Significance
Dendrites are highly polarized structures that are important for synaptic input and signal integration. However, the molecular and cellular mechanism underlying the polarized dendrite development in vivo remains largely unknown. This report shows that LKB1, a serine-threonine protein kinase known to be involved in axon formation in embryonic cortical pyramidal neurons, is essential for the polarized initiation and oriented extension of the primary dendrite in adult-born hippocampal granule cells, and provides further evidence that this morphogenic effect of LKB1 is mediated via regulation of polarized distribution of Golgi apparatus. These findings not only add a unique function to the growing list of LKB1 cellular actions, but also provide new clues to the subcellular mechanism of LKB1’s action.
Keywords: adult neurogenesis, neuronal polarization, Golgi deployment
Abstract
Adult-born granule cells in the dentate gyrus of the rodent hippocampus are important for memory formation and mood regulation, but the cellular mechanism underlying their polarized development, a process critical for their incorporation into functional circuits, remains unknown. We found that deletion of the serine-threonine protein kinase LKB1 or overexpression of dominant-negative LKB1 reduced the polarized initiation of the primary dendrite from the soma and disrupted its oriented growth toward the molecular layer. This abnormality correlated with the dispersion of Golgi apparatus that normally accumulated at the base and within the initial segment of the primary dendrite, and was mimicked by disrupting Golgi organization via altering the expression of Golgi structural proteins GM130 or GRASP65. Thus, besides its known function in axon formation in embryonic pyramidal neurons, LKB1 plays an additional role in regulating polarized dendrite morphogenesis in adult-born granule cells in the hippocampus.
Granule cells are continuously being generated in the dentate gyrus of the adult hippocampus (1). These adult-born neurons play an important role in memory formation (2–4) and mood regulation (5, 6). Typically, developing granule cells assume a bipolar morphology, with highly branched dendrites extending toward the molecular layer, and a long thin axon projecting into the hilar area (7). This asymmetric structure provides the anatomical basis for directional information flow within the neuron, receiving input from the entorhinal cortex at the dendrite and sending axonal output to the CA3 region. However, little is known about the mechanism responsible for the polarization of these newborn neurons within the adult tissue environment, including the specification of a proper number of axons and dendrites, as well as the extension of these processes with proper orientation relative to the layer structure of the dentate gyrus.
The serine/threonine protein kinase liver kinase B1 (LKB1) is a well-known regulator of cell polarity; it was originally identified as one of the six master regulators of anterior–posterior axis of the Caenorhabditis elegans zygote (8). Growing evidence indicates that LKB1 also plays important roles in cellular polarization in epithelial and other nonneural tissues in Drosophila and vertebrates (9–11). In the rodent central nervous system, LKB1 was shown to regulate axon formation and cell migration in embryonic cortical pyramidal neurons (12–14). However, it is unclear whether LKB1 also regulates other asymmetrical aspects of neuronal development, such as the polarized dendrite formation. Moreover, because LKB1 is also expressed in the adult brain, whether it plays a role in the morphogenesis of newborn neurons within the adult tissue environment remains to be determined.
In this study, we used a retrovirus-mediated gene-transfer approach (15) to delete the LKB1 allele in adult-born hippocampal granule cells, and found that LKB1 is essential for polarized initiation of a single primary dendrite from the soma and oriented growth of its arbor toward the molecular layer. We also obtained evidence that the effect of LKB1 on polarized dendrite development is mediated by regulating the distribution of the Golgi apparatus in the cytoplasm. Together, the data from this study demonstrate a unique function of LKB1 in dendrite morphogenesis and suggest a cellular mechanism underlying its action.
Results
Elevated LKB1 Expression in Newborn Neurons of Adult Dentate Gyrus.
We first examined the expression pattern of LKB1 in the dentate gyrus of the 8-wk-old adult mouse hippocampus. Immunostaining of hippocampal sections for LKB1 showed a high level of LKB1 expression in the nuclei of granule cells throughout the dentate gyrus, but the active form of LKB1 (phosphorylated LKB1 at the site Ser431, pLKB1S431) was mainly localized to the cytoplasm of granule cells (Fig. S1A). Coimmunostaining for LKB1 and cell-type–specific markers showed that LKB1 expression level was rather low in Sox2+GFAP+ neural stem cells, Sox2+Nestin+ neural progenitors, or minichromosome maintenance protein 2 and doublecortin (MCM2+DCX+) neuroblasts, in comparison with neighboring granule cells, and the level of LKB1 was highly increased in DCX+NeuN+ new mature neurons (Fig. S1B, white dotted circles). Moreover, the elevation of LKB1 occurred at the time interval between MCM2 and NeuN expression, as the level of LKB1 was rather high in MCM2−DCX+ and DCX+NeuN− cells, indicating increased LKB1 expression in immature postmitotic neurons (Fig. S1B, yellow full circles with arrows).
