Abstract
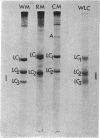
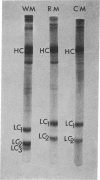
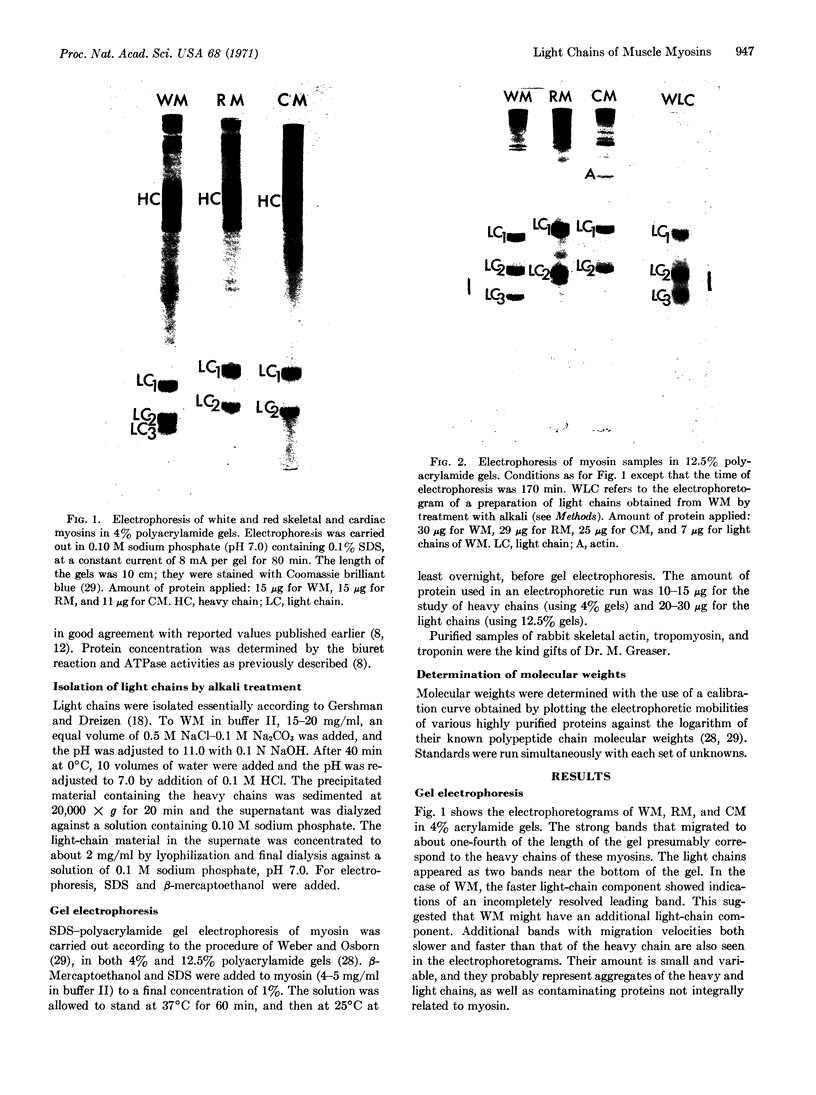
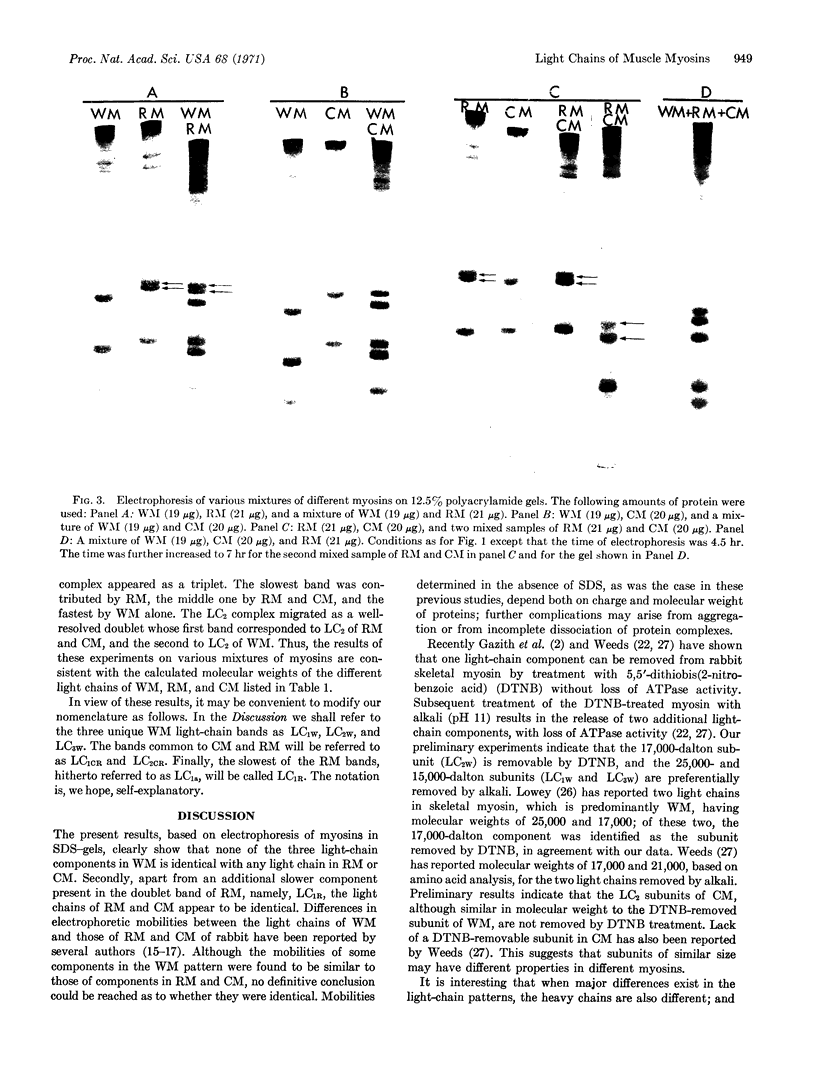
Purified preparations of rabbit skeletal white, red, and cardiac muscle myosin (WM, RM, and CM) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Significant differences in both the molecular weights and number of light chains in these myosins were found. WM has three distinct light-chain components (LC1W, LC2W, LC3W) having molecular weights of 25,500, 17,400, and 15,100, respectively. No component with a molecular weight around 15,000 is present in RM or CM. RM and CM contain components of identical molecular weights close to 25,000 and 17,000 (LC1CR and LC2CR) which, however, clearly differ in molecular weight from the corresponding subunits in WM. RM has an additional component (LC1R) having a slightly higher molecular weight than LC1W and LC1CR. Thus differences and similarities in many biochemical properties between WM, RM, and CM, which have been described earlier, are also reflected in the light-chain components. The present results support the hypothesis that different sets of genes are active in producing components of myosin that make up different isozymic forms characteristic of each muscle type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARANY M., BARANY K., RECKARD T., VOLPE A. MYOSIN OF FAST AND SLOW MUSCLES OF THE RABBIT. Arch Biochem Biophys. 1965 Jan;109:185–191. doi: 10.1016/0003-9861(65)90304-8. [DOI] [PubMed] [Google Scholar]
- Dreizen P., Gershman L. C. Relationship of structure to function in myosin. II. Salt denaturation and recombination experiments. Biochemistry. 1970 Apr 14;9(8):1688–1693. doi: 10.1021/bi00810a006. [DOI] [PubMed] [Google Scholar]
- Frederiksen D. W., Holtzer A. The substructure of the myosin molecule. Production and properties of the alkali subunits. Biochemistry. 1968 Nov;7(11):3935–3950. doi: 10.1021/bi00851a022. [DOI] [PubMed] [Google Scholar]
- Gaetjens E., Bárány K., Bailin G., Oppenheimer B. H., Bárány M. Studies on the low molecular weight protein components in rabbit skeletal myosin. Arch Biochem Biophys. 1968 Jan;123(1):82–96. doi: 10.1016/0003-9861(68)90106-9. [DOI] [PubMed] [Google Scholar]
