Significance
Gaucher disease (GD) is one of the most prevalent inherited metabolic storage disorders of humans. GD results from mutations in the gene coding for glucocerebrosidase (GCase) that often lead to protein misfolding and premature degradation. This study demonstrates that the small molecule celastrol prevents mutant GCase degradation by targeting the assembly of a heat-shock protein chaperone complex. Additionally, celastrol reorganizes the expression pattern of chaperone genes. Specifically, celastrol up-regulates genes coding for Heat-shock protein 70 (Hsp70), DnaJ homolog subfamily B members 1 and 9 (DNAJB1/9), and BAG family chaperone regulator 3 (BAG3). Celastrol stabilizes mutant GCase by specifically targeting BAG3. These findings provide insight into the molecular mechanisms underlying human disorders that result from abnormalities of protein stability.
Abstract
Gaucher disease is caused by mutations in the glucosidase, beta, acid gene that encodes glucocerebrosidase (GCase). Glucosidase, beta, acid mutations often cause protein misfolding and quantitative loss of GCase. In the present study, we found that celastrol, an herb derivative with known anticancer, anti-inflammatory, and antioxidant activity, significantly increased the quantity and catalytic activity of GCase. Celastrol interfered with the establishment of the heat-shock protein 90/Hsp90 cochaperone Cdc37/Hsp90-Hsp70-organizing protein chaperone complex with mutant GCase and reduced heat-shock protein 90-associated protein degradation. In addition, celastrol modulated the expression of molecular chaperones. Bcl2-associated athanogene 3 and heat shock 70kDa proteins 1A and 1B were significantly increased by celastrol. Furthermore, BAG family molecular chaperone regulator 3 assisted protein folding and maturation of mutant GCase. These findings provide insight into a therapeutic strategy for Gaucher disease and other human disorders that are associated with protein misfolding.
Gaucher disease (GD), one of the most prevalent human metabolic storage disorders, is caused by inadequate glucocerebrosidase (GCase) (1, 2). Loss of GCase activity leads to accumulation of toxic amounts of glucocerebroside and glucosylsphingosine, causing metabolic dysfunction that eventually results in hepatosplenomegaly, cytopenias, bone disease, and, in some patients, central nervous system manifestations. Mutations in the glucosidase, beta, acid (GBA) gene that encodes GCase are the most common causes of GD (3–5). These mutations commonly result in amino acid substitutions in GCase that significantly reduce protein stability without disrupting intrinsic catalytic activity (6). Changes in the GCase peptide sequence alter the conformation of the protein making it vulnerable to degradation mechanisms involving Parkin, casitas b-lineage lymphoma (Cbl), heat-shock protein 90 (Hsp90), and the endoplasmic reticulum-associated degradation (ERAD) pathway (7). Moreover, modulating the pathways involved in the folding and degradation of mutant GCase has been shown to be effective in increasing its activity (8).
Celastrol is derived from the root of Tripterygium Wilfordii (Thunder of God Vine) and Celastrus Regelii. It has been demonstrated to have antioxidant (9), anti-inflammatory (10), and anticancer (10–13) effects. Recent studies have shown that celastrol blocks protein degradation by inhibiting proteasomal function (13, 14) and prevents the degradation of mutant enzymes in certain lysosomal storage diseases (15). The molecular mechanisms by which celastrol exerts these effects, however, remain unknown. Zhang et al. (16, 17) showed that celastrol interferes with Hsp90 binding to Hsp90 cochaperone Cdc37 (Cdc37), suggesting that celastrol affects biologic processes by modulating molecular chaperones. Our recent discoveries in GD demonstrated that Hsp90 is not only a critical chaperone that assists protein folding but is also important in targeting the misfolded GCase for degradation (18, 19). Therefore, the potential therapeutic value of celastrol is of great interest in protein folding-related disorders.
We investigated the effect of celastrol on GCase folding and degradation. Using two common GBA mutations in type I (N370S/N370S) and type II/III (L444P/L444P) GD, we found that celastrol increased the catalytic activity of mutant GCase. Celastrol interfered with the recruitment of Cdc37 to Hsp90 halting the assembly of the requisite chaperone complex. Inhibition of Hsp90 reduced its recognition of mutant GCase and therefore limited the proteasomal degradation of the mutant protein. Additionally, celastrol triggered a reorganization of the gene expression pattern of molecular chaperones such as DnaJ homolog subfamily B members 1 and 9 (DNAJB1/9), heat shock 70kDa proteins 1A and 1B (HSPA1A/B), and Bcl2-associated athanogene 3 (BAG3). The presence of BAG family molecular chaperone regulator 3 (BAG3) further stabilized the nascent GCase protein and assisted its folding and catalytic activity.
Results
Celastrol Targets the Chaperone Function of Hsp90 and Inhibits Its Recognition of Mutant GCase.
