Significance
This paper provides a synthesis of results from experimental studies on plants and animals, and shows that there is strong evidence that genotypic and heritable phenotypic diversity improves establishment success and population persistence. An increased focus on among-individual variation may improve the success of conservation programs aiming to revitalize declining populations and species. Recognizing the role of diversity may also improve our ability to identify and ultimately prevent potential harmful invading species from spreading outside of their native ranges, and aid the development of means for improved protection against epizootics and epidemics.
Keywords: colonization, biological invasion, biodiversity, extinction
Abstract
There is abundant evidence that the probability of successful establishment in novel environments increases with number of individuals in founder groups and with number of repeated introductions. Theory posits that the genotypic and phenotypic variation among individuals should also be important, but few studies have examined whether founder diversity influences establishment independent of propagule pressure, nor whether the effect is model or context dependent. I summarize the results of 18 experimental studies and report on a metaanalysis that provides strong evidence that higher levels of genotypic and phenotypic diversity in founder groups increase establishment success in plants and animals. The effect of diversity is stronger in experiments carried out under natural conditions in the wild than under seminatural or standardized laboratory conditions. The realization that genetic and phenotypic variation is key to successful establishment may improve the outcome of reintroduction and translocation programs used to vitalize or restore declining and extinct populations. Founder diversity may also improve the ability of invasive species to establish and subsequently spread in environments outside of their native community, and enhance the ability of pathogens and parasites to colonize and invade the environment constituted by their hosts. It is argued that exchange of ideas, methodological approaches, and insights of the role of diversity for establishment in different contexts may further our knowledge, vitalize future research, and improve management plans in different disciplines.
Insight into factors that increase the ability of species to establish in novel environments is critical to improve conservation management actions aiming to protect biodiversity by (re)introducing individuals to locally extinct and declining populations, or by preventing the colonization and subsequent spread outside their native range of invading species (1–4). An improved understanding of what makes certain parasites and pathogens more prone to infect their hosts may also aid the development of means for improved protection against epizootics and epidemics (5, 6). Previous research suggests that species traits, such as reproductive life history characteristics, behavioral properties, and degree of ecological specialization influence establishment capacity and invasiveness (1, 7, 8). However, evidence is mounting that the outcome of founder events is also determined by emergent traits that exist only as collective properties of individuals. Accordingly, establishment has been found to increase with increasing number of individuals included in the founder group, and with increasing number of repeated introduction events (reviewed in refs. 1, 2, and 9). A positive effect of propagule pressure may arise both because larger populations are less vulnerable to extinction driven by demographic stochasticity, and because larger founder groups may harbor more genetic diversity and greater variation in functionally important phenotypic traits (1, 8, 10, 11).
There are multiple mechanisms by which genotypic and phenotypic diversity can positively influence establishment and population persistence (12–14). These include a higher probability that more diverse groups harbor preadapted phenotypes, i.e., a sampling effect (15, 16), niche complementarity resulting from reduced competition in groups where different phenotypes exploit different resources (17, 18), and facilitation, i.e., when the presence of one genotype or phenotype promotes the success of other phenotypes (19, 20). In addition, diverse populations of animals and plants may be less vulnerable to predators, diseases, and pathogens (21, 22). Finally, genetic and phenotypic variation may promote population persistence because it buffers against selection in changing environments and enables adaptations to novel and changing conditions (10, 23–25). The above mechanisms are neither exhaustive nor mutually exclusive. A positive effect of diversity on establishment has potentially important implications, both from theoretical and applied perspectives. However, few studies have experimentally examined how establishment success is influenced by founder diversity per se (i.e., independent of founder size), and it is not known whether the effect is model or context dependent.
