Significance
Lymph node swelling is a hallmark of adaptive immunity. Fibroblastic reticular cells form a fairly rigid scaffold throughout lymph nodes. They not only support organ structure and compartmentalization, but also guide lymphocyte trafficking. We describe how this rigid fibroblast network reacts to acute organ swelling. Rather than being disrupted or destroyed, the fibroblast network rapidly expands by proliferation and finally covers a much larger volume to accommodate many more lymphocytes. We identified naive lymphocyte trapping by innate triggers as an early fibroblast growth signal, with activated lymphocytes playing a role only in the later growth phase.
Keywords: lymph node swelling, fibroblasts, stromal cells, lymphotoxin, MyD88
Abstract
Adaptive immunity is initiated in T-cell zones of secondary lymphoid organs. These zones are organized in a rigid 3D network of fibroblastic reticular cells (FRCs) that are a rich cytokine source. In response to lymph-borne antigens, draining lymph nodes (LNs) expand several folds in size, but the fate and role of the FRC network during immune response is not fully understood. Here we show that T-cell responses are accompanied by the rapid activation and growth of FRCs, leading to an expanded but similarly organized network of T-zone FRCs that maintains its vital function for lymphocyte trafficking and survival. In addition, new FRC-rich environments were observed in the expanded medullary cords. FRCs are activated within hours after the onset of inflammation in the periphery. Surprisingly, FRC expansion depends mainly on trapping of naïve lymphocytes that is induced by both migratory and resident dendritic cells. Inflammatory signals are not required as homeostatic T-cell proliferation was sufficient to trigger FRC expansion. Activated lymphocytes are also dispensable for this process, but can enhance the later growth phase. Thus, this study documents the surprising plasticity as well as the complex regulation of FRC networks allowing the rapid LN hyperplasia that is critical for mounting efficient adaptive immunity.
Lymph nodes (LNs) are secondary lymphoid organs in which primary T- and B-cell responses to lymph-borne pathogens or tumors are induced. LNs contain three compartments characterized by distinct recirculating hematopoietic cells and resident stromal cells (1, 2). The B-cell zone is composed of CD35-positive follicular dendritic cells (FDCs), which produce CXCL13 to attract B cells. The T-cell zone (paracortex) is rich in gp38 (podoplanin)-positive fibroblastic reticular cells (FRCs) that express CCL19 and CCL21 to attract naive T cells and dendritic cells (DCs) (3). Finally, the medulla is rich in efferent lymphatic vessels and serves as exit zone for naive and activated lymphocytes. It expands during immune response to accommodate short-lived plasma cells (4–6).
Whereas FDCs are well-established players in B-cell responses, the importance of T-zone FRCs (TRCs) in adaptive immunity has emerged only recently. The extracellular matrix by TRCs forms conduits that transport small molecules to the T zone. TRCs enwrap these conduits and form a 3D cellular scaffold that allows DCs to adhere and recirculating T and B cells to migrate along it (1, 7), thereby enhancing the probability of successful encounters of activated DCs with cognate T cells. Besides CCL19/21, TRCs also produce IL-7 promoting naive T-cell survival (3), present self-antigens contributing to peripheral T-cell tolerance (2); express inducible nitric oxide synthase, limiting strong Th1 responses (8); and secrete VEGF, leading to growth of high endothelial venules (HEVs) (9). A second gp38+ FRC subset has been recently characterized, termed marginal reticular cells (MRCs) based on its specific localization below the subcapsular sinus and the selective expression of CXCL13, RANK-L, and MAdCAM-1 (10). Herein we refer to all gp38+ fibroblasts, including TRCs and MRCs, collectively as FRCs.
The size of the draining LNs increases dramatically during an immune response owing to the massive trapping of naive lymphocytes and the proliferation of antigen-specific lymphocytes (11, 12). This implies a rapid and extensive remodeling of the various stromal cell structures that form the backbone of LNs. Interestingly, various stromal cells appear to regulate this LN swelling. Immediately after infection or immunization, afferent lymphatic vessels expand, thereby enhancing recruitment of antigen-presenting DCs from the periphery (4, 13). Simultaneously, HEVs increase in number, size, and permeability (14, 15) to facilitate the entry of naïve lymphocytes. Furthermore, the exit of lymphocytes via efferent lymphatics is prevented by IFN-α/β–induced down-regulation of the egress receptor S1P1 on lymphocytes (11). Consequently, the number of antigen-presenting cells (APCs) and lymphocytes strongly increases in draining LNs, presumably to allow more efficient activation of the rare antigen-specific T cells. What happens to the dense, rigid FRC network and its function during acute LN hyperplasia is only partially understood, but is of great importance for the successful initiation of adaptive immunity. The existing evidence suggests an increase in the FRC network size (13, 16), owing at least in part to FRC proliferation (17). DCs have been proposed to trigger FRC proliferation directly by expressing lymphotoxin (LT) αβ (9, 17). RANK ligand also has been suggested as a growth signal for FRCs (18). It also has been suggested that the FRC network around HEVs might be disrupted (19), along with a transient loss of CCL19/21 and IL-7 expression (20, 21).
In this study, we performed a comprehensive analysis of FRC morphology, organization, phenotype, turnover, and function during immunization-induced LN hyperplasia. Our results demonstrate that FRCs are activated within hours after immunization and rapidly proliferate to maintain a dense FRC network throughout the enlarged T zone and medullary cords. Our data suggest that the early phase of FRC expansion depends largely on naive lymphocyte trapping and only indirectly on DCs. Antigen-specific immunity and the ligands of LTβ receptor (LTβR), LTαβ and LIGHT, positively regulate FRC expansion in a later phase.
Results
LN Hyperplasia Is Accompanied by Rapid FRC Activation and Expansion.