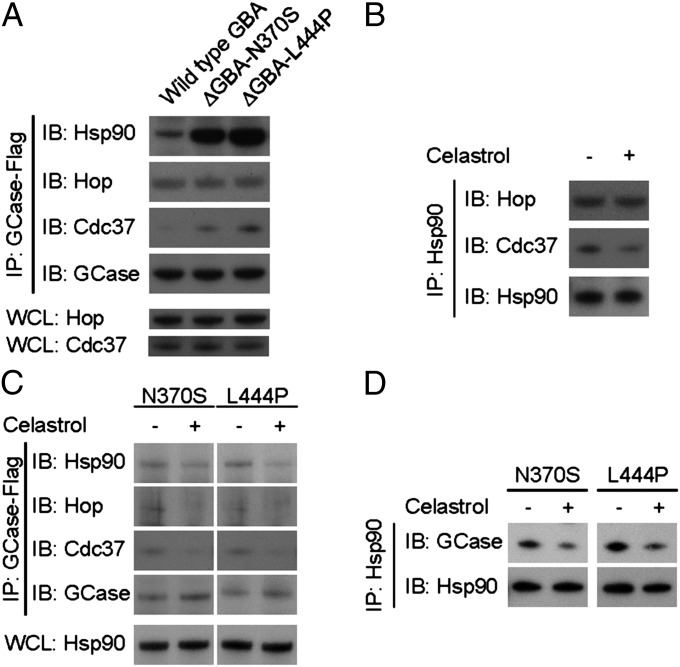
Our previous findings demonstrated that mutations in GCase result in its binding to Hsp90 (18, 19). Recognition by Hsp90 initiates protein degradation through the ERAD and valosin-containing protein (VCP)/protein 97 (p97)/proteasome pathways (7, 19–21). We confirmed that Hsp90 recognizes mutant GCase and investigated the formation of the Hsp90 chaperone complex (Fig. 1A). Consistent with previous findings, Hsp90 bound more avidly to both forms of mutant GCase versus wild-type GCase. Moreover, Cdc37, a cochaperone of Hsp90, likewise exhibited increased binding to mutant GCase. There was no observable difference in the binding of Hsp70–Hsp90-organizing protein (Hop) to mutant versus wild-type GCase.
Fig. 1.
Celastrol interferes with the formation of the Hsp90 chaperone complex and reduces its recognition of mutant GCase. (A) Immunoprecipitation demonstrated abnormal binding of the Hsp90/Cdc37/Hop chaperone complex to mutant GCases. Whole-cell lysate (WCL) was used as loading control. (B) Immunoprecipitation indicated that celastrol impaired the recruitment of Cdc37 to the Hsp90 chaperone complex. (C) Immunoprecipitation further showed that celastrol decreased recognition of the Hsp90/Cdc37/Hop complex to both N370S and L444P mutant GCases. (D) Reverse immunoprecipitation confirmed that celastrol decreased Hsp90 affinity for N370S and L444P mutant GCases.
Interfering with the function of Hsp90 has been shown to reduce the degradation of GCase (19). Zhang et al. (16) showed that celastrol interferes with Hsp90 function by occupying a specific domain in the Hsp90–Cdc37 interface. We hypothesized that recognition of GCase by Hsp90 is dependent on the establishment of the Hsp90/Cdc37/Hop complex. We hypothesized that blocking the assembly of the chaperone complex with the small molecular weight compound celastrol would inhibit Hsp90-associated GCase degradation. To test this hypothesis, we first confirmed the effect of celastrol on the assembly of the Hsp90 chaperone complex using immunoprecipitation (Fig. 1B). We found a reduction in Cdc37 binding to the Hsp90 chaperone complex in the presence of celastrol suggesting that celastrol interrupted the recruitment of Cdc37 to the protein complex. We then evaluated the impact of celastrol on Hsp90 recognition of mutant GCase (Fig. 1C). The Hsp90/Cdc37/Hop complex exhibited less affinity for both N370S and L444P GCase mutants in the presence of celastrol, confirming our hypothesis that celastrol inhibits the function of the Hsp90 chaperone complex and hence cotranslational degradation of mutant GCase. The impact of celastrol on Hsp90 recognition of mutant GCase was determined using reverse immunoprecipitation (Fig. 1D). Hsp90 exhibited less affinity for both N370S and L444P mutant forms of GCase in the presence of celastrol.
Celastrol Increases the Amount and Catalytic Activity of GCase by Reducing Protein Degradation.
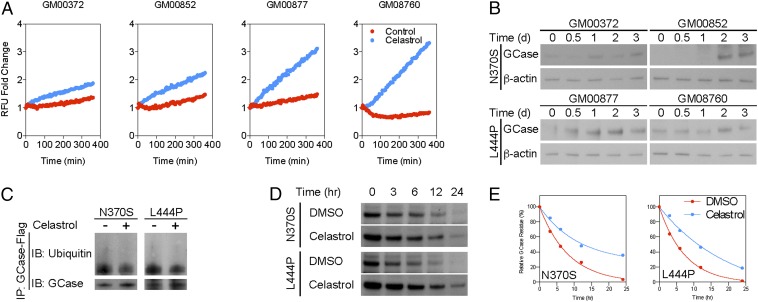
The potent inhibitory effect of celastrol on the establishment of the Hsp90 chaperone complex suggests that it may be useful for disorders related to abnormalities in Hsp90 function. GCase activity was measured in fibroblasts derived from either type I [GBA-mutant (GM) 00372 and GM00852] or type II/III (GM00877 and GM08760) GD patients using a fluorometric GCase assay. Celastrol consistently increased the catalytic activity of GCase in fibroblasts from both types of GD (Fig. 2A). In addition, a time-course study revealed that celastrol increased the amount of GCase in GD fibroblasts (Fig. 2B). Ubiquitination of both N370S and L444P GCase mutants was reduced following treatment with celastrol indicating that the increase in the amount of GCase was due to reduced protein degradation (Fig. 2C). A pulse–chase assay using cycloheximide further demonstrated that celastrol increased the stability of GCase mutants (Fig. 2 D and E).