Here, I review experimental studies on plants and animals and perform a metaanalysis to examine whether there is empirical support for the prediction (12–14, 26) that greater genotypic variation and phenotypic diversity in functionally important traits increases establishment success. Although high intraspecific variability may increase establishment and reduce risk of extinction, the ecological success and dynamics of populations may also affect patterns of genetic and phenotypic diversity, resulting in a complex interplay of ecoevolutionary dynamics (27, 28). Because of this feedback loop, firm demonstrations of causal relationships and mechanisms linking founder diversity to population fitness require experimental manipulations and replication. I therefore include in this review only studies in which the investigator first manipulated the level of genotypic or phenotypic diversity in experimental founder groups and then examined the effect on establishment success. In addition, I investigate whether the effect of diversity on establishment is manifest more strongly in experiments executed under natural conditions in the wild under the influence of multiple mechanisms, than in experiments carried out in less complex environments with fewer interactions under seminatural or under standardized laboratory conditions.
Review Findings
The literature search uncovered 18 studies (SI Results) in which experimental groups containing different numbers of clones, strains, or phenotypic variants (morphs) were used to test for effects of genetic or phenotypic diversity on establishment—or the reverse of establishment, viz extinction. These studies included both vascular plants (n = 9) and animals [n = 9: invertebrates (n = 8) and vertebrates (n = 1)]. Experimental durations ranged from less than one (e.g., ref. 29) to approximately eight (30) generations, depending on organism type. The number of diversity levels ranged from 2 to 7 (mean = 3.5, SD = 1.34). Seven studies reported on experiments performed under standardized conditions in the laboratory or greenhouse, three under seminatural conditions, and eight under natural field conditions in the wild. A brief summary of the experiments, sorted in chronological order, separately for plants and animals, is available in SI Results.
The results of all but 1 (31) of the 18 experiments (binomial test, P = 0.001) were in qualitative agreement with the prediction (12–14, 26) that higher diversity increases establishment and reduces extinction risk. Average effect size of diversity across all studies differed significantly from zero (mean Cohen’s d = 1.38 ± 1.30 SD, t = 4.49, df = 17, P = 0.0003). In three studies (32–34), the outcome fell short of statistical significance while the trend was in the positive direction predicted by theory. Higher levels of diversity did not significantly reduce establishment in any of the studies.
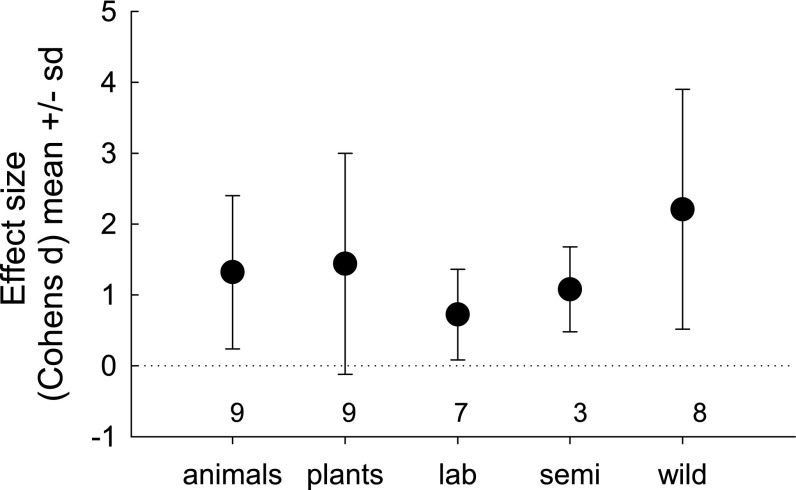
Comparisons of effect sizes indicate that higher levels of diversity promoted establishment success to similar extents in plants and animals, and that the effect was stronger in experiments carried out under natural conditions in the wild than in experiments performed under seminatural or standardized laboratory conditions [general linear model (GLM), effect of organism type: F(1,15) = 1.14, P = 0.30; effect of experimental setting: F(1,15) = 5.73, P = 0.030; Fig. 1]. There was no association across studies between the effect of diversity and the number of treatment levels in the experiment (r = −0.20, n = 18, P = 0.44). Effect size was not correlated with year of publication (r = −0.03, n = 18, P = 0.91).