To study the behavior of stromal cells in an acute LN swelling model and associate it with the T-cell response, ovalbumin (OVA)-specific TCR-transgenic CD8+ T cells (OT-1) and CD4+ T cells (OT-2) were adoptively transferred into mice that were then s.c. immunized with OVA antigen emulsified in an oil-in-water adjuvant, Montanide ISA-25 (Mont). A pool of six draining LNs was enzymatically digested to analyze both hematopoietic and stromal cells by flow cytometry. OVA injection induced rapid and strong LN swelling, peaking on day 8 and declining thereafter (Fig. 1A). Whereas OVA-specific T cells strongly expanded, ∼85% of cells found in swollen LNs were naive host-derived CD69− CD44− T and B lymphocytes. Continuous in vivo BrdU labeling showed that proliferation was limited largely to OVA-specific T cells and a small B-cell subset. The three major CD45− nonhematopoietic stromal cells—FRCs, lymphatic endothelial cells (LECs), and blood endothelial cells (BECs)—were distinguished based on their CD31 and gp38 expression (Fig. 1B) (3). Analysis of their numbers revealed a gradual expansion of all three subsets starting on day 3 and reaching a fivefold increase on day 8–14 (Fig. 1 C and D), comparable to the fivefold increase in lymphocyte number (Fig. 1A). The stromal cell growth was due, at least in part, to proliferation, given that up to 70% of FRCs, LECs, and BECs incorporated BrdU within the first 8 d after immunization (Fig. 1 C and D).
Fig. 1.
FRCs and endothelial cells expand rapidly during immune response in draining peripheral LNs. (A–D) Splenocytes from OT-1 (OVA-specific CD8+ TCR tg) and OT-2 (OVA-specific CD4+ TCR tg) C57BL/6 mice on a CD45.1+ background were transferred into C57BL/6 (CD45.2+) recipient mice, which then received s.c. injections of OVA/Mont or PBS, along with BrdU administration. The six draining peripheral LNs were isolated at the indicated time points after immunization, digested, and then analyzed by flow cytometry. (A) Number (Upper) and proliferation (BrdU incorporation; Lower) of indicated cell populations from PBS-control (open circles) and OVA/Mont-immunized (closed circles) mice. (B) Representative dot plots of DAPI−CD35−CD45− pregated stromal cells identifying FRCs (gp38+CD31−), LECs (gp38+CD31+), and BECs (gp38−CD31+). Percentages indicated show the cell frequency among all living cells. (C) Representative histograms showing FRCs stained with an antibody to BrdU (white) or an isotype-matched control (gray area) at 8.5 d after PBS or OVA/Mont injection. (D) Number (Upper) and proliferation (Lower) of FRCs, LECs, and BECs at indicated time points after OVA/Mont immunization. (E) WT C57BL/6 mice were infected with L. major in both footpads. On day 19, the two draining popliteal LNs were isolated, enzymatically digested, and analyzed as described above. Data are mean ± SD, representative of two or three independent experiments with n ≥3 mice per group.
To examine whether LN stromal expansion also occurs during infection, we inoculated the protozoan parasite Leishmania major in B6 mice and analyzed their LNs at 19 d postinfection. Similar expansions of FRCs, LECs, and BECs were observed (Fig. 1E), demonstrating that FRC expansion is a feature common to adaptive immunity induced by immunization or infection.
To define the sequence of early events in more detail, we analyzed LNs at 20 and 40 h after OVA/Mont immunization. Trapping of naive lymphocytes started as early as 20 h (Fig. S1A), whereas OVA-specific T-cell proliferation was first detected at 40 h (Fig. S1B). Strikingly, an increase in FRC number and proliferation was already evident at 40 h, in contrast to LECs and BECs, which both entered the cell cycle only around day 3.5 (Figs. 1D and 2A). Surprisingly, FRCs and LECs increased in size [forward scatter characteristics (FSCs)] and granularity [side scatter characteristics (SSCs)] as early as 20 h after immunization, with a peak at 40 h, followed by a slow decline over the next several weeks (Fig. 2 B and C and Fig. S1 C and D). Similarly, the numbers of FRCs and lymphocytes remained increased at 2 mo after immunization, but with no evidence of increased turnover (Fig. 2 B–D and Fig. S1E).
Fig. 2.
FRCs increase in size within 20 h and proliferate within 40 h after immunization. (A) Number and proliferation of FRCs, LECs, and BECs in the six draining LNs at 40 h after OVA/Mont immunization, as described in Fig. 1. (B and C) Representative histograms and kinetics for FSC (size; B) and SSC (granularity; C) flow cytometry profiles of FRCs at indicated time points after OVA/Mont immunization. The OVA/PBS ratio shows the FSC and SSC levels of FRCs from OVA/Mont-injected mice relative to PBS-injected mice. (D) Cell number and proliferation analysis of FRCs, LECs, and BECs at day 58 after OVA/Mont immunization. BrdU was administered to mice for only the last 5.5 d before sacrifice. Data are mean ± SD, representative of two or three independent experiments, with n ≥3 mice per group.
FRC lines have been shown to change their surface phenotype and function on treatment with proinflammatory molecules (8, 16, 22). In vivo, the mean fluorescence index (MFI) of gp38 expression, but not platelet-derived growth factor receptor (PDGF-R) α expression increased by 200% within 40 h and remained at that level until day 5.5 before declining sharply on day 8.5 (Fig. 3A). The 50% increase in cell size can only partially explain the strong increase in gp38 surface expression, particularly at day 5.5, when FRCs show only a 25% size increase. Increased gp38 levels were also seen in splenic FRCs after viral infection (23, 24), suggesting that gp38 may serve as an activation marker of FRCs. Furthermore, α-smooth muscle actin (α-SMA), a known myofibroblast marker (3, 25), was also induced on immunization and peaked at day 5.5 (Fig. 3B) suggesting that FRCs show signs of late activation distinct from proliferation or gp38 up-regulation. No significant changes were observed in transcripts for Il7, Ccl19, and Ccl21 by either quantitative RT-PCR (Fig. 3C) or in situ hybridization (Fig. 3D). Taken together, these data demonstrate that on immunization, FRCs are rapidly, yet transiently activated while maintaining expression of the cytokines important for naive T-cell recirculation and survival.
Fig. 3.
FRCs from swollen LNs exhibit an activated phenotype. (A) (Upper) Representative histograms and mean fluorescence intensity (MFI) for the surface expression of gp38 and PDGFRα on FRCs in the LNs of PBS-injected (black line) or OVA/Mont-injected (red line) mice at 5.5 d after immunization. Surface protein expression on CD45+ cells served as a negative control (gray area). (Lower) Kinetics of surface gp38 expression on FRCs at indicated time points after OVA/Mont immunization (closed circles) relative to PBS control (open circles). (B) Representative images showing expression of the myofibroblast marker α-SMA in LN sections obtained at the indicated time points after OVA/Mont immunization, as assessed by immunofluorescence labeling. Asterisks indicate HEVs surrounded by smooth muscle cells, and open arrows indicate reticular FRCs that costained for PDGFRβ. Labeling, exposure time, and image processing were identical for all time points shown. (C) mRNA expression of Il7, Ccl19, and Ccl21 were measured in stroma-enriched (white bars) and lymphocyte-enriched (gray bars) fractions from draining LNs at 0, 3.5, and 8.5 d after immunization and then normalized to two housekeeping genes, as described in Materials and Methods. (D) Representative images of in situ hybridization analysis showing Ccl19 and Ccl21 transcripts (green) in LN sections on days 0, 5.5, and 8.5 after OVA/Mont immunization, along with B220 antibody staining (red). All slides were treated similarly (ISH development, exposure time for photos, and processing of images). Data in A, B, and D are mean ± SD, representative of two or three independent experiments, with n ≥3 mice per group. n.s., statistically not significant. (Scale bars: 100 μm.)