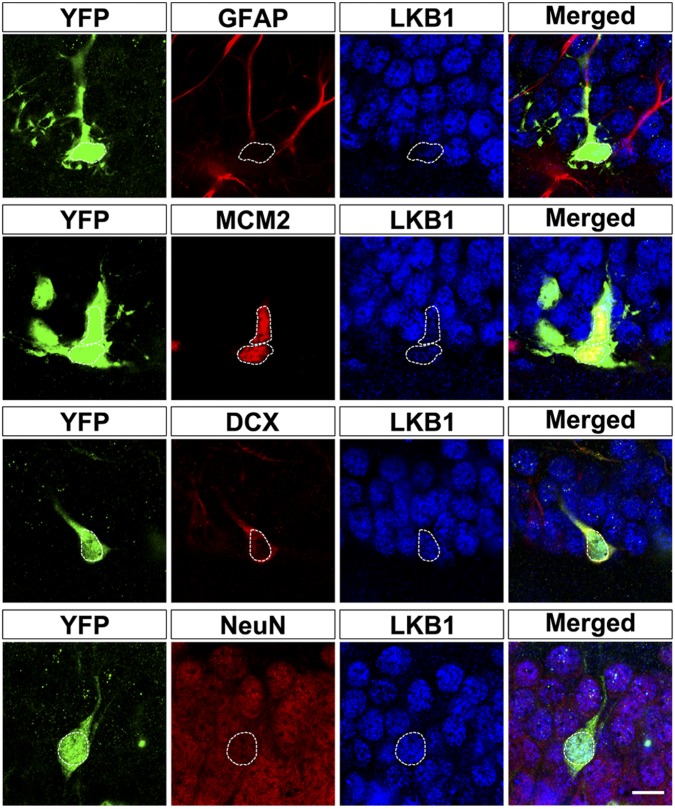
To determine the cell morphology more clearly, we used Nestin-CreERT2/Rosa26-YFP transgenic mice for inducible labeling (16). When we injected 180 mg/kg tamoxifen into the abdomen of 8-wk-old adult transgenic mice for three consecutive days and checked the labeling cells 1 mo after induction, we observed that LKB1 was expressed at a low level in GFAP+ radial glia-like cells and MCM2+ intermediate progenitor cells, but was highly expressed in DCX+ immature and NeuN+ mature newborn neurons (Fig. 1), consistent with that was observed in wild-type mice (Fig. S1B).
Fig. 1.
Expression pattern of LKB1 in the adult dentate gyrus. Sample confocal images of hippocampal slices in adult Nestin-CreER/Rosa26-YFP mice that were double-stained with antibodies against GFAP/LKB1, MCM2/LKB1, DCX/LKB1, and NeuN/LKB1. White dotted circles, the nuclei of colabeled cells. Observation time: 1-mo postinjection. (Scale bar, 10 μm.)
This expression pattern suggests that LKB1 may play a role in the development of adult-born granule cells in the mouse hippocampus.
LKB1 Regulates Oriented Dendrite Initiation and Extension in Granule Cells.
Because mice with LKB1-null mutation died between embryonic days 9 and 10 with defects in neural tube development (17), we used retrovirus injection to specifically delete the LKB1 allele (18) in proliferating cells and their progenies in the adult mice at 8 wk after birth. A retroviral vector was constructed to express Cre recombinase gene under the control of Ubiquitin (Ubi) promoter, with a downstream GFP gene linked by an E2A element (Fig. S2A). This process allowed GFP labeling of the cells in which targeted gene manipulation occurred. To assess the action of Cre recombinase, we stereotaxically injected this Ubi-Cre-E2A-GFP retrovirus into the hippocampus of Rosa26-tdTomato reporter mice, and found that GFP-labeled neurons also expressed Cre and tdTomato, indicating successful recombination (Fig. S2B).