Fig. 2.
Celastrol increases the quantity and catalytic activity of mutant GCase by reducing protein degradation. (A) Fluorometric GCase assay showed that celastrol increased catalytic activity in fibroblasts derived from type I and type II/III GD patients. (B) Western blot confirmed that prolonged exposure to celastrol also increased the amount of GCase in GD fibroblasts. (C) Immunoprecipitation further demonstrated that celastrol decreased ubiquitination of N370S and L444P mutant GCase. (D) A pulse–chase assay with cycloheximide indicated that celastrol increased the stability of N370S and L444P mutant GCases. (E) Quantitative analysis of the pulse–chase assay confirmed that celastrol increased the half-life GCase mutants.
Celastrol Induces a Heat-Shock Response and Reorganizes the Expression of Chaperone Genes.
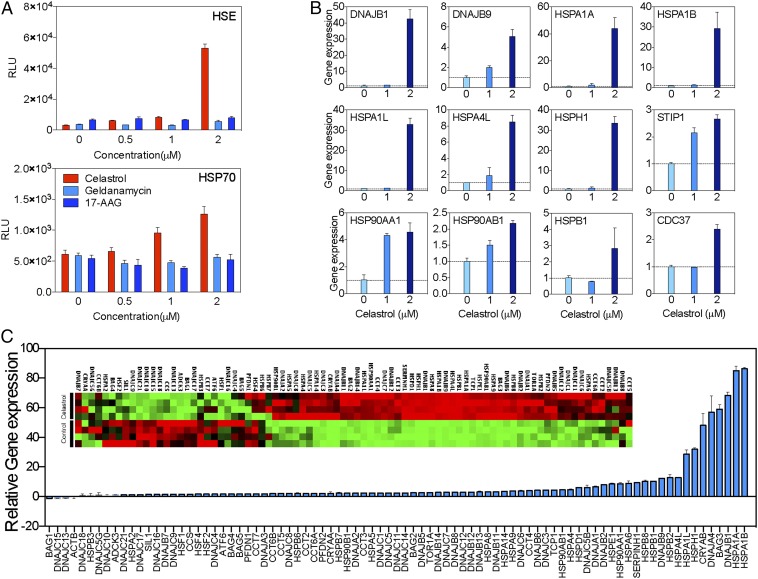
Protein misfolding and premature degradation is the major cause of quantitative loss of GCase in GD (6, 18, 19). Molecular chaperones play important roles in determining the fate of mutant GCase (8, 22). In addition to blocking the chaperone function of Hsp90, celastrol has been shown to affect the transcriptional activity of heat shock-related genes (23, 24), suggesting that celastrol may also affect additional molecular chaperone families such as those involved in GCase stabilization. To test this, the impact of celastrol on heat shock element (HSE)-associated gene transcription was assessed using a luciferase assay (Fig. 3A). Celastrol potently induced HSE-mediated gene transcription, which was not observed following treatment with Hsp90 inhibitors geldanamycin and tanespimycin (17-N-allylamino-17-demethoxygeldanamycin, 17-AAG). In addition, we confirmed the induction of a heat shock response by testing luciferase activity driven by the HSPA1A promoter. To confirm the effect of celastrol on genes coding for key chaperone proteins, HeLa cells were treated with varying amounts of celastrol, and chaperone gene expression was quantified using quantitative real-time PCR (qRT-PCR) (Fig. 3B). Indeed, celastrol increased the expression of multiple genes coding for molecular chaperones in a dose-dependent manner.
Fig. 3.
Celastrol modulates the expression pattern of molecular chaperones. (A) Luciferase assay showed that celastrol, but not geldanamycin or 17-AAG, increased transcriptional activation of the HSE (Top) and HSP1A1 (Bottom) promoters. (B) Quantitative real-time PCR indicated that celastrol increased the expression of molecular chaperones in a dose-dependent manner. (C) A quantitative PCR-based gene screening array demonstrated that celastrol also reorganized the expression of molecular chaperones in HeLa cells.
Finally, we analyzed the impact of celastrol on the expression of chaperone/cochaperone genes using high-throughput screening (Fig. 3C). Cells were treated with celastrol, and the mRNA expression profile was analyzed using a quantitative PCR-based screening assay. Celastrol induced a global change in the expression pattern of chaperone families: 91.8% (78 out of 85) of the chaperones were up-regulated by celastrol. Chaperones that assist protein folding/refolding were more potently induced by celastrol. For example, T-complex protein 1, subunit alpha (TCP-1), the gene coding for a key protein in the chaperone containing TCP-1 (CCT)/TCP-1 ring complex (TRiC) that has been shown to assist GCase folding (6), was up-regulated 4.5-fold by celastrol. The genes coding for DnaJ homolog subfamily B members 1 and 9 (DNAJB1/9), which have been shown to assist protein folding and suppress toxic protein aggregation (25, 26), were up-regulated 68.3- and 12.3-fold, respectively. HSPA1A and HSPA1B, which encode heat-shock protein 70 (Hsp70), were up-regulated by 85.1- and 86.3-fold, respectively.