The FRC Network in the T Zone Preserves Its Architecture During Immunization but Expands into the Medullary Area.
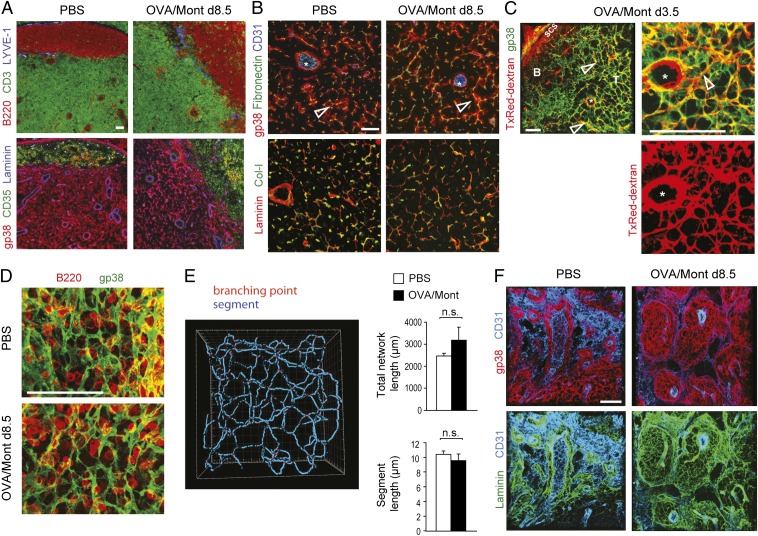
We next assessed histologically whether LN swelling leads to alterations in the integrity and organization of the LN FRC network. On day 8.5, as well as at earlier time points, the organization of the FRC and matrix network in the central T zone was comparable to that of naïve LNs, including their reticular morphology and association with conduits (Fig. 4 A and B). These conduits remained fully functional in activated LNs, with efficient lymph flow toward HEVs and no evidence of fluid leakage (Fig. 4C). Three-dimensional analysis of the central T zone indicated a comparable spacing of the FRC network in inflamed and naive LNs, fitting ∼5–10 lymphocytes within a 2D plane (Fig. 4D). Also comparable were the length of the total FRC network in a given volume or of individual FRCs (Fig. 4E), as was the space occupied by the FRC network in a given volume (PBS, 15.09 ± 5.68%; OVA, 18.44 ± 6.73%). Taken together, these data indicate that both the FRC network organization and the space available to lymphocytes and DCs remains fairly stable during this remodeling process. However, a strong increase in the volume covered by gp38+ FRC networks was observed in both the expanded T zone and the medulla (Fig. S2).
Fig. 4.
An expanding FRC network preserves its usual structure and function while extending into medullary cords. Immunofluorescence microscopy of cryostat sections (A and B) or 80-μm-thick vibratome sections (C–F) from draining LNs of PBS- or OVA/Mont-immunized mice were labeled with the indicated antibodies. (A) B220+ B cells and CD3+ T cells indicate B and T zones, respectively. LYVE-1 stains lymphatic vessels. Consecutive sections show FRC (gp38+CD35−) and FDC (gp38+/−CD35+) networks, as well as the laminin-positive basement membranes of vessels and conduits. (B) Higher-magnification image of the T zone showing CD31+ HEVs (asterisks) and reticular FRCs (gp38+) wrapped around fibronectin-positive conduits (open arrows). Conduits are composed of collagen I-positive (Col-I) fibrils surrounded by a laminin-positive basement membrane. (C) Texas (Tx) Red-dextran was injected s.c. at 3.5 d after OVA/Mont immunization, draining LNs isolated 30 min thereafter, followed by their processing for histological labeling. Open arrows highlight TxRed-dextran–positive conduits surrounded by gp38+ FRCs; the asterisk denotes an HEV with a perivascular space rich in TxRed-dextran. (D) Vibratome sections of the LN T zone in PBS- and OVA/Mont-immunized mice demonstrating a similar density and architecture as for the gp38+ FRC network. B220+ B cells are shown for a size comparison. (E) Filament tracer software was used to quantify the total network length and segment length of individual FRCs in images derived from gp38-labeled vibratome sections (mean ± SD), as shown in D. (F) Vibratome sections showing medullary cords displaying extensive gp38+ reticular FRC networks wrapping around laminin-positive structures and connecting with CD31high HEVs. The cords are demarcated by a thin layer of CD31intgp38+ lymphatic endothelium. Data are representative of two to three experiments with at least two mice and six LNs per mouse. (Scale bars: 100 μm.)
More detailed analysis of medullary cords revealed a 3D sponge-like network of laminin-positive fibers enwrapped by reticular cells with an FRC-like phenotype (gp38+CD31− LYVE-1−) that was much more pronounced in activated LNs than in naive LNs (Fig. 4F). These results demonstrate the astonishing capacity of the matrix and FRC network to adapt rapidly to the massive organ growth while preserving some of the principal FRC characteristics.
Dendritic Cells and Myd88-Dependant Signals Are Required to Initiate FRC Growth During LN Swelling.
To elucidate the sequence of events that control FRC proliferation, we first examined the relative role of innate versus adaptive immunity. Toward this end, we injected OVA/Mont or Mont alone into WT mice that had or had not received OT T cells. Surprisingly, injection of Mont induced lymphocyte trapping as well as FRC proliferation (Fig. 5A), indicating that inflammatory signals are sufficient to trigger both processes in the absence of notable lymphocyte activation. Although not required, adaptive immunity as induced by OVA/Mont led to a stronger lymphocyte trapping and FRC expansion than that induced by Mont, especially when OVA-specific T cells were present (Fig. 5A). Given that these activated lymphocytes are detectable starting only at 40 h after OVA/Mont immunization, only the later FRC expansion appears to be sustained by signals present during adaptive immunity.