Fig. 1.
Comparison of effect sizes in experiments testing whether genotypic and phenotypic diversity promotes establishment success. Mean (±SD) effect size measured as Cohen’s d in plants and animals, and for experiments performed in the laboratory, under seminatural conditions or under field conditions in the wild. The numbers above the horizontal axis indicate sample sizes. The figure shows raw data, but the statistical analyses were based on log(x + 1)-transformed data.
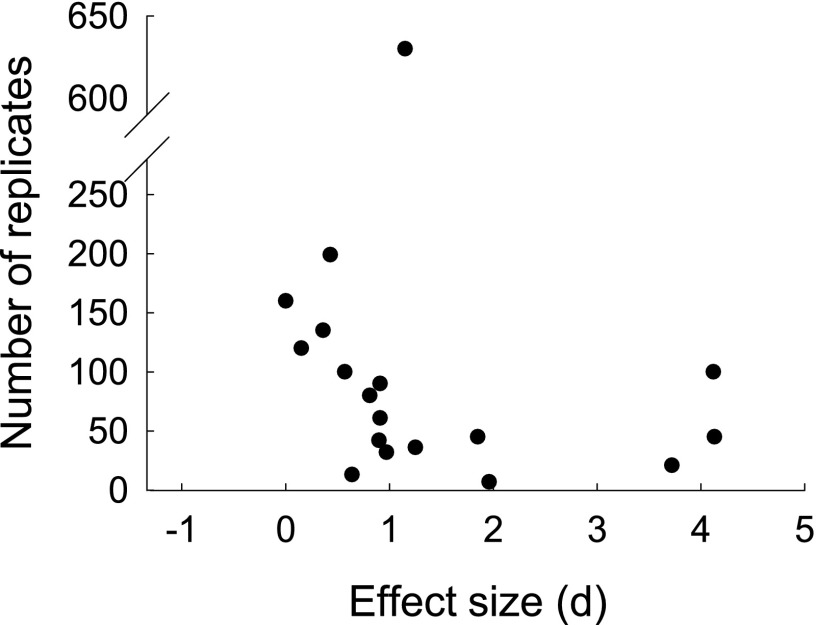
The funnel plot indicates that there is no strong publication bias toward small (in terms of number of replicates) studies reporting high effects of founder diversity on establishment, as evidenced by the presence also of small studies that report low effect sizes (Fig. 2). Effect size was not associated with sample size (regression analysis of effect size on log number of replicates; F(1,16) = 1.63, P = 0.22, R2 = 0.09).
Fig. 2.
Funnel plot to assess publication bias in experimental tests for effects of founder diversity on establishment success. The figure shows the relationship between study size, measured as number of replicates, and effect size measured as Cohen’s d. Publication bias might be suspected if small studies (with few replicates) reporting low effect sizes are absent but small studies reporting high effect sizes are present. Effect size was not significantly related to study size [regression of effect size on log number of replicates; F(1,16) = 1.63, P = 0.22, R2 = 0.09].
Discussion
Previous research implicates propagule pressure as a determinant of initial establishment success (1, 2, 9), but few studies have used an experimental approach to examine whether there is an important contribution of founder diversity per se, given a certain founder size. Furthermore, no previous systematic comparison has evaluated whether the effect of diversity is model or context dependent. Overall, the 18 experimental studies included in the metaanalysis provide firm almost invariable evidence, in agreement with predictions from theory (12–14, 26), that higher levels of among individual genotypic and phenotypic variation promotes establishment, in both plants and animals.