Celastrol-Induced BAG3 Expression Stabilizes Mutant GCase and Assists in Its Maturation.
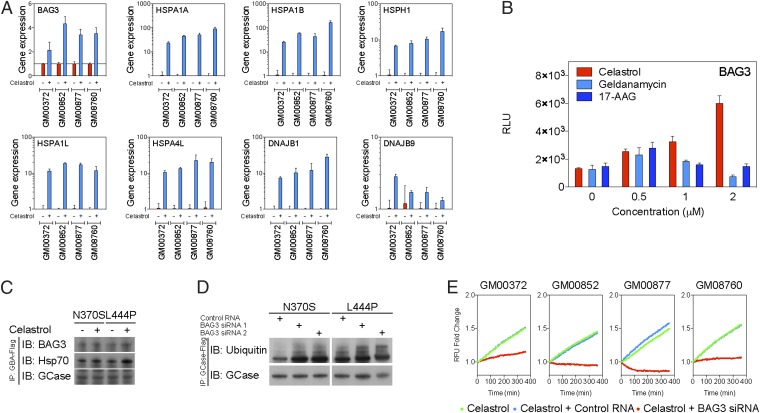
Among chaperone genes that may affect GCase stability, we found BAG3 was robustly up-regulated (∼60-fold) by celastrol. BAG family chaperone regulator 3 (BAG3) has been found to compete with Hip-1 for binding to the heat-shock cognate 71kDa protein (Hsc70)/Hsp70 ATPase domain and promote substrate release (27). Several recent discoveries indicate that BAG3 also regulates the proteasomal and lysosomal protein elimination pathways (28–30). However, the involvement of BAG3 in GCase folding and maturation is unclear. We hypothesized that BAG3 stabilizes misfolded GCase and thus increases the amount of GCase. To test this hypothesis, gene expression of BAG3 and other chaperones was measured in type I and type II/III GD fibroblasts using qRT-PCR (Fig. 4A and Fig. S1). Consistent with the findings presented above, celastrol induced expression of chaperones that assist protein folding in GD fibroblasts including a 115–330% increase in BAG3 expression. Likewise, celastrol increased BAG3 expression by 89–348% in a luciferase assay, whereas no change in BAG3 expression was observed following treatment with geldanamycin or 17-AAG (Fig. 4B).
Fig. 4.
BAG3 is induced by celastrol and stabilizes mutant GCase. (A) Quantitative real-time PCR of molecular chaperone expression in GD fibroblasts. Celastrol increased the expression of BAG3, HSPA1A, HSPA1B, heat shock 105kDa/110kDa protein 1 (HSPH1), heat shock 70kDa protein 1L (HSPA1L), heat shock 70kDa protein 4L (HSPA4L), and DNAJB1/9 in GD fibroblasts. (B) Luciferase assay demonstrated that celastrol, but not geldanamycin or 17-AAG, increased BAG3 transcription in a dose-dependent manner. (C) Immunoprecipitation further showed that celastrol enhanced BAG3 and Hsp70 binding to mutant GCase. (D) Small interfering RNA inhibition of BAG3 expression increased ubiquitination of mutant GCase. (E) A fluorometric enzyme assay further indicated that siRNA inhibition of BAG3 reduced GCase activity in GD fibroblasts treated with celastrol.
Next, we investigated the role of celastrol-induced BAG3 expression in GCase misfolding and degradation. The affinity of BAG3 for GCase mutants was assessed using immunoprecipitation (Fig. 4C). Celastrol increased the binding of BAG3/Hsp70 chaperones to N370S and L444P GCase mutants, indicating that celastrol not only up-regulated expression of BAG3/Hsp70 but also enhanced their binding to mutant GCase. Dicer-substrate siRNAs targeting BAG3 were then implemented to assess the importance of BAG3 in GCase degradation. All siRNAs reduced BAG3 expression by ∼80% (Figs. S2 and S3). Ubiquitination of GCase mutants was significantly increased in cells that were cotransfected with BAG3 siRNAs, suggesting that BAG3 is essential for stabilization of mutant GCases (Fig. 4D). Finally, the importance of BAG3 in mutant GCase activity was confirmed by measuring GCase activity in GD fibroblasts transfected with BAG3 siRNAs (Fig. S4). Cells were exposed to celastrol, and GCase activity was measured using a fluorometric assay (Fig. 4E). Cotransfection with BAG3 siRNAs, but not control siRNA, significantly reduced GCase activity in both type I and type II/III GD fibroblasts, suggesting that the therapeutic effect of celastrol depends on the function of BAG3.