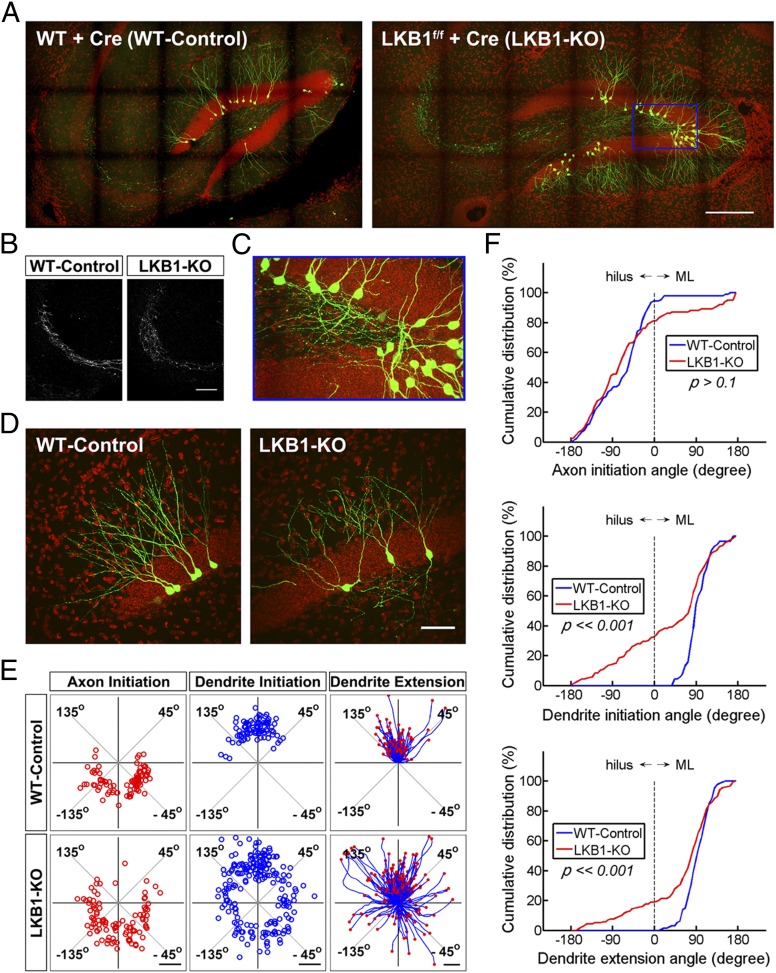
We then injected this same Ubi-Cre-E2A-GFP retrovirus into the hippocampus of LKB1-flox (LKB1f/f) mice to delete the LKB1 allele in newborn granule cells (LKB1-KO) and examined the morphology of infected cells at 3 wk after injection, when most dendritic arbors are being established (7). As the control, the same retrovirus was injected into wild-type (WT) mice where LKB1 allele remained intact (WT-Control). Large-scale montage images showed that, in WT-Control mice, somata of GFP-expressing neurons were located at the inner granule cell layer, with all their dendrites extending toward the molecular layer and axons projecting along the mossy fiber pathway toward the CA3 region (Fig. 2A). In LKB1-KO mice, we found no obvious difference in the axon morphology and their projections (Fig. 2 A and B), suggesting LKB1 is not required for axon formation and pathfinding of these neurons at this stage of development. However, many aberrant primary dendrites were found to ectopically project into the hilar region in LKB1-KO mice (Fig. 2 A and C).
Fig. 2.
LKB1 deletion in adult-born granule cells affects oriented initiation and extension of the primary dendrite. (A) Large-scale montage images of hippocampal sections in wild-type and LKB1-flox mice that were infected with Ubi-Cre-E2A-GFP retroviruses. Red, DAPI; green, GFP. Observation time: 3-wk postinjection. (Scale bar, 200 μm.) (B) Sample confocal images of Mossy fibers in the CA3 region of WT and LKB1-flox mice infected with Ubi-Cre-E2A-GFP, respectively. (Scale bar, 100 μm.) (C) Confocal image of aberrant dendrites extending into the hilus, a higher magnification view (10:3) of the region marked by the blue box in A. (D) Sample confocal images of retrovirus-infected granule cells in WT and LKB1-flox mice at a higher resolution. (Scale bar, 50 μm.) (E) Angular distribution of the axon and dendrite initiation sites on the soma, and tracing of primary dendrites. [Scale bars, 5 μm (Left, Center) 20 μm (Right).] (n = 80–120 cells from four to six mice for each plot.) (F) Cumulative percentage plots for the angular distribution of axon and dendrite initiation sites, and end points of primary dendrites (P value as marked; K-S test).