The main virtues of experimental approaches in ecological research are to reduce or eliminate the contribution to variation in the response variable of uncontrolled environmental factors (i.e., noise) that may obscure true effects, establish causal relationships, and identify underlying mechanisms. In view of this, it may seem counterintuitive that the effect of diversity on establishment and population persistence appears to be stronger (not weaker) in experiments performed in the wild than in experiments performed under seminatural or laboratory conditions (Fig. 1). A possible resolution to this apparent paradox is that a larger fraction of the multifarious pathways and mechanisms by which diversity may contribute to increased establishment success and population fitness (12–14, 16, 20, 26) are operative under complex natural environmental conditions that encompass a broader array of ecological interactions than in simpler, standardized laboratory settings. In the wild, individuals are faced with competitors, predators (or herbivores), and heterogeneous sometimes adverse abiotic conditions. Therefore, selection differentials are likely more variable, and the buffering effect of diversity and need for evolutionary potential more severe under natural settings. Moreover, inbreeding depression is usually greater in more stressful environments (35).
Experimental durations were generally short, ranging from less than one to approximately eight generations. There is therefore a need for more long-term studies to determine whether the positive effect of diversity on initial establishment translates into increased persistence (36). Similarly, the taxonomic bias among the studied species leaves some uncertainty with regard to generality of the findings. The consequences of variation depend on the spatiotemporal scale of change relative to the life span and dispersal capacity of the organism (37). The studies on vascular plants reviewed here generally used annual or biannual species, and eight of the nine studies on animals used small-sized, short-lived invertebrates (SI Results). It remains an open question whether the positive effects of diversity on establishment demonstrated here are manifest also in more long-lived taxa with overlapping generations.
Implications for Conservation Biology and (Re)introduction Programs.
Assisted colonization, translocations, and reintroductions are often used in conservation biology to overcome endangerment and risks of extinction associated with habitat loss, habitat fragmentation, and reproductive isolation (36). That increased genotypic and phenotypic diversity offers a means to increase establishment success illustrates that the role of individual variation deserves increased consideration by designers, managers, and practitioners involved in conservation biology (3, 38). In this context, it should be emphasized that mixing individuals from different populations to increase diversity can actually reduce population fitness (39). It is particularly promising that diversity seems to contribute to successful establishment most strongly in experiments performed under natural conditions in the wild, because in those studies the fate of the introduced individuals and their progeny may be influenced by a plethora of abiotic factors and biotic interactions beyond the control of the experimenter, as in reintroduction and translocation programs. Further support for the pivotal role of diversity for population fitness comes from comparative studies across different species, indicating that greater variability in morphological, life history, and color pattern traits buffers against extinction in bivalves, ostracods, frogs, snakes, lizards, birds, and mammals (24, 40–44). The results reported here also support the notion that founder diversity is potentially important in biological invasions (3, 25).
Implications for Invasion Biology.
In addition to boosting establishment in novel environments, diversity may enable evolutionary rescue, allow for local adaptations in response to new selective regimes, and thereby promote long-term persistence (10, 25, 45, 46). A downside of this is that some species that successfully establish and spread in novel environments may negatively affect local communities and species (47, 48). Studies aiming to develop tools for identifying potentially invading species before they invade have focused largely on behavioral traits and life history characteristics (3). The results reported here, and in previous reviews (1), implicate founder diversity as important in biological invasions, and suggest that adding the level of genotypic and phenotypic variation to the list of characteristics may help us identify and foresee which species may constitute future outbreaks.
Most invasive populations have reduced genetic diversity (measured as allelic richness and heterozygosity) compared with their source populations (49). However, a positive effect of diversity on establishment may be one explanation as to why some of the most well-known invasive animal species that have undergone extreme range expansions (e.g., the harlequin ladybird, Harmonia axyridis, the zebra mussel, Dreissena polymorpha, the Spanish slug, Arion vulgaris, the lizard, Anolis sagrei, the brown tree snake, Boiga irregularis, the cane toad, Bufo marinus, and the Trinidadian guppy, Poecilia reticulata) all show high color pattern variability (7, 47, 48, 50–53). However, these examples are suggestive only, and the proposed role of color polymorphism for invasiveness needs systematic evaluation. It should also be emphasized here that it is not necessarily color pattern variation per se that has promoted invasiveness in these species. Coloration is genetically, developmentally, and functionally associated with several important ecological traits in many types of animals (for examples, see reviews in refs. 54 and 55), and this leaves opportunity for alternative mediating mechanisms.