Discussion
Our results reveal that celastrol is an effective small molecular weight compound that may be useful for the treatment of GD. It increases the quantity and catalytic activity of mutant GCase in GD fibroblasts by targeting molecular chaperones and inhibiting protein degradation. Celastrol induces a heat-shock response in GD fibroblasts and up-regulates genes coding for molecular chaperones BAG3, Hsp70, and DNAJB1/9. Moreover, celastrol-induced BAG3 assists in stabilizing mutant GCase by modulating protein-folding machinery (Fig. 5). Thus, celastrol in GD may serve as a valuable therapeutic paradigm for treating diseases caused by protein misfolding.
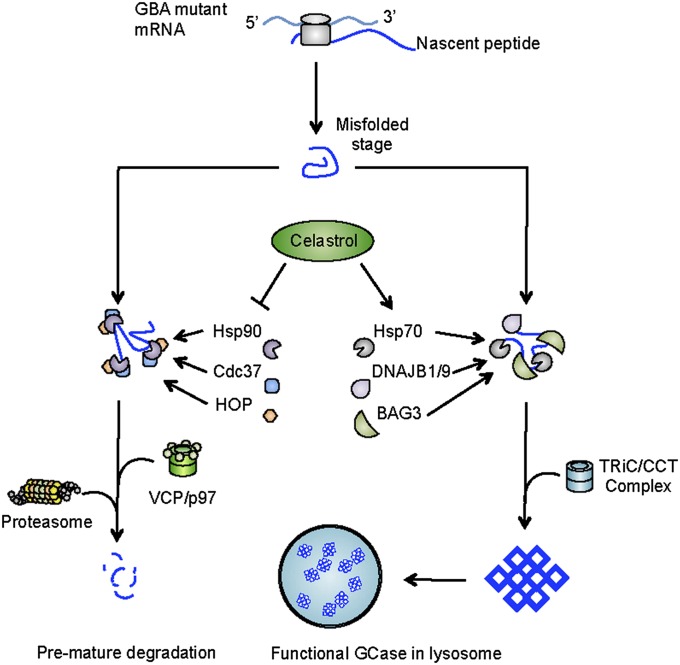
Fig. 5.
Celastrol rescues mutant GCase from degradation by modulating molecular chaperones. Mutant GCase proteins assume an unnatural conformation during protein translation. Misfolded GCase is targeted for proteasomal degradation by the Hsp90/Cdc37/Hop chaperone complex. Celastrol inhibits the assembly of the Hsp90/Cdc37/Hop complex and reduces the degradation of mutant GCase. In addition, celastrol increases the expression of chaperone genes such as DNAJB1/9, HSPA1A/B, and BAG3. Reorganization of molecular chaperones further stabilizes GCase mutants by enhancing protein folding and maturation.
Recent investigations have provided critical insights concerning the pathogenic mechanisms of inherited disorders such as GD. In the past, it was presumed that alterations in the amino acid sequence of GCase reduced the catalytic activity of the enzyme. These mutations appear more likely to affect the stability of the protein without inducing catastrophic changes in enzymatic function (6, 18), and quantitative reduction of the enzyme is determined by molecular chaperones (19). Therefore, targeting molecular chaperones and protein misfolding has recently been proposed as a therapeutic approach for this disease and others resulting from premature protein degradation (31).
Our previous findings demonstrated that Hsp90 serves as a key molecule that determines the fate of mutant GCase (19). Hsp90 recognizes the unnatural conformation of mutant GCase during protein translation and targets the misfolded protein for degradation. Hsp90 has been targeted using various molecular inhibitors in an attempt to restore the expression of mutant GCase. For example, 17-AAG was shown to inhibit the N-terminal ATP-binding pocket of Hsp90 which effectively reduced the ubiquitination of GCase (19). In addition, histone deacetylase inhibitors (HDACi) inhibit the deacetylation of the middle domain of Hsp90, reducing its chaperone function and increasing the amount of GCase (18). However, it is not known whether a subpopulation of Hsp90 is preferentially overexpressed in GBA-mutant cells or whether cells overexpressing Hsp90 can be targeted using a specific Hsp90 inhibitor, as has been demonstrated in certain types of cancer (32–39). Nevertheless, our data suggest that celastrol is a Hsp90 inhibitor that targets the formation of the associated chaperone complex.
Enzyme replacement therapy is currently the gold standard for treating GD (40, 41), but the inability of the enzyme to cross the blood–brain barrier demands that unique approaches be taken to treat the neuronopathic subtypes. Small molecular weight compounds that modulate protein chaperones offer a promising alternative to enzyme replacement therapy. However, additional considerations must be taken into account before clinical testing. For example, clinically available HDACi, Hsp inhibitors, and celastrol itself are associated with significant toxicities (42–47). Our results demonstrate that HDACi and celastrol could have undesirable “off-target” effects that should be identified before clinical trials in GD are pursued (48). Further work is required to determine whether route of administration, dose, molecular structure, or other aspects of these compounds can be selected to maximize therapeutic effect and minimize toxicity. Another noteworthy consideration regarding our findings is the involvement of BAG3 in the genesis, progression, and therapeutic resistance of multiple cancers (49–51), which may limit the therapeutic implications of these findings. Furthermore, it is not currently known whether GBA-mutant cells endogenously express higher levels of BAG3 or whether an increase in BAG3 expression contributes to the elevated risk of malignancy in GD (52). Although these considerations must be taken into account before further clinical investigations, proteostasis regulators such as HDACi and celastrol offer an alternative to enzyme replacement therapy for the treatment of neuronopathic subtypes of GD.
