization when they lacked AOAH was reflected in their reciprocal ability to enhance Treg induction compared with Aoah+/+ DCs (Fig. 4C).
Fig. 4.
Impaired Th17 polarization ability of AOAH-deficient CD103+CD11b+ALDH− cLP DCs. Supernatant IL-17 levels (A and B) or Treg induction (C) after OT-II cell culture with indicated Aoah+/+ (dark bars, A and B) and Aoah−/− (white bars, A and B) cLP DC subsets loaded with OVA with or without LPS (400 ng/mL). Data are mean ± SD of triplicate samples or flow cytometry plots and are representative of three independent experiments.
CD103+CD11b+ALDH− cLP DCs also were able to polarize naïve T cells to secrete Th2 cytokines (IL-4, IL-5, and IL-13), but at much lower amounts than IL-17 (Fig. S5). Th1 polarization, characterized by IFN-γ, was preferentially driven by CD103+CD11b−ALDH− cLP DCs (Fig. S5), which expressed low amounts of AOAH (Fig. 2I). AOAH deficiency had minimal effects on this bias (Fig. S5). Thus, CD103+CD11b+ALDH− cLP DCs, uniquely characterized by high AOAH expression, were also uniquely capable of driving Th17 polarization, and AOAH deficiency impaired this ability.
Impaired Th17 Polarization by Aoah−/− CD103+CD11b+ALDH− cLP DCs Is Due to Low IL-6 Production.
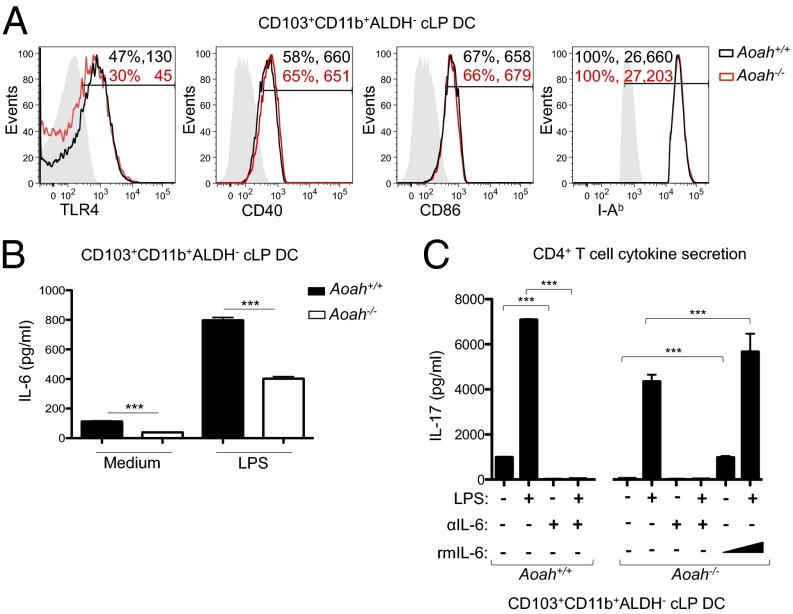
To elucidate the mechanistic basis for impaired Th17 polarization by Aoah−/− cLP DCs, we examined their expression of cell surface molecules and cytokines that could influence T-cell activation. CD103+CD11b+ALDH− cLP DCs from Aoah+/+ and Aoah−/− littermate mice similarly displayed cell surface costimulatory and antigen presentation molecules, such as CD40, CD86, and I-Ab (Fig. 5A). Both also expressed TLR4, although cLP DCs from Aoah−/− mice displayed less (Fig. 5A). Consistent with this TLR4 expression pattern and the previously reported LPS tolerance of Aoah−/− macrophages (34, 35), CD103+CD11b+ALDH− cLP DCs from both sets of mice induced IL-6 secretion in response to LPS, but the Aoah−/− DCs secreted ∼100 and 400 pg/mL less IL-6 when cultured overnight in the absence or presence of LPS, respectively (Fig. 5B). Aoah+/+ and Aoah−/− cLP DCs did not differ in their ability to produce TGF-β1 or other Th17-promoting cytokines, including IL-23, IL-1β, and TNF-α, which were detected at only low levels (Fig. S6). Neutralization of IL-6 during coculture of naïve OT-II CD4+ T cells with either OVA-loaded Aoah+/+ or Aoah−/− cLP DCs (CD103+CD11b+ALDH−) abrogated Th17 polarization, even when DCs were stimulated with LPS (Fig. 5C). The addition of recombinant murine IL-6 (rmIL-6; 100 or 400 pg/mL) completely rescued the ability of this Aoah−/− DC subset to polarize Th17 cells (Fig. 5C). These data show that the impaired Th17 polarization ability of Aoah−/− cLP DCs is the result of diminished IL-6 secretion.