Further examination of individual neurons revealed that most neurons (88%) in WT-Control mice had only one primary dendrite and the rest (12%) exhibited multiple primary dendrites, with all their dendritic arbors extending toward the molecular layer (Fig. 2D). In LKB1-KO mice, however, more than 80% of the neurons bore multiple primary dendrites, with many dendritic arbors extending in parallel to the granule layer or even toward the hilus (Fig. 2D). Quantitative analysis of oriented dendrite initiation and extension relative to the granule layer was further performed as follows. For dendrite initiation, the orientation was determined by defining the angular location of the initiation site on the soma (19), with the origin at the soma center, and the x (0° to ± 180°) and y (±90°) axes parallel and perpendicular to the granule layer, respectively (Fig. S3A). For the orientation of the dendrite, we determined the angular location of the end point of the first segment of the primary dendrite (20), with the origin at the dendrite initiation site on the soma surface and same coordinate as that for dendrite initiation sites (Fig. S3B). These analyses showed that a great majority of dendrite initiation sites in WT-Control neurons were preferentially oriented toward the molecular layer (within 45–135°). In contrast, virus-infected LKB1-KO neurons showed a clear dispersal of initiation sites toward lateral directions (within ±45° and ±135°) and the hilus (from −135° to −45°) (Fig. 2E). Tracing of primary dendrites also showed that the preference for dendrite orientation toward the molecular layer was largely lost in LKB1-KO neurons (Fig. 2E). Cumulative percentage plots of the distribution of dendrite initiation angles and the end points of primary dendrites showed marked differences between WT-Control and LKB1-KO neurons [P < < 0.001, Kolmogorov–Smirnov (K-S) test] (Fig. 2F). In contrast to the abnormality in dendrite initiation and extension, the axon initiation site from the soma was not significantly different between WT-Control and LKB1-KO neurons (P > 0.1, K-S test), with most axons initiated toward the hilus (Fig. 2 E and F). These results indicate that LKB1 is critical for both the polarized initiation and extension of dendrites, but not for axon initiation and projection, in newborn granule cells of the adult mouse hippocampus.
We further expressed exogenous LKB1 in LKB1-KO neurons by injecting CAG-Cre mixed with Ubi-LKB1-E2A-GFP retroviruses into LKB1f/f mice for the rescue test. To examine the efficiency of virus-mediated LKB1 manipulation, we immunostained virus-injected hippocampal slices for LKB1. We found that LKB1 expression was completely abolished in cells infected with CAG-Cre alone (LKB1-deleted cells). In comparison with neighboring uninfected cells (wild-type cells), cells infected with Ubi-LKB1-E2A-GFP alone (LKB1-overexpressed cells) showed a much higher LKB1 level, whereas those expressing both CAG-Cre and Ubi-LKB1-E2A-GFP (LKB1 deletion restored by overexpression cells) showed a slightly higher LKB1 level (Fig. S4). This expression pattern supports the validity of virus-mediated LKB1 gene manipulation.
In comparison with the LKB1f/f mice injected with CAG-Cre and Ubi-LKB1-E2A-GFP retroviruses mixture (Cre+LKB1), we also injected the same CAG-Cre retroviruses mixed with Ubi-GFP (Cre+GFP) into LKB1f/f mice as the control for the rescue experiment. Examination of GFP-labeling and Cre-expression neurons at 3 wk after injection revealed marked defects of dendrite morphogenesis in Cre+GFP mice, in which polarized initiation of primary dendrite from the soma was reduced and oriented dendrite growth toward the molecular layer was disrupted, similar to that were found in LKB1-KO mice (Fig. S5 A and B). As expected, these phenotypes totally disappeared in Cre+LKB1 mice [Fig. S5 A and B (P < < 0.001, K-S test) and Fig. S5C], indicating the specificity in the action of LKB1 deletion on dendrite morphogenesis.
In addition to retrovirus-mediated LKB1 knockout in the dentate gyrus, we also used an inducible Nestin-CreERT2/LKB1f/f/Rosa26-tdTomato (LKB1-CKO) gene knockout system to specifically delete LKB1 allele in the adult stage (16). Accordingly, Nestin-CreERT2/Rosa26-tdTomato mice were used as the control. Tamoxifen was injected into the abdomen of 8-wk-old transgenic mice at 180 mg/kg for three consecutive days, and the morphology of the tdTomato labeling cells was examined 2 mo later when most labeled cells had differentiated into neurons and established their dendritic arbors (21). We found the adult-born granule cells exhibited defective phenotypes in both dendrite initiation and extension similar to that were found in retrovirus-mediated LKB1-KO mice. In control mice, most neurons exhibited only one primary dendrite, with dendritic arbors initiating toward the molecular layer (Fig. S6 A and B). In contrast, nearly 40% of LKB1-CKO neurons had multiple primary dendrites, with their initiation sites dispersing around the soma and arbors extending in all directions (Fig. S6 A and B). The remaining 60% of the neurons represented normal morphology similar to control neurons, which might be because of the leakage of Rosa26-tdTomato mice or the low efficiency of the inducible gene-knockout system. Quantitative analysis showed marked differences in both dendrite initiation and extension between LKB1-CKO neurons with multiple primary dendrites and control neurons (P < < 0.001, K-S test) (Fig. S6C).