- Gazith J., Himmelfarb S., Harrington W. F. Studies on the subunit structure of myosin. J Biol Chem. 1970 Jan 10;245(1):15–22. [PubMed] [Google Scholar]
- Gershman L. C., Dreizen P. Relationship of structure to function in myosin. I. Subunit dissociation in concentrated salt solutions. Biochemistry. 1970 Apr 14;9(8):1677–1687. doi: 10.1021/bi00810a005. [DOI] [PubMed] [Google Scholar]
- Gershman L. C., Dreizen P., Stracher A. Subunit structure of myosin, II. Heavy and light alkali components. Proc Natl Acad Sci U S A. 1966 Sep;56(3):966–973. doi: 10.1073/pnas.56.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman L. C., Stracher A., Dreizen P. Subunit structure of myosin. 3. A proposed model for rabbit skeletal myosin. J Biol Chem. 1969 May 25;244(10):2726–2736. [PubMed] [Google Scholar]
- Locker R. H., Hagyard C. J. A correlation of various small sub-units of myosin. Arch Biochem Biophys. 1967 Apr;120(1):241–244. doi: 10.1016/0003-9861(67)90628-5. [DOI] [PubMed] [Google Scholar]
- Locker R. H., Hagyard C. J. Small subunits in myosin. Arch Biochem Biophys. 1967 May;120(2):454–461. doi: 10.1016/0003-9861(67)90264-0. [DOI] [PubMed] [Google Scholar]
- Locker R. H., Hagyard C. J. The myosin of rabbit red muscles. Arch Biochem Biophys. 1968 Sep 20;127(1):370–375. doi: 10.1016/0003-9861(68)90238-5. [DOI] [PubMed] [Google Scholar]
- Locker R. H., Hagyard C. J. Variations in the small subunits of different myosins. Arch Biochem Biophys. 1967 Nov;122(2):521–522. doi: 10.1016/0003-9861(67)90230-5. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Markert C. L. The molecular basis for isozymes. Ann N Y Acad Sci. 1968 Jun 14;151(1):14–40. doi: 10.1111/j.1749-6632.1968.tb11876.x. [DOI] [PubMed] [Google Scholar]
- Murphy A. J., Morales M. F. Number and location of adenosine triphosphatase sites of myosin. Biochemistry. 1970 Mar 31;9(7):1528–1532. doi: 10.1021/bi00809a008. [DOI] [PubMed] [Google Scholar]