Fig. 5.
DC- and MyD88-dependent signals regulate FRC growth during the immune response. The total cellularity, number of FRCs, and proliferation of FRCs were measured in six draining LNs by flow cytomety at day 3 (B and D) or day 5.5 (A, C, and E) after immunization. (A) Mice that received OT T cells (closed circles) or did not receive OT T cells (open circles) were immunized with PBS, Mont, or OVA/Mont. Statistics were calculated on a pool of all data points. (B) CD11c-DTR tg or ntg littermate mice received OT T cells, were injected with a single dose of DT to deplete DCs, and finally were immunized with PBS or OVA/Mont. (C) WT mice were immunized s.c. with WT or MyD88 KO BMDCs activated with either LPS or CpG without loading of OVA antigen. (D) CD11c-DTR tg or ntg littermate mice were treated with a single dose of DT and then immunized s.c. with PBS or WT BMDCs activated with CpG without OVA antigen. (E) WT or MyD88 KO mice were immunized with WT BMDCs activated with CpG without loading of OVA antigen. Data are mean ± SD from at least two experiments, with n ≥3 mice per experiment.
Migratory DCs are known to transmit antigen and inflammatory signals to LNs and thereby start the swelling process (14, 26). As expected, mice depleted of DCs demonstrated strongly reduced lymphocyte trapping and T-cell expansion on day 3 after OVA/Mont immunization (Fig. 5B and Fig. S3 A and B) (14). Strikingly, the number and proliferation of FRCs were also significantly reduced (Fig. 5B), indicating a requirement for DCs, consistent with a recent report (17).
Because both migratory and LN-resident DCs are depleted in CD11c-diphtheria toxin receptor (DTR) mice, we investigated whether the transfer of migratory DCs is sufficient to trigger FRC expansion. Injection of LPS-matured bone marrow-derived DCs (BMDCs) with or without OVA protein into WT mice efficiently induced LN swelling and FRC expansion (Fig. 5C and Fig. S3C). Similar results were obtained with CpG-matured BMDCs (Fig. 5C). These results demonstrate that migratory DCs can induce LN swelling as well as FRC expansion, with a potential contribution by adaptive immunity elicited by OVA or bovine serum proteins present in the medium. BMDCs are not direct inducer cells for LN swelling and FRC expansion, however; transfer of BMDCs into mice depleted of endogenous DCs had little effect (Fig. 5D), suggesting that migratory DCs require LN-resident DCs to trigger both processes.
To explore whether signaling via Toll-like receptor (TLR) or IL-1 cytokine family receptors (IL-1/18/33) is required in migratory DCs, we injected MyD88 KO BMDCs matured with either LPS or CpG. Surprisingly, they triggered an FRC expansion comparable to that seen with WT BMDCs (Fig. 5C). In contrast, injection of WT BMDCs into MyD88 KO mice reduced lymphocyte trapping and FRC expansion relative to WT mice (Fig. 5E). However, this reduction was not observed in mice deficient in signaling via TLR2, TLR4, IL-1, or IL-33 (T1-Fc) or deficient in IL-1/18 processing (caspase-1 KO) (Fig. S3D), suggesting redundancy among these pathways or alternative pathways. Similarly, cytokines induced downstream of TLR signals, including STAT-1–dependent IFN-αβ and IFN-γ, demonstrated no limiting role in this process, as did the other TLR adaptor molecule, TRIF (Fig. S3D). Taken together, these findings indicate that endogenous cells, possibly DCs or FRCs, may respond to TLR or IL-1R signaling via MyD88 to detect danger signals, eventually leading to FRC proliferation.
Naive Lymphocyte Accumulation Is Required and Sufficient to Trigger FRC Growth.
Based on our finding that the increase in FRC number closely followed the increase in lymphocyte number over the course of the immune response (Fig. S4A), we tested the hypothesis that inflammatory DCs trigger FRC growth indirectly by inducing naive lymphocyte trapping. In support of this hypothesis, immunization of lymphocyte-deficient RAG2 KO mice with activated BMDCs did not trigger FRC growth (Fig. 6A), despite the presence of endogenous DCs. To further test this model, we induced lymphocyte trapping in the absence of inflammatory signals by injecting WT mice with IL-7/α-IL7 complexes that induce abundant homeostatic T-cell proliferation (27). Within 5 d, the number of total LN cells and T cells increased by several fold (Fig. 6B and Fig. S4B) in the absence of significant T-cell activation (Fig. S4C). An increase in B cells, as well as in resident and migratory DCs, was also detected (Fig. S4 B, D, and E). Importantly, strong FRC expansion was also observed in this setting, indicating that an increase in naive lymphocytes owing to LN trapping may be sufficient to drive FRC proliferation with no need for inflammatory signals or adaptive immunity.
Fig. 6.
Naive lymphocytes and LTβR signaling are required to trigger FRC expansion. Total LN cellularity, as well as the number and proliferation of FRCs, were measured by flow cytometry in six draining LNs at 5.5–6 d (A, B, D–F) or 3 d (C) after the indicated immunization. (A) WT or T/B-cell-deficient (RAG2 KO) mice were immunized s.c. with PBS or WT BMDCs activated with CpG without OVA antigen. (B) WT mice received i.p. injections of PBS or IL-7/α-IL-7 complexes each day for the first 4 d. (C and D) Mice that received OT T cells were injected with LTβR-Fc or control (ctrl) IgG and then immunized with OVA/Mont or PBS, followed by the flow cytometry analysis of six draining LNs at day 3 (C) or day 5.5 (D) after immunization. (E) Representative histogram (Left) and mean fluorescence intensity (MFI; Right) for the surface expression of LTβR on FRCs in LNs of PBS-injected (black line) or OVA/Mont-injected (dashed line) mice. Surface protein expression on CD45+ cells served as a negative control (gray area). (F) OT T cells were adoptively transferred into CD11c-DTR tg or ntg littermate mice, which were then immunized s.c. with OVA/Mont and received a single dose of DT at 3 d after immunization. Data are pooled from two independent experiments that are represented by circles and triangles. Data are mean ± SD, either representative of (A, C, and E) or compiled from (B, D, and F) at least two experiments, with n ≥3 mice per experiment.
Lymphotoxin-αβ/LIGHT Signals Drive the Later FRC Growth Phase.