Taken together, these results indicate that LKB1 is critical for both the polarized initiation and extension of the single primary dendrite in newborn granule cells of the adult mouse hippocampus.
Site-Specific Phosphorylation of LKB1 Is Important for Dendrite Morphogenesis.
To further study LKB1 function, we overexpressed LKB1 (WT-LKB1) in the adult-born neurons using retrovirus injection strategy. Because PKA-dependent phosphorylation at Ser431 is important for LKB1 activation (9), we also generated the dominant-negative LKB1 (DN-LKB1) and constitutively-active LKB1 (CA-LKB1) by point mutation of Ser431 to alanine and glutamine, respectively.
At 3 wk after injection, we found that the expression of DN-LKB1 led to dendrite defects similar to that observed in LKB1-KO neurons: the majority of neurons exhibited multiple dendrites, with aberrant dendrites extending in parallel to the granule layer or toward the hilar region. Orientation analysis showed a reduction of polarized dendrite initiation from the soma and polarized dendrite growth toward the molecular layer (P < < 0.001, K-S test) (Fig. S7). However, the effects of DN-LKB1 expression on dendrite polarity were weaker than those observed in LKB1-KO neurons. In contrast, expression of CA-LKB1 did not result in any obvious dendrite abnormality (P > 0.1, K-S test) (Fig. S7). Overexpressing WT-LKB1 in these adult-born neurons led to occasional abnormal dendrite initiation and oriented extension (Fig. S7 A and B), but there was no statistically significant difference from those observed in GFP-control neurons (P > 0.1, K-S test) (Fig. S7C).
These findings reveal that site-specific phosphorylation of LKB1 is important for normal dendrite polarization in the adult brain. The lack of effect found in mice overexpressing CA-LKB1 and WT-LKB1 may be attributed to the fact that the LKB1’s action requires the presence of the cofactor STE20-related adaptor (STRAD) (22, 23), the level of which may be limited in these neurons.
LKB1 Is Required for Polarized Golgi Distribution in Granule Cells.
How does LKB1 regulate dendrite polarity? In Drosophila peripheral dendritic arborization sensory neurons, Golgi-dependent membrane supply is important for dendrite growth (24), and Golgi outposts within the dendrite mediate microtubule nucleation and are crucial for dendrite branch formation (25). In cultured rat hippocampal neurons, polarized distribution of Golgi apparatus in the cytoplasm mediates asymmetric dendrite growth (26). In the present study, we found that the Golgi apparatus was predominantly accumulated at the base and within the initial segment of the primary dendrite of the DCX+ immature granule cells in the 8-wk-old mouse dentate gyrus (Movie S1).
The Golgi distribution was further examined in LKB1-KO adult-born neurons by immunostaining of hippocampal slices for the Golgi structure protein GRASP65 (27) at 3 wk after injection of Ubi-Cre-E2A-GFP retrovirus into LKB1f/f mice (LKB1-KO). As the control, the same retrovirus was injected into wild-type mice (WT-Control). High-resolution z-stack confocal images were collected from brain slices of these mice, and a custom-made Matlab-based software was used to remove Golgi signals in surrounding cells that obscured Golgi signals in the infected cells in the 2D projection (Fig. S3C). We found that, although the Golgi apparatus accumulated at the base and within the initial segment of the primary dendrite in WT-Control granule cells, it was dispersed over the entire soma in LKB1-KO neurons, with no clear Golgi outposts in any dendrite (Fig. 3A). The angular distribution of Golgi was determined by a method similar to that used in dendrite initiation analysis, with the origin at the soma center and x/y axis along the line parallel/perpendicular to the granule layer, respectively (Fig. S3D). Quantitative analysis showed that the Golgi distribution exhibited a clear preference toward the molecular layer (within quadrant I) in WT-Control neurons, and this preference was largely lost in LKB1-KO neurons. This result was shown by the cumulative fluorescence intensity of GRASP65 staining for all neurons (Fig. 3B) (26) and by measuring the average percentage of GRASP65 staining intensity in each of four quadrants of the cytoplasm (P < < 0.001, t test) (Fig. 3C). Thus, LKB1 is required for the polarized Golgi distribution in adult-born granule cells.
Fig. 3.
LKB1 deletion leads to dispersion of Golgi apparatus. (A) Sample images of immunostained Golgi apparatus within retrovirus-labeled cells after removing signals within surrounding cells. Green, GFP; red, GRASP65. Observation time: 3-wk postinjection. (Scale bar, 10 μm.) (B) Summation of immunofluorescence intensities of GRASP65-labled Golgi apparatus for all retrovirus-infected neurons (n = 60–80 cells, from four mice each). (Scale bar, 5 μm.) (C) Average percentage of total fluorescence in each of four quadrants (marked by dashed lines, see B), calculated for each cell before averaging. The same dataset as in B. Values represent mean ± SEM (***P < < 0.001; t test).