It has been argued that additional evidence that greater genetic diversity promotes establishment and spread comes from the many examples in which hybridization has stimulated the evolution of invasiveness, because recombination in hybrids generates novel heritable variation (1, 25, 56). However, genetic admixture resulting from interbreeding between individuals from different source populations may have either positive or negative effects on population fitness, depending on the evolutionary history, genetic architecture, and environmental contexts of the populations involved (1, 4, 39, 57, 58). I therefore excluded from the present review studies in which individuals from different populations were intermixed to create the diverse treatments.
Implications for Infection Biology and Disease.
The key challenges and mechanisms of importance in invasion biology are similar in many respects to those of interest for researchers and practitioners concerned with ecology and evolution of host–parasite interactions, infectious diseases, and epidemiology. Support for the notion that diversity promotes population fitness comes also from experimental demonstrations that genetic diversity makes populations more resistant to infectious diseases (22, 59–61). Conversely, evidence is mounting that genetically diverse viruses, bacteria, and parasites are better able to infect their vectors and hosts. Simultaneous exposure to diverse genotypes or strains may lead to more efficient exploitation of host resources and faster parasite multiplication (62). Simultaneous attack and coinfection may also compromise the immune system of the host (i.e., facilitation) (63, 64). For instance, surveys of genetic diversity among avian influenza viruses in wild birds frequently show evidence of a high proportion of mixed subtype infections (65, 66) consistent with the notion that subtype diversity may allow for host-adaptation and promote the rate of infection. Furthermore, most pandemic viruses have originated through coinfections, for example, the latest H1N1 swine flu (67), although firm experimental evidence that coinfections increase establishment success and virulence seems to be lacking. Experimental coinfections have been performed both on bacteria and on parasites with complex life cycles. Wilson et al. (68) experimentally demonstrated a strong correlation between genetic diversity of Campylobacter jejuni bacteria subpopulations and their ability to colonize the gastrointestinal tract of broiler chickens and mice.
That diversity may facilitate host colonization has been demonstrated also in multicellular parasites with complex life cycles. For instance, Ferguson and Read (69) investigated the importance of parasite genetic variation for vector virulence and found that, in standard conditions, mortality was highest in Anopheles stephensi mosquitoes infected with mixed genotypes of the rodent malaria parasite Plasmodium chabaudi. In a later study of the same model system, mixed genotype infections were also found to reduce mosquito fecundity more than did single-genotype infections (70). Ganz and Ebert (59) experimentally exposed Daphnia magna hosts to different genetic diversity levels (one, two, three, or four strains) of the microsporodian gut parasite Ordospora colligata and found that parasite prevalence increased strongly with increasing number of parasite strains. Karvonen et al. (71) experimentally exposed rainbow trout Oncorhynchus mykiss fishes to cercariae of the trematode Diplostomum pseudopathaceum that originated from Lymnaea stagnalis snail vectors infected with either a single or with two different trematode genotypes. The proportion of parasites that established in the fish was higher (25% difference) if cercariae originated from double-genotype–infected snails than from single-genotype–infected snails, supporting the facilitation hypothesis. Additionally, artificial genotype mixtures had a higher infection success than did single genotypes (71). These examples indicate that knowledge of the roles of founder diversity for colonization and establishment is essential for a better understanding of host–parasite dynamics and spread of infectious disease, and of relevance from a human health perspective.