To further examine which molecular signals delivered by lymphocytes or DCs trigger FRC expansion, we tested inflammatory and growth factor signals known to regulate stromal cell activation and proliferation (12, 28). We found that a deficiency in TNFα, LTα3, PDGF, or VEGF signaling pathways did not strongly affect FRC expansion (Fig. S5A). Notably, however, LTβR-Fc treatment blocking LTαβ and LIGHT led to a marked reduction in the fold increase of FRC numbers and proliferation despite normal LN swelling on day 5.5, but not on day 3 (Fig. 6 C and D), pointing to a role for LTαβ or LIGHT only in the later phase of FRC expansion. Consistent with this finding, FRCs were found to express increased levels of surface LTβR on day 5.5 after immunization (Fig. 6E).
Several hematopoietic sources of LTαβ/LIGHT have been described that could be responsible for stimulating the second growth phase of LTβR-expressing FRCs, including lymphoid tissue inducer (LTi) cells, lymphocytes, and DCs (29, 30). Lack of LTi cells in RORγ KO bone marrow chimera mice did not affect FRC expansion on OVA/Mont immunization (Fig. S5B). Surprisingly, mice deficient in either T cells (TCRβδ KO) or B cells (JH KO) also showed normal FRC proliferation (Fig. S5 C and D). This is in stark contrast to RAG2 KO mice, which did not demonstrate FRC expansion on immunization with OVA/Mont (Fig. S5E), reminiscent of our observations after BMDC transfer (Fig. 6A).
We took two approaches to testing a role of LTαβ/LIGHT on DCs. LTβ-deficient BMDCs injected into WT mice induced a normal FRC expansion (Fig. S5F). Similarly, DC depletion starting on day 3 of the response, when T-cell expansion had become largely DC independent (31) (Fig. S5G), did not markedly change FRC number and proliferation (Fig. 6F). Both experiments suggest that DCs are not required as an LTαβ/LIGHT source.
Taken together, our findings point to a key role of T and B lymphocytes in providing signals for the early and late FRC growth phases, including possibly LTαβ/LIGHT and mechanical stress signals.
Discussion
In this study, we provide a comprehensive analysis of FRC number, phenotype, and function and correlate it with the antigen-specific T-cell response over time. We show that, rather than being disrupted or functionally altered, FRCs increase in size and number to cover a much larger volume while maintaining their vital functions. Within less than 1 d, FRCs are activated in a process dependent on naive lymphocyte trapping induced by DCs. In a later phase, activated lymphocytes further enhance this FRC expansion, presumably in a LTαβ/LIGHT-dependent but DC-independent manner.
FRCs are activated at the onset of LN swelling and T-cell priming, with the first signs of activation evident after 20 h. This suggests that FRCs can sense the increase in lymph flow, inflammatory cytokines, or local DCs early in the response. Alternatively, FRCs may sense mechanical stress or other signals, such as hypoxia, owing to the large number of recently trapped naive lymphocytes. FRC proliferation began at 40 h after immunization, with 50–70% of FRCs incorporating BrdU at the peak of LN swelling, comparable to previous findings with complete Freund’s adjuvant immunization (13, 17). Thus, the strong expansion of the FRC network is related largely to local cell proliferation.
Our findings do not exclude a contribution by circulating fibroblasts or transdifferentiation from other cell types (32, 33). Given the lack of striking morphological alteration of the existing FRC network, we propose that the network expands at its boundaries, most prominently in the strongly inflated medullary area, where FRC-like cells were observed in conjunction with matrix proteins. This expansion must go along with enhanced matrix synthesis, because the conduit network appears to remain functional throughout the growing T zone (34).
In contrast to previous reports investigating mostly the spleen (20, 21), we observed only a moderate and very transient decrease in relative transcript levels of Il7, Ccl19, and Ccl21 during the immune response, suggesting that most FRCs in the LN T zone maintain not only their structural characteristics, but also their functional characteristics. The precise function of medullary FRCs remains to be established, however, given that they colocalize with plasma cells rather than with T cells. Medullary FRCs also localize next to LECs, suggesting that they may promote lymphatic vessel growth by providing VEGF. Consistent with earlier reports (4, 9, 35), we observed a strong increase in both LEC and BEC numbers and proliferation during LN swelling. Thus, LN hyperplasia is associated not only with an increase in lymphocyte numbers, but also with an equivalent increase in all three major stromal cell populations that provide the organ infrastructure and organization for both naive and activated lymphocytes. Therefore, we postulate that the generation of a protective adaptive immune response strongly depends on an efficient expansion of the FRC network that provides the niches for the rapid selection, expansion, and differentiation of antigen-specific lymphocytes.
How is FRC proliferation triggered during LN swelling? We observed a strong dependence of FRC expansion on migratory and resident DCs, although at present we cannot formally exclude a role for subcapsular sinus macrophages, which are also depleted on injection of diphtheria toxin (DT) into CD11c-DTR mice (36). Comparable findings were reported by Lu et al. (17) using CD11c-DTR and CCR7 KO mice. They concluded that migratory DCs transmit signals to CCR7-negative resident DCs, which then directly trigger FRC growth.
We obtained several lines of evidence supporting an alternative model in which lymphocyte numbers control FRC expansion with only an indirect role for DCs. First, the number of FRCs closely correlated with the number of total lymphocytes during LN swelling, but showed no clear correlation with activated T-cell numbers. Second, at 3 d after DC depletion, we observed a 40–70% decrease in lymphocyte numbers, which correlated with the reduction in FRC numbers. This observation is consistent with reports showing that resident DCs regulate naive lymphocyte recirculation and LN cellularity by modifying HEVs (15). Third, we and others (17) detected FRC expansion in immunized WT mice but not in RAG2 KO mice, indicating that activated DCs are ineffective in promoting FRC growth in the absence of lymphocytes. Fourth, DC depletion after successful T-cell priming did not interfere with FRC expansion. Fifth, homeostatic T-cell expansion induced by IL-7/α-IL-7 immune complexes was sufficient to trigger FRC growth by inducing LN swelling without the aid of activated migratory DCs or inflammatory signals. In conclusion, we propose a model in which migratory DCs transmit a signal to resident DCs that then trigger HEV/LEC changes, leading to naive lymphocyte trapping, which is critical for mediating FRC expansion (Fig. S6). Only in a later phase did activated lymphocytes appear to further boost FRC expansion.