Recent studies have shown that celastrol occupies the interface of Hsp90/Cdc37-binding site, blocking the establishment of Hsp90 complex and thus its function as a molecular chaperone (16, 17). The present study demonstrates the importance of Hsp90 in the folding and degradation of mutant GCase. The potent inhibitory effect of celastrol on Hsp90 suggests that it may enhance GCase stability. Others have demonstrated that celastrol inhibits the degradation of mutant GCase in GD fibroblasts and mutant β-hexosaminidase A in Tay–Sachs disease fibroblasts by activating the heat shock and unfolded protein responses (15), but the molecular mechanism by which this occurs has not been well-addressed. We demonstrated that binding of the Hsp90/Cdc37 complex to mutant GCase was disrupted upon treatment of GD fibroblasts with celastrol. Celastrol decreased recognition of mutant GCase by the Hsp90 chaperone complex and therefore reduced GCase degradation. Importantly, celastrol increased GCase activity by inducing not only a heat shock response but also transcription of key chaperone genes including DNAJB1/9 and BAG3.
BAG3 is potently induced in the presence of celastrol and assists in the folding and maturation of GCase by reorganizing molecular chaperones, thereby increasing the stability and catalytic activity of mutant GCase. Celastrol targets Hsp90/Cdc37 heterodimerization limiting the affinity for GCase mutants and thus blocking protein degradation. Additionally, celastrol induces a heat shock response and increases the expression of molecular chaperones that assist GCase stabilization thereby increasing the amount of mature GCase and catalytic activity of the enzyme. Celastrol may be a valuable therapeutic option for GD, especially the neuronopathic phenotypes. Additionally, inhibition of the Hsp90 chaperone system and induction of a heat shock response by celastrol may be useful in additional human disorders related to protein misfolding such as type C Niemann–Pick disease, cystic fibrosis, neurofibromatosis type 2, succinate dehydrogenase B-associated neuroendocrine tumors, and von Hippel Lindau disease.
Materials and Methods
Cell Culture.
Foreskin fibroblasts derived from GD patients were maintained as previously described (19). Fibroblasts from type I GD (GM00372 and GM00852) and type II GD (GM00877 and GM08760) were used (Coriell Institute for Medical Research). HeLa cells were purchased from American Type Culture Collections (ATCC). Cells were incubated in Eagle’s minimum essential medium alpha (MEMα, Invitrogen) supplemented with 10% (vol/vol) FBS. For each treatment condition, cells were incubated in either celastrol (Sigma–Aldrich) dissolved in DMSO (Sigma–Aldrich) at the indicated concentration or in DMSO alone overnight in a humidified chamber at 37 °C unless otherwise indicated.
Pulse–Chase Assay.
The pulse–chase assay of mutant GCases was performed using cycloheximide as previously described (53).
Immunoprecipitation.
Cell pellets were lysed in Nonidet P-40 lysis buffer supplemented with a Halt proteasome inhibitor mixture (Thermo Scientific). One milligram of total protein was precipitated with DynaBeads Protein G Immunoprecipitation Kit (Invitrogen). The antibodies for immunoprecipitation included monoclonal antibodies against Flag-tag (Origene) and Hsp90 (Cell Signaling Technology).
Western Blot.
Cell pellets were lysed with radioimmunoprecipitation (RIPA) lysis buffer supplemented with a Halt proteasome inhibitor mixture. Protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad). Samples were separated on a NuPAGE Bis–Tris 4–12% (vol/vol) gel (Invitrogen). Protein was transferred to a PVDF membrane (Millipore) and probed with the primary antibody. The primary antibodies used in this study included those to Hsp90, Hop, and Cdc37 (Cell Signaling Technology); GCase (Abcam and Sigma–Aldrich); β-actin (Sigma–Aldrich); ubiquitin and BAG3 (Abcam); and Flag-tag (Origene). The amount of protein was determined by visualizing the primary antibody using HRP-conjugated species-specific secondary antibody and SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
GCase Activity Assay.
The fluorometric GCase enzyme activity assay was performed as described previously (19).
RNA Interference.
RNA interference was carried out with dicer-substrate RNAs (Integrated DNA Technologies). The RNA oligomers used in present study were siBAG3.1: 5′-GCU GUA GAC AAC UUU GAA GGC AAG A-3′; siBAG3.2: 5′-CCC AUG ACC CAU CGA GAA ACU GCA C-3′; and siBAG3.3: 5′-GCC AUA GGA AUA UCU GUA UGU UGG A-3′. Fifty to 100 pmol of siRNA oligomer was transfected into HeLa cells or GD fibroblasts with lipofectamine RNAiMAX Reagent (Invitrogen). Cells were used for in vitro assays 48–72 h after transfection. Efficacy of siBAG3 oligomers in blocking BAG3 protein expression was confirmed using Western blot against BAG3 (Figs. S2 and S3).
Quantitative Real-Time PCR.