Fig. 5.
Low IL-6 secretion by AOAH-deficient cLP DCs abates Th17 polarization. (A) Percentage of positive cells and mean fluorescence intensity for indicated molecules on Aoah+/+ (black) and Aoah−/− (red) CD103+CD11b+ALDH− cLP DCs. Isotype control antibodies are shaded. (B) Supernatant IL-6 from Aoah+/+ (dark bars) and Aoah−/− (white bars) CD103+CD11b+ALDH− cLP DCs cultured overnight without or with LPS (400 ng/mL). (C) Supernatant IL-17 from OT-II cell cultures with Aoah+/+ and Aoah−/− CD103+CD11b+ALDH− cLP DCs loaded with OVA with or without LPS (400 ng/mL). Indicated cultures included neutralizing antibodies against IL-6 and IL-6R (αIL-6; 20 μg/mL each) or rmIL-6 (100 or 400 pg/mL). Data are shown as flow cytometry histograms or mean ± SD of triplicate samples and are representative of at least three independent experiments.
Aoah−/− Mice Exhibit an Altered Balance of Th17 and Treg Cells.
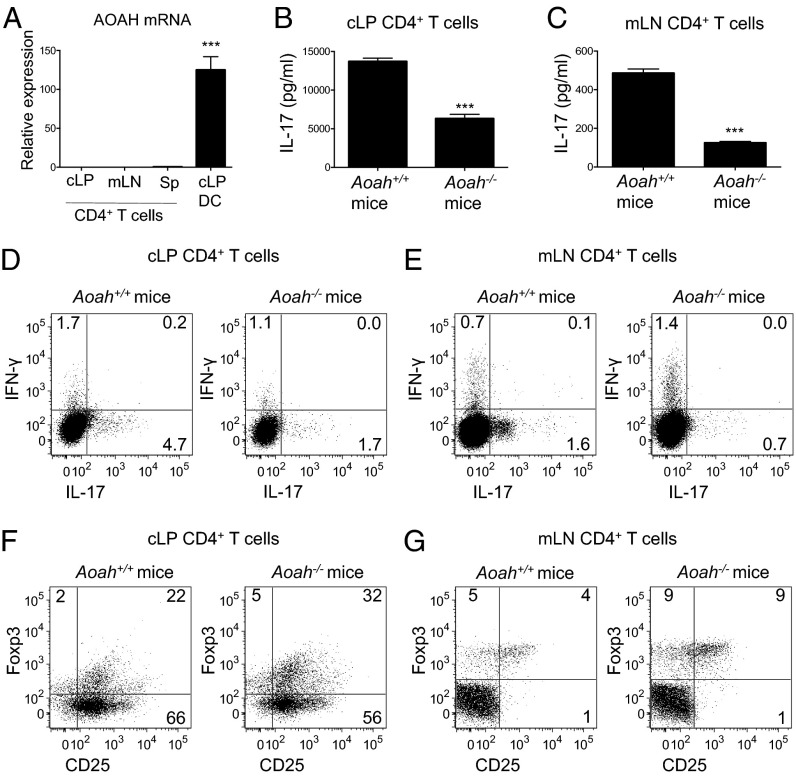
To investigate whether AOAH deficiency similarly affects T-cell polarization in vivo, we assessed the cytokine profile of CD4+ T cells isolated directly from the cLP and mLN of Aoah+/+ and Aoah−/− mice. AOAH mRNA was not detected in CD4+ T cells isolated from the cLP, mLN, or spleens of Aoah+/+ mice (Fig. 6A), implying that any differences in T-cell polarization observed in Aoah+/+ and Aoah−/− mice are likely related to the influence of Ag-presenting DC populations in vivo. TLR4 deficiency had minimal effects on Th17 and Treg frequency (Fig. S7A), possibly because of compensatory mechanisms in the KO mice. However, depletion of LPS-containing commensals with antibiotics resulted in reduced frequency of Th17 cells and increased Treg frequency in the colon (Fig. S7B), suggesting that AOAH regulation of commensal LPS may play a role in modulating Th17 polarization in vivo under homeostatic conditions. Consistent with this, CD4+ T cells from the cLP and mLN of Aoah−/− mice secreted less IL-17 (Fig. 6 B and C) and had a lower frequency of IL-17-secreting CD4+ cells (Fig. 6 D and E) compared with WT counterparts. In contrast, more CD4+ Treg cells (CD25+FoxP3+) were detected in the cLP and mLN of Aoah−/− mice (Fig. 6 F and G and Fig. S8). Collectively, these results are consistent with our ex vivo data on the impact on T-cell polarization of AOAH expression in cLP DCs, and they indicate that polarization of Th17 cells at the mucosa is impaired in vivo in the absence of AOAH.