What are the signals triggering FRC growth? Our results suggest that MyD88 is involved as key sensor of inflammation, with a role for this adaptor protein in endogenous cells rather than in the transferred BMDCs. Similar to DCs and other APCs, FRCs and vascular cells express transcripts for MyD88 (37), and both cell types may participate in sensing early signals of danger and inflammation. The signals leading to MyD88 activation await further exploration, given that mice deficient in the TLR-2, TLR-4, IL-1, IL-18, or IL-33 pathway demonstrated normal FRC expansion during LN swelling.
The second positive regulator of FRC expansion that we identified is LTβR, considering that LTβR-Fc partially inhibited FRC expansion by blocking the ligands LTαβ and LIGHT. Because LIGHT also binds to HVEM, a role for this alternative receptor cannot be formally ruled out. Given that this pathway has little effect on stromal cell biology, we favor a model involving the LTαβ-LTβR pathway (38). Naive lymphocytes and NK cells express low levels of LTαβ, which are strongly up-regulated during activation (29). Recirculating LTαβ+VEGF+ B cells are known to contribute to stromal cell growth (4, 39), but, surprisingly, neither B lymphocytes nor T lymphocytes were required in our experimental setting. Migratory DCs have been proposed to express LTαβ and VEGF, thereby regulating the growth of HEVs and LECs once they have homed into the T zone, both in homeostasis and in the immune response (15). LTαβ+ DCs also stimulate HEV-proximal FRCs to express more CCL21 and VEGF, which may then indirectly increase lymphocyte recirculation and vessel growth (19, 40, 41).
Here we show that LN FRC numbers in homeostasis depend on both DCs and B cells, but without a limiting role for LTβR ligands. In contrast, on immunization, we observed a requirement for DCs in combination with either T lymphocytes or B lymphocytes for FRC proliferation, leading us to propose that DCs trigger this process via naive lymphocyte trapping. Given that LTβR-Fc inhibited only the later phase of FRC expansion, a role for LTαβ expression by DCs or LTi cells appears less likely, and instead may be related to activated lymphocytes expressing high levels of LTαβ and LIGHT in this later phase of the response (29). Similarly, HEV expansion during lymphocytic choriomeningitis virus infection is dependent on activated LTαβ+ B cells (42). Interestingly, noncognate B cells provide LTβR signals to remodel the medullary cords during immune response (6). Thus, the later LTαβ/LIGHT-dependent growth phase of FRCs may occur predominantly in that zone, with contributions by naive B cells.
Besides chemical signals, physical signals also may trigger FRC expansion. Fibroblasts are known to be mechanosensitive (25). Given that FRCs form a network throughout the T zone, it is conceivable that early in the response, FRCs sense the physical pressure of trapped lymphocytes (3). In addition, FRCs may detect the increase in lymph flow within conduits that accompanies skin inflammation (43). Indeed, FRC lines can respond to fluid flow with increased proliferation and CCL21 expression (44). The incoming lymph could allow the transport of proinflammatory mediators from inflammatory sites to LN T zones within minutes (43), possibly explaining the observed FRC activation after 20 h and the partial MyD88 dependence of this process. Consistent with LN FRCs acting as early sensors of infection, in vivo LN FRCs showed marked transcriptional changes at 12 h after LPS injection (37). Similarly, FRC lines responded rapidly to proinflammatory cytokines, including IL-1, IFN-αβ, IFN-γ, and TNF-α (16, 45). Whereas FRC size, granularity, and gp38 expression were up-regulated within hours after immunization, intracellular α-SMA expression peaked 2–4 d later, suggesting different stages in FRC activation and possibly function.
In conclusion, our data demonstrate that FRCs are early sensors of inflammation and rapidly adapt structurally and functionally to accommodate more lymphocytes in inflamed LNs. It seems likely that several cells and signals collaborate to regulate FRC numbers in the T zone and medulla and thereby the number of lymphocyte niches, as summarized in our model (Fig. S6). Whether the opposite process of LN shrinking is regulated by the absence of those positive signals or by specialized anti-inflammatory factors remains an exciting open question of high clinical relevance.
Materials and Methods
Animals.
C57BL/6 mice were obtained from Janvier. OT-1 and OT-2 mice were obtained from The Jackson Laboratory and bred onto a CD45.1+ C57BL/6 background. RAG2 KO, Caspase-1 KO, TCRβδ KO, TNF-R1 KO, and CD11c-DTR/GFP transgenic mice were obtained from The Jackson Laboratory. MyD88 KO (46), TRIF KO (47), TLR2 KO (48), and TLR4 KO mice (49) were kindly provided by S. Akira (Osaka University, Osaka). T1-Fc mice (50) were kindly provided by D. Pinschewer (University of Basel, Basel); RORγ KO mice (51), by D. Littman (New York University, New York); LTβ KO mice (52), by M. Heikenwälder (Technical University of Munich, Munich); and IFNαR1 KO mice (53), by H. Acha-Orbea (University of Lausanne, Lausanne, Switzerland). STAT1 KO and JH KO mice were obtained from Taconic.
Mouse Treatments.
A total of 106 OT-1 and 106 OT-2 splenocytes were injected i.v. at 24 h before immunization. OVA protein (Sigma-Aldrich) was dissolved in PBS and then mixed with Montanide (Seppic) at a 3:1 ratio by vortexing. Mice received six s.c. OVA injections of 50 μg in areas drained by axial, brachial, and inguinal LNs. Alternatively, BMDCs were generated as described previously (54) and activated with 0.1 µg/mL LPS (Sigma-Aldrich) or 5 µg/mL CpG (ODN1668; Trilink) for 16 h before injection. BDMCs were labeled with 2 μM CFSE (Invitrogen) and resuspended in PBS, and 106 BMDCs were injected s.c. into each site as described above. For BrdU administration, mice were injected with 100 μL of 10 mg/mL BrdU in PBS and then maintained on BrdU-containing drinking water (0.8 mg/mL). For IL-7/α-IL-7 immune complex injection, anti-human IL-7 antibodies (M25; 50 μg/mL) were mixed with mouse IL-7 (10 μg/mL) in the same volume, and 200 µL was administered i.p. to mice on days 0, 1, 2, and 3.