Total RNA was extracted from HeLa cells or GD fibroblasts using an RNeasy Mini Kit (Qiagen). Genomic DNA was removed by RNase-free DNase I (Qiagen). Total RNA was reverse-transcribed into cDNA using SuperScript III first-strand synthesis supermix (Invitrogen) according to the manufacturer’s protocol. The cDNA products were analyzed by qRT-PCR. The gene expression of molecular chaperones was determined using heat shock proteins and chaperones RT2 profiler PCR array (Qiagen). The primer sets used in this study are described in Table S1.
Luciferase Assay.
The luciferase assay was performed as previously described (54).
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NIH) and the NIH Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer, Inc., the Doris Duke Charitable Foundation, Alexandria Real Estate Equities, Inc., and Mr. and Mrs. Joel S. Marcus and the Howard Hughes Medical Research Institute, as well as other private donors. For a complete list, please visit http://fnih.org/work/education-training-0/medical-research-scholars-program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321341111/-/DCSupplemental.
References
- 1.Brady RO, Kanfer JN, Shapiro D. Metabolism of Glucocerebrosides. II. Evidence of an enzymatic deficiency in Gaucher's disease. Biochem Biophys Res Commun. 1965;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- 2.Brady RO, Kanfer JN, Bradley RM, Shapiro D. Demonstration of a deficiency of glucocerebroside-cleaving enzyme in Gaucher’s disease. J Clin Invest. 1966;45(7):1112–1115. doi: 10.1172/JCI105417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mistry PK, et al. Genetic diagnosis of Gaucher’s disease. Lancet. 1992;339(8798):889–892. doi: 10.1016/0140-6736(92)90928-v. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz M, Zimran A. Mutations causing Gaucher disease. Hum Mutat. 1994;3(1):1–11. doi: 10.1002/humu.1380030102. [DOI] [PubMed] [Google Scholar]
- 5.Stone DL, et al. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15(2):181–188. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, et al. Decreased glucocerebrosidase activity in Gaucher disease parallels quantitative enzyme loss due to abnormal interaction with TCP1 and c-Cbl. Proc Natl Acad Sci USA. 2010;107(50):21665–21670. doi: 10.1073/pnas.1014376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ron I, Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet. 2005;14(16):2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 8.Zheng W, et al. Three classes of glucocerebrosidase inhibitors identified by quantitative high-throughput screening are chaperone leads for Gaucher disease. Proc Natl Acad Sci USA. 2007;104(32):13192–13197. doi: 10.1073/pnas.0705637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trott A, et al. Activation of heat shock and antioxidant responses by the natural product celastrol: Transcriptional signatures of a thiol-targeted molecule. Mol Biol Cell. 2008;19(3):1104–1112. doi: 10.1091/mbc.E07-10-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109(7):2727–2735. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 11.Chang FR, et al. Antitumor agents. 228. five new agarofurans, Reissantins A-E, and cytotoxic principles from Reissantia buchananii. J Nat Prod. 2003;66(11):1416–1420. doi: 10.1021/np030241v. [DOI] [PubMed] [Google Scholar]
- 12.Nagase M, et al. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci Biotechnol Biochem. 2003;67(9):1883–1887. doi: 10.1271/bbb.67.1883. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66(9):4758–4765. doi: 10.1158/0008-5472.CAN-05-4529. [DOI] [PubMed] [Google Scholar]
- 14.Walcott SE, Heikkila JJ. Celastrol can inhibit proteasome activity and upregulate the expression of heat shock protein genes, hsp30 and hsp70, in Xenopus laevis A6 cells. Comp Biochem Physiol A Mol Integr Physiol. 2010;156(2):285–293. doi: 10.1016/j.cbpa.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Mu TW, et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134(5):769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7(1):162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 17.Zhang T, et al. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J Biol Chem. 2009;284(51):35381–35389. doi: 10.1074/jbc.M109.051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, et al. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc Natl Acad Sci USA. 2011;108(52):21200–21205. doi: 10.1073/pnas.1119181109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, et al. Histone deacetylase inhibitors increase glucocerebrosidase activity in Gaucher disease by modulation of molecular chaperones. Proc Natl Acad Sci USA. 2013;110(3):966–971. doi: 10.1073/pnas.1221046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendikov-Bar I, Ron I, Filocamo M, Horowitz M. Characterization of the ERAD process of the L444P mutant glucocerebrosidase variant. Blood Cells Mol Dis. 2011;46(1):4–10. doi: 10.1016/j.bcmd.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Bendikov-Bar I, Horowitz M. Gaucher disease paradigm: From ERAD to comorbidity. Hum Mutat. 2012;33(10):1398–1407. doi: 10.1002/humu.22124. [DOI] [PubMed] [Google Scholar]
- 22.Ong DS, Kelly JW. Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases. Curr Opin Cell Biol. 2011;23(2):231–238. doi: 10.1016/j.ceb.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerheide SD, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279(53):56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 24.Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem. 2005;280(39):33097–33100. doi: 10.1074/jbc.R500010200. [DOI] [PubMed] [Google Scholar]
- 25.Vos MJ, Hageman J, Carra S, Kampinga HH. Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry. 2008;47(27):7001–7011. doi: 10.1021/bi800639z. [DOI] [PubMed] [Google Scholar]