Fig. 6.
AOAH-deficient mice have decreased mucosal Th17 cells in vivo. (A) Relative AOAH expression in cLP DCs and CD4+ T cells (CD45+CD3+CD4+) from the cLP, mLN, or spleen (Sp) of Aoah+/+ mice. (B and C) Supernatant IL-17 levels after anti-CD3/CD28 stimulation of sorted CD4+ T cells from the cLP (B) or mLN (C) of Aoah+/+ and Aoah−/− mice. (D–G) Flow cytometry of viable CD45+CD3+CD4+ T cells from the cLP (D and F) or mLN (E and G) of Aoah+/+ and Aoah−/− mice. Cytokine production (D and E) was assessed after PMA/ionomycin activation. Data are shown as mean ± SD of triplicate samples or flow cytometry plots and are representative of at least three independent experiments.
IL-6 Rescues Attenuated Th17 Responses After Oral Immunization in Aoah−/− Mice.
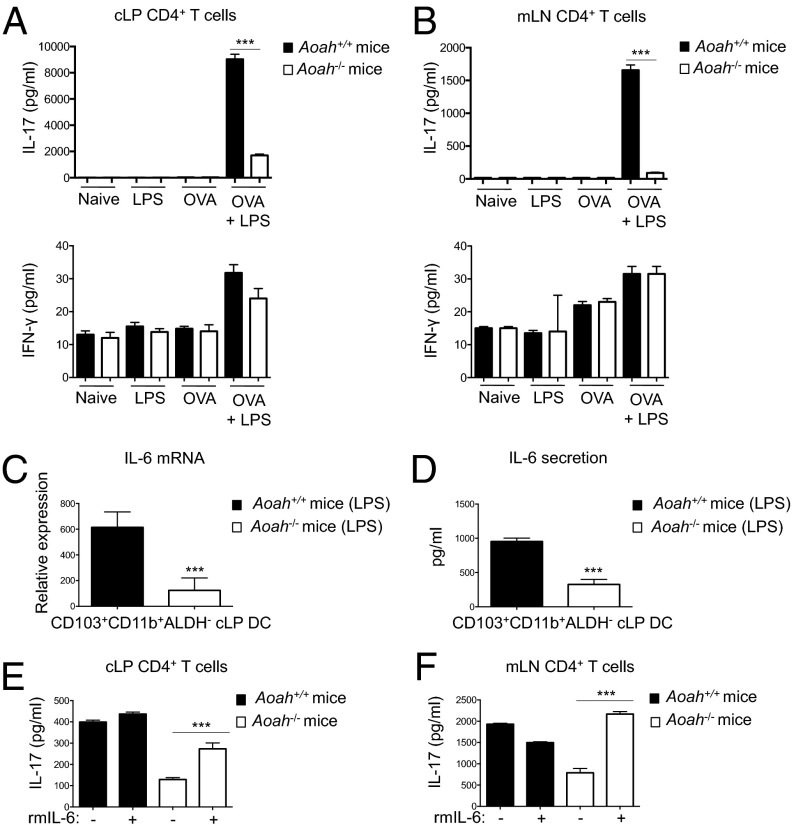
To determine whether AOAH deficiency would impair development of Ag-specific Th17 responses in vivo, we investigated the polarization of CD4+ T cells after oral immunization by gavage with OVA and LPS. CD4+ T cells isolated from the cLP (Fig. 7A) and mLN (Fig. 7B) of Aoah−/− mice immunized with OVA and LPS demonstrated significantly less OVA-specific IL-17 secretion compared with CD4+ T cells from similarly immunized Aoah+/+ mice. OVA-specific IFN-γ production was extremely low and did not differ between Aoah+/+ and Aoah−/− mice (Fig. 7 A and B, Lower), demonstrating the preferential induction of Th17 immunity after oral immunization and the specificity of AOAH to uniquely modulate Th17 bias. CD103+CD11b+ADLH− cLP DCs in Aoah−/− mice had impaired IL-6 mRNA (Fig. 7C) and protein (Fig. 7D) induction after oral delivery of LPS. Administration of recombinant IL-6 at the time of oral immunization rescued the ability of Aoah−/− mice to promote Ag-specific mucosal Th17 immunity (Fig. 7 E and F). These results suggest that AOAH function impacts the generation of Ag-specific Th17 responses in vivo after mucosal immunization by optimizing IL-6 production from Th17-skewing CD103+CD11b+ADLH− cLP DCs.