To deplete DCs, DT (Sigma-Aldrich) dissolved in PBS was injected i.p. once at 4 ng/g body weight at 16 h before immunization. For L. major infection, 3 × 106 stationary LV39 L. major promastigotes were injected s.c. into the hind limb footpads. For LTαβ/LIGHT blocking, 200 μg of mLTβR-Fc or control human IgG1 antibody (Biogen) was injected at 1 d before immunization, followed by 100 μg i.p. every 2 d. For PDGFRα blocking in vivo, 200 µg of anti-PDGFRα (APA5; hybridoma kindly provided by S.-I. Nishikawa, Riken Center, Kobe, Japan) and control IgG2a antibody (2A3; BioXCell) were injected i.p. at 1 d before immunization, followed by 100 µg every 2 d. For IL-1β blocking, 200 μg of neutralizing antibody (Novartis Pharmaceuticals) were injected i.p. at 1 d before and 1 d after immunization. For anakinra (soluble IL-1R; Kineret), WT mice were injected i.p. with 200 µg of anakinra every 12 h. Imatinib (Novartis) was dissolved in water and injected i.p. at 50 μg/g daily. Nilotinib (55) and AAL993 (56) (both from Novartis) were dissolved in N-methylpyrrolidinone and diluted 1/10 in PEG300, and mice were given 150 mg/kg or 100 mg/kg, respectively, orally each day. All animal experiments were authorized by the Swiss Federal Veterinary Office.
Isolation of Stromal Cells and Flow Cytometry Analysis.
Unless stated otherwise, the six peripheral LNs (axillary, inguinal, and brachial) were collected and completely digested using collagenase IV (1 mg/mL; Worthington), followed by collagenase D (1 mg/mL; Roche), and then labeled for flow cytometry analysis, as described previously (3). Dead cells were excluded using 7-AAD or DAPI (Invitrogen). Data were acquired on an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software (TreeStar). BrdU (Sigma-Aldrich) administration and surface staining (using doubled antibody concentrations) and fixation of cells were performed as described previously (3), except that cells were incubated with 70 Kunitz U/mL DNase I (Roche) for 45 min at 37 °C and with FITC-conjugated anti-BrdU mAb or an isotype-matched control mAb (BD Biosciences) for 60 min at room temperature. Antibodies used for flow cytometry are listed in Table S1.
Immunofluorescence Staining and Imaging.
Cryostat sections (8–10 μm) of Tissue-Tek optimum cutting temperature (OCT) compound (Sakura)-embedded LNs were collected on Superfrost/Plus glass slides (Fisher Scientific), then air-dried overnight, fixed in ice-cold acetone for 10 min, and rehydrated in PBS. Sections were quenched with 0.3% H2O2 in PBS and blocked with 0.1% BSA and 1–4% (vol/vol) animal serum in PBS, followed by a streptavidin-biotin blocking kit (Vector Laboratories), as described previously (3). Stainings were performed for 60 min at room temperature using the antibodies listed in Table S2. For gp38, the staining was revealed using HRP-conjugated secondary reagents followed by tyramide signal amplification (Molecular Probes Kit 22) according to the manufacturer’s instructions, but using a borate buffer (0.1 M in PBS; pH 8.5) for tyramide dilution. Images were acquired on a Leica DM5500 microscope with a Leica DFC320 camera or on a Leica DM IRE2 microscope with a Leica TCS SP2 AOBS laser scanning confocal head. To visualize functional conduits, 20 µL of Texas Red-labeled 10-kDa dextran (Invitrogen) was injected s.c. at 5 mg/mL into the footpad, and mice were killed 30 min later. LNs were then removed and fixed in 4% (wt/vol) paraformaldehyde at 4 °C for 2 h, then saturated in 30% (wt/vol) sucrose for 3 h at 4 °C before being embedded in OCT compound and frozen in an ethanol dry ice bath.
Vibratome Sections.
Isolated LNs were fixed overnight at 4 °C in freshly prepared 1% paraformaldehyde in PBS, washed, and embedded in 4% (wt/vol) low-gelling agarose (Sigma-Aldrich) in PBS. Then 100- to 200-µm sections were cut with a vibratome (Microm HM 650V). Sections were blocked with 1% BSA for 1 h and stained for at least 3–12 h with the antibodies listed in Table S2, then washed extensively in PBS and embedded using Elvanol (Mowiol; Calbiochem). Images were obtained with a Zeiss Axio Imager upright microscope. Three-dimensional image reconstructions were created with Imaris software, and segment length was measured using the FilamentTracer plugin (both from Bitplane).
RNA Isolation and Quantitative RT-PCR Analysis.
LNs were mashed through a 40-µm filter using a plunger, with the filtered cells representing the soluble fraction and the remaining white matter the nonsoluble fraction. RNA was extracted using TRIzol reagent (Invitrogen). First-strand cDNA synthesis (Superscript II; Invitrogen) was performed according to the manufacturer’s instructions using random nonamer primers (Microsynth). cDNA was purified with the NucleoSpin Extract II Kit (Macherey-Nagel), and individual transcripts were assessed by quantitative RT-PCR using the Light Cycler-FastStart DNAMaster SYBR Green I Kit (Roche Diagnostics) on a Light Cycler 2.0 machine (Roche Diagnostics). Efficiency-corrected gene expression of target genes was normalized with the geometric mean of expression of two housekeeping genes, hypoxanthine guanine phosphoribosyl transferase (hprt1) and TATA-binding protein (tbp). Primer sequences (Microsynth) are listed in Table S3.
In Situ Hybridization.
The Ccl19, Ccl21, and Il7 riboprobes were cloned and used as described previously (3). In brief, frozen 8-μm-thick sections were fixed in 4% (wt/vol) paraformaldehyde, washed in PBS, and incubated with prehybridization solution, followed by overnight incubation at 60 °C with sense or antisense digoxigenin-labeled riboprobes. After several washes, sections were incubated with sheep anti-digoxigenin antibody, followed by alkaline phosphatase-coupled donkey anti-sheep antibody. The color reaction was achieved using nitro blue tetrazolium (Bio-Rad) and 5-bromo-4-chloro-3-indolylphosphate (Sigma-Aldrich), followed by antibody staining for B220 and laminin. Hybridization signals were imaged by transmission microscopy and false-colored in green.
Statistical Analysis.
Statistical significance was determined using an unpaired two-tailed Student t test for unequal variances. P values are indicated as *P < 0.05, **P < 0.01, ***P < 0.001, and ns, statistically not significant.