- Oppenheimer H., Bárány K., Hamoir G., Fenton J. Succinylation of myosin. Arch Biochem Biophys. 1967 Apr;120(1):108–118. doi: 10.1016/0003-9861(67)90604-2. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin structure as revealed by simultaneous electrophoresis of heavy and light subunits. Biochemistry. 1970 Oct 13;9(21):4094–4105. doi: 10.1021/bi00823a010. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M. K., Young M. Studies on the isolation and molecular properties of homogeneous globular actin. Evidence for a single polypeptide chain structure. J Biol Chem. 1967 Oct 10;242(19):4449–4458. [PubMed] [Google Scholar]
- Richards E. G., Chung C. S., Menzel D. B., Olcott H. S. Chromatography of myosin on diethylaminoethyl-Sephadex A-50. Biochemistry. 1967 Feb;6(2):528–540. doi: 10.1021/bi00854a022. [DOI] [PubMed] [Google Scholar]
- Samaha F. J., Guth L., Albers R. W. Differences between slow and fast muscle myosin. Adenosine triphosphatase activity and release of associated proteins by p-chloromercuriphenylsulfonate. J Biol Chem. 1970 Jan 25;245(2):219–224. [PubMed] [Google Scholar]
- Sarkar S., Cooke P. H. In vitro synthesis of light and heavy polypeptide chains of myosin. Biochem Biophys Res Commun. 1970 Nov 25;41(4):918–925. doi: 10.1016/0006-291x(70)90171-3. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Studies on myosin from red and white skeletal muscles of the rabbit. II. Inactivation of myosin from red muscles under mild alkaline conditions. J Biol Chem. 1967 Dec 10;242(23):5623–5629. [PubMed] [Google Scholar]
- Sreter F. A., Seidel J. C., Gergely J. Studies on myosin from red and white skeletal muscles of the rabbit. I. Adenosine triphosphatase activity. J Biol Chem. 1966 Dec 25;241(24):5772–5776. [PubMed] [Google Scholar]
- Stracher A. Evidence for the involvement of light chains in the biological functioning of myosin. Biochem Biophys Res Commun. 1969 May 22;35(4):519–525. doi: 10.1016/0006-291x(69)90377-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G. Light chains of myosin. Nature. 1969 Sep 27;223(5213):1362–1364. doi: 10.1038/2231362a0. [DOI] [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]
- Young M. The molecular basis of muscle contraction. Annu Rev Biochem. 1969;38:913–950. doi: 10.1146/annurev.bi.38.070169.004405. [DOI] [PubMed] [Google Scholar]