Fig. 7.
AOAH-deficient mice develop diminished Ag-specific Th17 immunity after oral immunization. (A and B) Supernatant IL-17 and IFN-γ levels from restimulated CD4+CD3+CD45+ T cells from the cLP (A) and mLN (B) of Aoah+/+ (dark bars) and Aoah−/− (white bars) mice orally immunized with OVA (250 μg) and/or LPS (50 μg) or naïve mice. (C and D) IL-6 mRNA (C) and protein (D) from CD103+CD11b+ALDH− cLP DCs harvested at 24 h after oral gavage of Aoah+/+ mice (dark bars) and Aoah−/− mice (white bars) with LPS. (E and F) Supernatant IL-17 levels from restimulated CD4+CD3+CD45+ T cells from the cLP (E) and mLN (F) of Aoah+/+ mice (dark bars) and Aoah−/− mice (white bars) orally immunized with OVA and LPS. Indicated mice received i.p. rmIL-6 with each round of oral immunization. Data are mean ± SD of triplicate samples.
Discussion
Our data identify the ability of AOAH, a host LPS inactivation mechanism, to impact mucosal T-cell polarization. The influence of microbial products, particularly commensal organisms, on the regulation and dysregulation of immunity has been increasingly recognized in recent years (7, 8), providing mechanistic insight into how factors such as those proposed in the hygiene hypothesis may contribute to the development of immune-mediated diseases. Our studies now implicate host processing of LPS by AOAH as a determinant of these previously recognized influences on T-cell function, and suggest that alterations in this host enzymatic machinery may affect T-cell-mediated health and disease.
Th17 cells have been implicated in host defense and immune-mediated pathology at mucosal sites. Induction of Th17 responses has been best described in the small intestines, where a role for commensal Gram-positive SFB has been identified (11). Commensal Gram-negative bacteria may further amplify these Th17 responses (5, 25, 26). Our studies suggest the colon as a site where LPS from Gram-negative commensal bacteria influences Th17 induction, given that depletion of Gram-negative bacteria or host Tlr4 diminished the ability of cLP DCs to polarize Th17 cells. Compared with siLP DCs, cLP DCs secreted more IL-6 in response to LPS, suggesting that the colon, with its high microbial load, may be a unique site for Gram-negative commensal LPS to drive differentiation of Th17 cells. In accordance with this idea, we found that the Th17-skewing population of cLP DCs (CD103+CD11b+ALDH−) was also the population that expressed AOAH, and that AOAH deficiency negatively impacted the ability of these DCs to polarize Th17 cells. Evocative of the impaired recovery of Aoah−/− mice and macrophages after LPS stimulation owing to persistent exposure to bioactive LPS (34, 35), the altered Th17 polarization ability of Aoah−/− cLP DCs appeared to be related to their attenuated IL-6 response to LPS. This suggests a cell-intrinsic function of AOAH to influence the ability of cLP DCs to respond to commensal LPS and drive Th17 polarization.
We demonstrate that within cLP DC subsets, AOAH expression and Th17 polarization capability is uniquely present in the ALDH− and CD11b+ fractions, and is greatly enriched in the CD103+CD11b+ALDH− population. This finding expands on the previously recognized contribution of distinct LP DC subsets to the development of effector and regulatory T-cell function in the gut necessary to maintain the balance between immunity and oral tolerance (38, 39). AOAH was minimally expressed in CD103+CD11b−ALDH− cLP DCs, and AOAH deficiency did not impact the preferential polarization of Th1 cells by this subset, suggesting that sustained responsiveness to commensal LPS may have a specific role in the regulation of Th17 immunity.