Conclusions
The recognition of the key role of founder genotypic and heritable phenotypic diversity for successful establishment has important implications for different areas and calls for some changes in policy and management. For instance, conservation programs that use reintroductions and translocations to vitalize or restore declining and locally extinct populations and species should focus at least as much on founder diversity as on propagule pressure and degree of environmental match between the habitat occupied by the source population and the properties at the introduction site. From the perspective of invasive species management, an increased focus on the role of diversity may help improve our ability to identify and protect against potential harmful invaders. Substantial research has attempted to identify traits and ecological characteristics that typify invasive species and properties that make environments susceptible or resistant to colonization and invasion. The results reported here suggest that founder diversity may influence the ability of invasive species to establish and subsequently spread outside of their native community, as well as the ability of pathogens and parasites to colonize and invade the environment constituted by their hosts. It therefore seems likely that an exchange of ideas, methodological approaches, and insights of the role of diversity for establishment in different contexts may further our knowledge, vitalize future research, and improve management plans in different areas.
Methods
Protocol for Literature Search.
The objective of the study was to review experimental studies to investigate the primary question whether there is empirical support for the proposition that higher levels of phenotypic and genetic variation among individuals included in experimental founder groups promotes establishment success. The study was carried out in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, but did not adhere to all formal requirements for systematic reviews; e.g., the review and review protocol was not registered, and the number of studies identified and removed through the different phases was not recorded. I used the Institute for Scientific Information (ISI) Web of Knowledge [Science Citation Index Expanded (1945 to present)] database (72) to search for experimental studies to include in the review. The following combination of search terms was used (an asterisk denotes a wild-card search term allowing for several permutations of each intervention type): (propagule OR founder OR group OR popul*) AND (genet* OR phenot*) AND (varia* OR divers* OR richness OR polymorph*) AND (establ* OR coloni* OR extinc* OR persist*) AND (experiment* OR manipul*).
| Document type = | Article |
| Language = | English |
| Research area = | Environmental Sciences Ecology |
| Marine Freshwater Biology | |
| Evolutionary Biology | |
| Entomology | |
| Biodiversity Conservation | |
| Plant Sciences | |
| Microbiology | |
| Zoology |
The search criteria used are listed in the display table below.
The initial search generated nearly 2,000 returns. Studies were initially selected by Title, but if there was any doubt of the studies relevance, the Abstract was also acquired and judged. When the relevance of the study could not be assessed based on Title and Abstract, the full-text version was examined. For each included study, I used the reference citation map in ISI forward and backward to find additional studies.
Inclusion Criteria and Study Quality Assessment.
I included studies that report on controlled experiments in which the effect of phenotypic and/or genetic variation was investigated based on comparisons between replicated treatment groups in which the levels of among individual variation had been manipulated. The means used to achieve this varied among studies depending on type of organism. In several studies, comparisons were made between treatments that contained different number of clones, strains, or morphs. I included studies that tested for effects of either genetic or phenotypic variation. Studies on phenotypic variation were included regardless the type of trait (e.g., morphology, physiology, behavior, or life history) and regardless whether the variation reflected an underlying genetic polymorphism, developmental plasticity, phenotypic flexibility, or combinations thereof. I included only studies in which the response variables was measured in a quantitative manner, and in which the outcome of comparisons between treatments was reported in a way that allowed for unambiguous interpretation and extraction of results.
I excluded studies in which all of the diverse treatments were obtained by mixing individuals that originated from different source populations. The rationale for excluding such experiments is that genetic admixture resulting from interbreeding between individuals from different populations may have either positive or negative effects on population fitness depending on the evolutionary history, genetic architecture, and environmental contexts of the populations involved, rather than on the degree of genetic and phenotypic variation per se (1, 39, 57, 58). This is because even though immigration may alleviate the effects of inbreeding depression, it may also disrupt local adaptations and result in the breakup of coadapted gene complexes and favorable trait–value combinations (i.e., outbreeding depression) (23, 73).
I included studies on both plants and animals. Whether studies should be included in or excluded from the review was determined without consideration of type of organism and species.