- 26.Hageman J, et al. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell. 2010;37(3):355–369. doi: 10.1016/j.molcel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3(10):E237–E241. doi: 10.1038/ncb1001-e237. [DOI] [PubMed] [Google Scholar]
- 28.Doong H, et al. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: Accumulation of poly-ubiquitinated Hsp90 client proteins. J Biol Chem. 2003;278(31):28490–28500. doi: 10.1074/jbc.M209682200. [DOI] [PubMed] [Google Scholar]
- 29.Gamerdinger M, et al. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28(7):889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammirante M, et al. IKKgamma protein is a target of BAG3 regulatory activity in human tumor growth. Proc Natl Acad Sci USA. 2010;107(16):7497–7502. doi: 10.1073/pnas.0907696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brady RO, Yang C, Zhuang Z. An innovative approach to the treatment of Gaucher disease and possibly other metabolic disorders of the brain. J Inherit Metab Dis. 2013;36(3):451–454. doi: 10.1007/s10545-012-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breinig M, et al. Targeting heat shock protein 90 with non-quinone inhibitors: A novel chemotherapeutic approach in human hepatocellular carcinoma. Hepatology. 2009;50(1):102–112. doi: 10.1002/hep.22912. [DOI] [PubMed] [Google Scholar]
- 33.Caldas-Lopes E, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci USA. 2009;106(20):8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerchietti LC, et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat Med. 2009;15(12):1369–1376. doi: 10.1038/nm.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huntoon CJ, et al. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway. Cancer Res. 2010;70(21):8642–8650. doi: 10.1158/0008-5472.CAN-10-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marubayashi S, et al. HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans. J Clin Invest. 2010;120(10):3578–3593. doi: 10.1172/JCI42442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usmani SZ, Bona RD, Chiosis G, Li Z. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J Hematol Oncol. 2010;3:40. doi: 10.1186/1756-8722-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen W, Sin SH, Wen KW, Damania B, Dittmer DP. Hsp90 inhibitors are efficacious against Kaposi Sarcoma by enhancing the degradation of the essential viral gene LANA, of the viral co-receptor EphA2 as well as other client proteins. PLoS Pathog. 2012;8(11):e1003048. doi: 10.1371/journal.ppat.1003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moulick K, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7(11):818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beutler E, Dale GL, Guinto DE, Kuhl W. Enzyme replacement therapy in Gaucher’s disease: Preliminary clinical trial of a new enzyme preparation. Proc Natl Acad Sci USA. 1977;74(10):4620–4623. doi: 10.1073/pnas.74.10.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brady RO. Enzyme replacement for lysosomal diseases. Annu Rev Med. 2006;57:283–296. doi: 10.1146/annurev.med.57.110104.115650. [DOI] [PubMed] [Google Scholar]
- 42.Supko JG, Hickman RL, Grever MR, Malspeis L. Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol. 1995;36(4):305–315. doi: 10.1007/BF00689048. [DOI] [PubMed] [Google Scholar]
- 43.Egorin MJ, et al. Metabolism of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) by murine and human hepatic preparations. Cancer Res. 1998;58(11):2385–2396. [PubMed] [Google Scholar]
- 44.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12(10):1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Liu K, Wang X, He Q, Chen X. Toxic effects of celastrol on embryonic development of zebrafish (Danio rerio) Drug Chem Toxicol. 2011;34(1):61–65. doi: 10.3109/01480545.2010.494664. [DOI] [PubMed] [Google Scholar]
- 46.Fukutomi A, et al. A phase I study of oral panobinostat (LBH589) in Japanese patients with advanced solid tumors. Invest New Drugs. 2012;30(3):1096–1106. doi: 10.1007/s10637-011-9666-9. [DOI] [PubMed] [Google Scholar]
- 47.Kusy S, Ghosn EE, Herzenberg LA, Contag CH. Development of B cells and erythrocytes is specifically impaired by the drug celastrol in mice. PLoS ONE. 2012;7(4):e35733. doi: 10.1371/journal.pone.0035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, et al. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc Natl Acad Sci USA. 2013;110(26):10747–10752. doi: 10.1073/pnas.1308950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Festa M, et al. BAG3 protein is overexpressed in human glioblastoma and is a potential target for therapy. Am J Pathol. 2011;178(6):2504–2512. doi: 10.1016/j.ajpath.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosati A, et al. Role of BAG3 protein in leukemia cell survival and response to therapy. Biochim Biophys Acta. 2012;1826(2):365–369. doi: 10.1016/j.bbcan.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Zhu H, Liu P, Li J. BAG3: A new therapeutic target of human cancers? Histol Histopathol. 2012;27(3):257–261. doi: 10.14670/HH-27.257. [DOI] [PubMed] [Google Scholar]
- 52.Shiran A, Brenner B, Laor A, Tatarsky I. Increased risk of cancer in patients with Gaucher disease. Cancer. 1993;72(1):219–224. doi: 10.1002/1097-0142(19930701)72:1<219::aid-cncr2820720139>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 53.Yang C, et al. Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas. Blood. 2013;121(13):2563–2566. doi: 10.1182/blood-2012-10-460972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang C, Huntoon K, Ksendzovsky A, Zhuang Z, Lonser RR. Proteostasis modulators prolong missense VHL protein activity and halt tumor progression. Cell Rep. 2013;3(1):52–59. doi: 10.1016/j.celrep.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.