The reduced Th17 polarization seen in CD4+ T cells isolated from Aoah−/− mice reflects altered T-cell priming under homeostatic conditions in vivo that parallel the effects seen in vitro with Aoah−/− DCs. The in vivo effects of AOAH were further confirmed in the context of Ag-specific Th17 responses that developed after oral immunization with OVA and LPS. The decreased Th17 bias of Aoah−/− DCs ex vivo and Aoah−/− mice in vivo were both restored after administration of rmIL-6, identifying an impaired DC IL-6 response to commensal LPS as the mechanistic basis for altered Th17 polarization in Aoah−/− mice. The ability of LPS to drive gut-associated Th17 responses is consistent with reported effects of LPS on Th17 effector cell expansion (5). Promotion of Th17 immunity by LPS stimulation of colonic DC populations has been implicated in the pathogenesis of inflammatory bowel disease (40, 41) and host defense against colonic pathogens (42). Our data suggest that the ability of AOAH to regulate IL-6 production by Th17-skewing DCs may influence responses to LPS during immunization and infection.
In conclusion, we have demonstrated that AOAH, a host mechanism for inactivating LPS that previously was shown to limit LPS tolerance by preventing the persistence of bioactive LPS, can influence T-cell polarization in the gut. This reveals a unique mechanism of interaction between the host and its microbiota that shapes adaptive immunity. In particular, we have shown that cell-intrinsic AOAH in specific colonic DC subsets determines the ability of those DCs to respond to LPS and govern the balance of Th17 and Treg polarization. Furthermore, we have identified that commensal-derived LPS is crucial for cLP DCs to prime antigen-specific Th17 immunity in the colon, adding to the previously recognized role for Gram-positive SFB in driving Th17 polarization in the small intestine. These effects of AOAH on Th17 polarization were evident in Ag-specific immunity that developed after oral immunization, suggesting a role for AOAH function in determining T-cell–mediated host defense and disease predisposition. Indeed, recent human studies have correlated Aoah polymorphisms with rhinosinusitis and asthma (43, 44), and have identified an HLA polymorphism linked to both colitis and Aoah expression (45, 46). Thus, the influence of AOAH on mediating commensal effects on immune function identified in our murine studies may play a role in human susceptibility to disease.
Materials and Methods
Mice.
Animal experiments were conducted in compliance with the guidelines of the National Institute of Allergy and Infectious Diseases Institutional Animal Care and Use Committee. Further information is provided in SI Materials and Methods.
In Vitro CD4+ T-Cell Polarization Assays.
DC subsets sorted from spleens, mLN, or cLP of mice were incubated with endotoxin-free OVA (1 mg/mL; BioVendor) and/or 0–400 ng/mL LPS (List Biological Laboratories) before coculturing with OT-II naïve CD4+ T cells (1:10 ratio) in DMEM (Life Technologies) for 3 d at 37 °C. No exogenous cytokines were added to DC:T-cell cocultures unless specified otherwise. Some cultures included rmIL-6 (100 or 400 pg/mL) (R&D Systems) or neutralizing antibodies to murine IL-6R and soluble IL-6 (Bio X Cell). Bioplex (Bio-Rad) and ELISA (eBioscience) analyses were performed to quantify cytokines in supernatants. CD4+ Treg frequency was assessed as described in SI Materials and Methods.
Mucosal Immunization.
Mice were orally gavaged with endotoxin-free OVA (250 μg), LPS (50 μg), OVA and LPS, or sodium bicarbonate buffer (naïve mice) on day 0. Mice were boosted on day 14 and day 28. Some mice received rmIL-6 (2 μg i.p.) on days 0, 1, 14, 15, 28 and 29. At 3 d after the last boost, mLN and cLP were harvested from naïve and immunized mice, and total CD4+ T cells were isolated and sorted. CD4+ T cells were restimulated with unloaded or OVA-loaded BMDCs for 2 d to assess Ag-specific T-cell cytokine secretion by Bioplex analysis. Some mice were euthanized at 24 h after oral gavage of LPS (50 μg), and sorted CD103+CD11b+ALDH− cLP DCs were cultured overnight to assess IL-6 cytokine secretion by Bioplex analysis. Experimental groups included between three and six mice.