We also examined LKB1’s function on dendrite morphogenesis and Golgi distribution at earlier time points during adult-born granule cell development (3, 5, and 7 d after virus injection). At day 3, we found that most WT control neurons exhibited two or more neurites at the beginning, and the Golgi apparatus was found to accumulate at the base of only one neurite. However, by days 5 and 7 we found increasing percentages of neurons exhibiting various processes, with the Golgi outpost at the base and within the initial segment of the thickest process, suggesting the formation of a single primary dendrite, presumably from the neurite with initial Golgi accumulation (Fig. S8). This result also indicates that asymmetric distribution of Golgi apparatus occurs before the differentiation of the primary dendrite. In LKB1-deleted neurons, however, we found that the Golgi apparatus was dispersed within the soma and neurons had multiple equivalent neurites with no apparent primary dendrite (Fig. S8). These results suggest that LKB1 was required for the selective stabilization of the primary dendrite.
Asymmetric Golgi Distribution Is Essential for Dendrite Polarity.
To determine the role of polarized Golgi accumulation in dendrite development of adult-born neurons, we disrupted the Golgi distribution by either down-regulating the Golgi matrix protein GM130 (with RNA interference) or overexpressing GRASP65 in the adult hippocampus via stereotaxic retrovirus injection (26, 28, 29). For down-regulation of GM130, we constructed retroviral vector containing both the U6 promoter-driven specific shRNA and EF1α promoter-driven GFP gene (Fig. S9A) (30). The knockdown efficiency was confirmed by Western blot analysis in vitro and Golgi distribution analysis in vivo (Fig. S9 C and D). Experiments were performed using each one of four kinds of effective shRNAs against different regions of GM130 mRNA, and the neuronal phenotypes of infected granule cells were pooled. We found significant defects in both the initiation and extension of dendrites in GM130-knockdown and GRASP65-overexpressing neurons, compared with neurons expressing control vectors that contained scrambled shRNA and GFP gene alone, respectively (P < 0.05, K-S test) (Fig. 4). For phenotype comparison, we also examined newborn neurons infected with the retrovirus construct that expressed GFP and specific shRNAs against LKB1 expression (Fig. S9B). We found that dendritic defects in LKB1-knockdown neurons were weaker than those found in LKB1-KO neurons, but were remarkably similar to those found in GM130-knockdown and GRASP65-overexpressing neurons (P > 0.05, K-S test) (Fig. 4). These results support the idea that Golgi polarity is important for asymmetric dendrite morphogenesis in adult-born granule cells, and suggest that LKB1’s function in dendrite development is mediated by regulating Golgi organization.
Fig. 4.
Down-regulating LKB1 and manipulating of Golgi structural proteins led to similar impairment of dendrite morphognesis. (A and C) Sample confocal images of neurons infected with retroviruses that expressed scrambled-shRNA, LKB1-shRNA, or GM130-shRNA, as well as that expressed GFP or GRASP65. Observation time: 3-wk postinjection. (Scale bar, 50 μm.) (B and D) Angular distribution of dendrite initiation sites and tracing of primary dendrites. [Scale bars, 5 μm (Upper) 20 μm (Lower).] (n = 160–240 cells, from 8 to 12 mice each.) (E) Cumulative percentage plots for the angular distribution of dendrite initiation sites and end points of primary dendrites for all data in B and D. (P value as marked; K-S test).
Discussion
In the present study, we demonstrated that LKB1 regulates polarized Golgi distribution and polarized formation of the single dendrite in adult-born hippocampal granule cells. Our findings provide unique evidence for the LKB1’s function on polarized initiation and oriented growth of the primary dendrites, and add to the increasing evidence that the Golgi apparatus plays important role in dendrite morphogenesis.
A striking aspect of neuronal development is the large amount of plasma membrane required for constructing a typical neuron, which has a surface area 10,000-times greater than typical animal cells, capable of sustaining a highly branched structure hundreds of microns from the soma. The major sites for the synthesis of plasma membrane components are the organelles of the secretory pathway, including the endoplasmic reticulum and Golgi apparatus (31). Thus, the distribution of the Golgi apparatus is likely to determine the location of dendrite initiation from the soma and the specific dendrite that receives preferential trafficking of dendrite-destined secretory cargos. This idea is supported by our finding that the Golgi apparatus is accumulated toward the molecular layer of the dentate gyrus, where the only primary dendrite is initiated, and disruption of Golgi polarity leads to the generation of multiple primary dendrites in the granule cell.