Supplementary Material
Acknowledgments
We thank J. Stein and V. Kumar for help with the segment length analysis; H. Acha-Orbea, I. Ferrero, D. Pinschewer, T. Roger, and J. Tschopp for the KO mice; O. Boyman and C. Krieg for IL-7 complexes; A. So for IL-1 inhibitors; Novartis for pharmacological inhibitors; J. Browning and Biogen Idec for LTβR-Fc and TNFR1-Fc; C. Ronet for L. major preparation; G. Guarda and D. Zehn for discussions; J.-C. Stehle (Mouse Pathology Facility) for histological sections; D. Labes, A. Wilson (Flow Cytometry Facility), F. Morgenthaler, and Y. Krempp (Cellular Imaging Facility) for technical expertise; J. Perrin and B. Bujisic for assistance with immunofluorescence microscopy; and K. Schäuble and Ania Zuba for a critical read of the manuscript. This study was supported by the Swiss National Science Foundation (Grants PPOOA-116896/1 and 31003A-130488/1, to S.A.L.), Boehringer Ingelheim Fonds (T.K.V.), and the Taiwan National Science Council (H.-Y.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312585111/-/DCSupplemental.
References
- 1.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9(9):618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol. 2010;10(12):813–825. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
- 3.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 4.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Mohr E, et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182(4):2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 6.Abe J, et al. B cells regulate antibody responses through the medullary remodeling of inflamed lymph nodes. Int Immunol. 2012;24(1):17–27. doi: 10.1093/intimm/dxr089. [DOI] [PubMed] [Google Scholar]
- 7.Bajénoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25(6):989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegert S, Luther SA. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol. 2012;3:285. doi: 10.3389/fimmu.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chyou S, et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol. 2008;181(6):3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katakai T, et al. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181(9):6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 11.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012;30:69–94. doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- 12.Zhu MZ, Fu YX. The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling. Immunol Rev. 2011;244(1):75–84. doi: 10.1111/j.1600-065X.2011.01061.x. [DOI] [PubMed] [Google Scholar]
- 13.Tan KW, et al. Expansion of cortical and medullary sinuses restrains lymph node hypertrophy during prolonged inflammation. J Immunol. 2012;188(8):4065–4080. doi: 10.4049/jimmunol.1101854. [DOI] [PubMed] [Google Scholar]
- 14.Webster B, et al. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203(8):1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 16.Katakai T, Hara T, Sugai M, Gonda H, Shimizu A. Lymph node fibroblastic reticular cells construct the stromal reticulum via contact with lymphocytes. J Exp Med. 2004;200(6):783–795. doi: 10.1084/jem.20040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chyou S, et al. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J Immunol. 2011;187(11):5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess E, et al. RANKL induces organized lymph node growth by stromal cell proliferation. J Immunol. 2012;188(3):1245–1254. doi: 10.4049/jimmunol.1101513. [DOI] [PubMed] [Google Scholar]
- 19.Tzeng TC, et al. CD11c(hi) dendritic cells regulate the re-establishment of vascular quiescence and stabilization after immune stimulation of lymph nodes. J Immunol. 2010;184(8):4247–4257. doi: 10.4049/jimmunol.0902914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller SN, et al. Regulation of homeostatic chemokine expression and cell trafficking during immune responses. Science. 2007;317(5838):670–674. doi: 10.1126/science.1144830. [DOI] [PubMed] [Google Scholar]
- 21.Scandella E, et al. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9(6):667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 22.Vega F, et al. Tissue-specific function of lymph node fibroblastic reticulum cells. Pathobiology. 2006;73(2):71–81. doi: 10.1159/000094491. [DOI] [PubMed] [Google Scholar]
- 23.Benedict CA, et al. Specific remodeling of splenic architecture by cytomegalovirus. PLoS Pathog. 2006;2(3):e16. doi: 10.1371/journal.ppat.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekiaris V, et al. Ly49H+ NK cells migrate to and protect splenic white pulp stroma from murine cytomegalovirus infection. J Immunol. 2008;180(10):6768–6776. doi: 10.4049/jimmunol.180.10.6768. [DOI] [PubMed] [Google Scholar]
- 25.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Fontecha A, et al. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J Exp Med. 2003;198(4):615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T cell expansion without lymphopenia. J Immunol. 2008;180(11):7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 28.Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev. 2004;202:49–66. doi: 10.1111/j.0105-2896.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 30.Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479(7374):542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- 31.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203(9):2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bénézech C, et al. Lymphotoxin-β receptor signaling through NF-κB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 2012;37(4):721–734. doi: 10.1016/j.immuni.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: Emerging effector cells in chronic inflammation. Nat Rev Immunol. 2011;11(6):427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gretz JE, Norbury CC, Anderson AO, Proudfoot AEI, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192(10):1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177(5):3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- 36.Probst HC, et al. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141(3):398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra D, et al. Immunological Genome Project Consortium Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol. 2012;13(5):499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ware CF. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev. 2008;223:186–201. doi: 10.1111/j.1600-065X.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194(11):1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wendland M, et al. Lymph node T cell homeostasis relies on steady-state homing of dendritic cells. Immunity. 2011;35(6):945–957. doi: 10.1016/j.immuni.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Ngo VN, et al. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189(2):403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar V, et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin–dependent pathway. Blood. 2010;115(23):4725–4733. doi: 10.1182/blood-2009-10-250118. [DOI] [PubMed] [Google Scholar]
- 43.Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20(12):1483–1487. doi: 10.1093/intimm/dxn110. [DOI] [PubMed] [Google Scholar]
- 44.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol. 2009;183(7):4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 45.Siegert S, et al. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS ONE. 2011;6(11):e27618. doi: 10.1371/journal.pone.0027618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi O, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 49.Hoshino K, et al. Cutting edge. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–3752. [PubMed] [Google Scholar]
- 50.Senn KA, et al. T1-deficient and T1-Fc-transgenic mice develop a normal protective Th2-type immune response following infection with Nippostrongylus brasiliensis. Eur J Immunol. 2000;30(7):1929–1938. doi: 10.1002/1521-4141(200007)30:7<1929::AID-IMMU1929>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Sun Z, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 52.Koni PA, et al. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6(4):491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 53.Müller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 54.Vogt TK, Link A, Perrin J, Finke D, Luther SA. Novel function for interleukin-7 in dendritic cell development. Blood. 2009;113(17):3961–3968. doi: 10.1182/blood-2008-08-176321. [DOI] [PubMed] [Google Scholar]
- 55.Manley PW, et al. Structural resemblances and comparisons of the relative pharmacological properties of imatinib and nilotinib. Bioorg Med Chem. 2010;18(19):6977–6986. doi: 10.1016/j.bmc.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 56.Manley PW, et al. Anthranilic acid amides: A novel class of antiangiogenic VEGF receptor kinase inhibitors. J Med Chem. 2002;45(26):5687–5693. doi: 10.1021/jm020899q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.