Statistical Analysis.
Mean values of two groups were compared using the unpaired Student t test (two-tailed) with Prism software (GraphPad). Statistically significant differences between indicated or comparator groups are indicated with ns (nonsignificant), *P < 0.05, **P < 0.001, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank R. Munford for a critical reading of the manuscript. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1311987111/-/DCSupplemental.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone, I: Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 2.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 4.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAleer JP, et al. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. J Leukoc Biol. 2010;88(1):21–31. doi: 10.1189/jlb.0909631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 10.Niess JH, Leithäuser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180(1):559–568. doi: 10.4049/jimmunol.180.1.559. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17–producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455(7214):808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19(6):377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 17.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: Intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177(5):1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13(12):1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209(1):139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz O, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206(13):3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 24.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uematsu S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9(7):769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 26.Davila E, Kolls J. A “Toll” for Th17 cell expansion. J Leukoc Biol. 2010;88(1):5–7. doi: 10.1189/jlb.0110057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009;30(10):475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita H, et al. Endotoxin tolerance attenuates airway allergic inflammation in model mice by suppression of the T-cell stimulatory effect of dendritic cells. Int Immunol. 2010;22(9):739–747. doi: 10.1093/intimm/dxq062. [DOI] [PubMed] [Google Scholar]
- 29.Wolk K, Kunz S, Crompton NE, Volk HD, Sabat R. Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J Biol Chem. 2003;278(20):18030–18036. doi: 10.1074/jbc.M207714200. [DOI] [PubMed] [Google Scholar]
- 30.Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169(9):5209–5216. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- 31.Munford RS, Hall CL. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- 32.Munford R, Lu M, Varley A. Chapter 2: Kill the bacteria...and also their messengers? Adv Immunol. 2009;103:29–48. doi: 10.1016/S0065-2776(09)03002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitchens RL, Munford RS. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270(17):9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 34.Lu M, Varley AW, Ohta S, Hardwick J, Munford RS. Host inactivation of bacterial lipopolysaccharide prevents prolonged tolerance following gram-negative bacterial infection. Cell Host Microbe. 2008;4(3):293–302. doi: 10.1016/j.chom.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu M, Varley AW, Munford RS. Persistently active microbial molecules prolong innate immune tolerance in vivo. PLoS Pathog. 2013;9(5):e1003339. doi: 10.1371/journal.ppat.1003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu M, et al. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat Immunol. 2005;6(10):989–994. doi: 10.1038/ni1246. [DOI] [PubMed] [Google Scholar]
- 37.Lu M, et al. Stimulus-dependent deacylation of bacterial lipopolysaccharide by dendritic cells. J Exp Med. 2003;197(12):1745–1754. doi: 10.1084/jem.20030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwata M, Yokota A. Retinoic acid production by intestinal dendritic cells. In: Litwack G, editor. Vitamins and Hormones. Vol 86. Waltham, MA: Academic Press; 2011. pp. 127–152. [DOI] [PubMed] [Google Scholar]
- 39.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241(1):241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogino T, et al. Increased Th17-inducing activity of CD14(+) CD163(low) myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology. 2013;145(6):1380–1391, e1. doi: 10.1053/j.gastro.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 41.Baumgart DC, et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin Exp Immunol. 2009;157(3):423–436. doi: 10.1111/j.1365-2249.2009.03981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiber HA, et al. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. J Exp Med. 2013;210(10):2025–2039. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. Polymorphisms in RYBP and AOAH genes are associated with chronic rhinosinusitis in a Chinese population: A replication study. PLoS ONE. 2012;7(6):e39247. doi: 10.1371/journal.pone.0039247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes KC, et al. Polymorphisms in the novel gene acyloxyacyl hydroxylase (AOAH) are associated with asthma and associated phenotypes. J Allergy Clin Immunol. 2006;118(1):70–77. doi: 10.1016/j.jaci.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Fairfax BP, et al. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet. 2012;44(5):502–510. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fehrmann RS, et al. Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA. PLoS Genet. 2011;7(8):e1002197. doi: 10.1371/journal.pgen.1002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.