Polarized distribution of membrane components and cytosolic organelles is central to the ability of polarized cells to perform specialized functions. Additionally, the protein kinase LKB1 is among a group of proteins known to contribute to polarized cellular events, such as cell division, apical-basal axis formation, cell migration, and axon formation (32, 33). For its subcellular action, LKB1 is essential for spindle cleavage, centrosome localization, and cytoskeleton arrangement (33), all of which directly contribute to the establishment of asymmetric cellular organization. The kinase LKB1 and its downstream synapses of amphids defective (SAD) have been shown to phosphorylate the zinc finger protein MEX-5, resulting in its asymmetric distribution of RNA-rich P-granules, a critical step in the polarization of one-cell C. elegans embryos (34, 35). This contribution of LKB1 and SAD kinase to cytoplasmic patterning and cell polarity is also found during the development of embryonic cortical pyramidal neurons, where LKB1 and SAD kinases were found to phosphorylate and alter the location of Tau and other microtubule-associated proteins (MAPs), leading to cytoskeleton rearrangement required for axon specification during neuronal polarization (12, 36). Our results that LKB1 regulated asymmetric Golgi distribution and thus regulated polarized dendrite formation of the granule cells add to the cumulating evidence that asymmetric distribution of intracellular components is essential for the establishment of cell polarity. Furthermore, we note that LKB1 deletion led to a switch from bipolar to multipolar neuronal morphology by increasing the number of primary dendrites. Thus, abnormal neuronal polarity could originate not only from the absence of axon or dendrite, but also from changes in dendrite number and orientation, which in turn alter the pattern of neuronal integration and information processing.
The LKB1 gene is ubiquitously expressed in many embryonic and adult tissues (37); it encodes a highly conserved 50-kDa serine/threonine kinase, with the kinase domain and a regulatory domain at its N terminus and another regulatory domain at its C terminus. Two additional nuclear localization signals in the N-terminal region allow its predominant nuclear localization. Activation of LKB1 requires the formation of a complex with its cofactors STRAD and MO25 that leads to the translocation of the complex to the cytoplasm (22, 23, 38). Active cytoplasmic PKA then phosphorylates LKB1 at the site Ser431, and phosphorylated LKB1 further activates downstream signaling pathways (9, 39). Two of LKB1’s downstream effectors, Mst4 and Stk25 (also named Ysk1), bind to the Golgi matrix protein GM130. These effectors are enriched in the Golgi apparatus and essential for Golgi organization (29, 40, 41). Therefore, LKB1 may regulate Golgi deployment through Stk25 or Mst4. In addition, microtubule affinity-regulating kinases, major regulators of microtubule cytoskeletal organization through phosphorylation of MAPs, are identified as the main downstream kinases of LKB1 (36, 42). Thus, LKB1 may regulate Golgi deployment through its direct action on cytoskeleton rearrangement. Moreover, because the Golgi apparatus in mammalian cells is located near the centrosome-based microtubule-organization center, and LKB1 is known to be essential for centrosome localization in cortical pyramidal neurons (14), LKB1 could also regulate Golgi deployment through its effect on centrosome positioning. Taken together, these findings suggest multiple signaling pathways could mediate LKB1’s regulation of Golgi deployment.
Interestingly, reduction of the scaffolding protein DISC1 (disrupted in schizophrenia 1) also results in defects in dendrite morphogenesis in adult-born hippocampal granule cells (43). However, there are differences in dendrite abnormalities compared with those reported here. Although multiple primary dendrites were observed in both cases, LKB1-deleted granule cells showed aberrant orientation toward the hilar region, whereas all dendrites in DISC1-reduced cells showed proper orientation toward the molecular layer. Unlike the effects of DISC1-reduction (44), we found that the effects of LKB1-deletion began at an earlier stage (before 7 d postinfection) and there was no increase in the total dendrite length. These differences suggest distinct cellular actions of LKB1 and DISC1 in dendrite morphogenesis.
We did not observe any obvious axon defect in LKB1f/f mice. This finding may be attributed to the delayed time course or extent of LKB1 deletion associated with viral expression, which allowed us to reduce the LKB1 level at a time beyond the period of axonal actions of LKB1 or to a level that still permits axon formation. Interestingly, a recent study using in utero electroporation of the Cre and NEX-Cre transgenic line instead of Emx1-Cre line to knockout LKB1 in cortical pyramidal neurons showed that LKB1 is important in regulating axon terminal branching rather than axon formation, indicating that the neuronal phenotype caused by LKB1 deletion depends on the time of Cre action (45). Furthermore, intrinsic neuronal properties or the tissue environment may differ between the embryo and adult, so that LKB1 preferentially acts on dendrite development of adult-born hippocampal granule cells. Changes in the expression of LKB1 effectors in the adult-born vs. embryonic neurons may also account for differential actions of LKB1 in these two types of neurons. This result was suggested by the finding in C. elegans that LKB1 acts on actin binding protein UNC-115 instead of SAD to regulate dendrite growth in motor neurons, without affecting axon development (46).
Materials and Methods
Virus Injection.
Eight-week-old mice were anesthetized, and retroviruses (2 μL per hemisphere) were stereotaxically injected into the dentate gyrus in each hemisphere at the site with the following coordinates: posterior: 2 mm from Bregma; lateral: 1.5 mm; ventral: 2.3 mm from the brain surface. All animal care followed the institutional guidelines, and the procedures were approved by the Animal Care Facilities of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
Data Analysis.
Matlab-based programs were designed to analyze dendrite orientation and Golgi accumulation (details are in SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank F. H. Gage (Salk Institute) for providing plasmids for viral packaging, and A. J. Eisch (University of Texas Southwestern Medical Center) for providing Nestin-CreERT2 transgenic mice. This work was supported by grants from Ministry of Science and Technology (973 Program, 2011CBA00400) and Chinese Academy of Sciences (Strategic Priority Research Program, XDB02020001).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321454111/-/DCSupplemental.
References
- 1.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 2.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 6.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52(3):311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 9.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421(6921):379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 10.Ossipova O, Bardeesy N, DePinho RA, Green JB. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat Cell Biol. 2003;5(10):889–894. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- 11.Baas AF, et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116(3):457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 12.Barnes AP, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129(3):549–563. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129(3):565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007;27(43):11769–11775. doi: 10.1523/JNEUROSCI.1938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27(46):12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ylikorkala A, et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001;293(5533):1323–1326. doi: 10.1126/science.1062074. [DOI] [PubMed] [Google Scholar]
- 18.Bardeesy N, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419(6903):162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 19.Shelly M, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327(5965):547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 20.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404(6778):567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 21.Ables JL, et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30(31):10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baas AF, et al. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003;22(12):3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326(5960):1707–1711. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye B, et al. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130(4):717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76(5):921–930. doi: 10.1016/j.neuron.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton AC, et al. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48(5):757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Barr FA, Puype M, Vandekerckhove J, Warren G. GRASP65, a protein involved in the stacking of Golgi cisternae. Cell. 1997;91(2):253–262. doi: 10.1016/s0092-8674(00)80407-9. [DOI] [PubMed] [Google Scholar]
- 28.Puthenveedu MA, Bachert C, Puri S, Lanni F, Linstedt AD. GM130 and GRASP65-dependent lateral cisternal fusion allows uniform Golgi-enzyme distribution. Nat Cell Biol. 2006;8(3):238–248. doi: 10.1038/ncb1366. [DOI] [PubMed] [Google Scholar]
- 29.Matsuki T, et al. Reelin and stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell. 2010;143(5):826–836. doi: 10.1016/j.cell.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol. 2004;6(7):585–591. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Ohno S. The PAR-aPKC system: Lessons in polarity. J Cell Sci. 2006;119(Pt 6):979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 33.Nakano A, Takashima S. LKB1 and AMP-activated protein kinase: Regulators of cell polarity. Genes Cells. 2012;17(9):737–747. doi: 10.1111/j.1365-2443.2012.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tenlen JR, Molk JN, London N, Page BD, Priess JR. MEX-5 asymmetry in one-cell C. elegans embryos requires PAR-4- and PAR-1-dependent phosphorylation. Development. 2008;135(22):3665–3675. doi: 10.1242/dev.027060. [DOI] [PubMed] [Google Scholar]
- 35.Griffin EE, Odde DJ, Seydoux G. Regulation of the MEX-5 gradient by a spatially segregated kinase/phosphatase cycle. Cell. 2011;146(6):955–968. doi: 10.1016/j.cell.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307(5711):929–932. doi: 10.1126/science.1107403. [DOI] [PubMed] [Google Scholar]
- 37.Jenne DE, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18(1):38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 38.Boudeau J, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22(19):5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 40.ten Klooster JP, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16(4):551–562. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Preisinger C, et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164(7):1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23(8):307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 43.Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JY, et al. Interplay between DISC1 and GABA signaling regulates neurogenesis in mice and risk for schizophrenia. Cell. 2012;148(5):1051–1064. doi: 10.1016/j.cell.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courchet J, et al. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153(7):1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teichmann HM, Shen K. UNC-6 and UNC-40 promote dendritic growth through PAR-4 in Caenorhabditis elegans neurons. Nat Neurosci. 2011;14(2):165–172. doi: 10.1038/nn.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.