Data Extraction.
I used full-text articles to classify studies with regard to whether they were performed either under standardized conditions in the laboratory, under seminatural conditions, or under natural conditions in the wild; whether the outcome was in qualitative agreement or in conflict with the prediction from theory; and whether the outcome was statistically significant (P < 0.05). The outcome, or response variable, of the experiments used as a measure of establishment success or population persistence differed depending on the type of study organism (SI Results). In some studies, establishment was determined based on data on survival, growth, and maturity of the introduced propagules after a given time period. In other studies, establishment was determined according to individuals that resulted from recruitment of offspring produced in situ by the propagules included in the experimental founder groups. Examples of response variables used in the included studies were as follows: number or proportion of introduced individuals alive; proportion of introduced populations alive (or extinct); population size; total biomass; number of recruited adults; number of seedlings; number of recruits; or percentage growth (or maximum spread distance in some plants) after a given time (SI Results).
Metaanalysis.
I investigated whether the effect of diversity on establishment was different in plants and animals, and whether it was condition dependent and manifest more strongly under natural conditions in the wild under the influence of multiple complex mechanisms, compared with less heterogeneous environments with fewer interactions. To this end, I estimated and compared effects sizes, Cohen’s d (74, 75), between studies on plants and animals, and between studies performed under standardized conditions in the laboratory, under seminatural or under natural conditions in the wild. Before analyses, effect sizes were log(x + 1) transformed to normalize distributions and homogenize variances. To examine whether effect size differed between plants and animals, and whether it increased with increasing ecological complexity of the experimental setup (i.e., from laboratory via seminatural to natural conditions), I used a GLM approach (74, 76), as implemented with procedure GLM in SAS 9.1.3 for Windows (SAS) software package. Pearson correlation analysis was used to test (in separate analyses) whether effect size was associated with number of diversity treatments used in the experiment, or with year of publication. Statistical combination approaches, whether simple or based on sophisticated algorithms, can be trusted only if it is known with certainty that all studies that have been carried out are included in the review (77). Because this is virtually impossible, and as the number of studies included in this review is relatively small, the result from the comparison of effect sizes is tentative and should be evaluated with care.
A funnel plot (a graph of effect size versus number of replicates in each study) was used to assess whether it is likely that publication or availability bias (74, 75) has influenced to an important degree the results and conclusions. This approach is based on the assumption that, in the absence of publication or availability bias, average effect size is expected to be similar in small- and large-sample studies, but vary more in smaller studies due to a greater influence of sampling error (74). Because it may be difficult to correctly identify publication bias from visual inspection of funnel plots (78), I used the funnel plot data to test for a relationship between effect size and sample size (log number of replicates) with regression analysis.
Supplementary Material
Acknowledgments
I am grateful to D. Agashe for sharing unpublished information and to N. Latorre-Margalef, J. Waldenström, and two anonymous reviewers for comments on an earlier draft of the manuscript. Financial support was provided by The Swedish Research Council and Linnaeus University.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1317745111/-/DCSupplemental.
References
- 1.Simberloff D. The role of propagule pressure in biological invasions. Annu Rev Ecol Syst. 2009;40:81–102. [Google Scholar]
- 2.Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20(5):223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Allendorf FW, Lundquist LL. Introduction, population biology, evolution and control of invasive species. Conserv Biol. 2003;17(1):24–30. [Google Scholar]
- 4.Frankham R, Ballou JD, Briscoe DA. Introduction to Conservation Genetics. Cambridge, UK: Cambridge Univ Press; 2010. [Google Scholar]
- 5.Grenfell BT, Dobson AP. Ecology of Infectious Diseases in Natural Populations. Cambridge, UK: Cambridge Univ Press; 1995. [Google Scholar]
- 6.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 7.Deacon AE, Ramnarine IW, Magurran AE. How reproductive ecology contributes to the spread of a globally invasive fish. PLoS One. 2011;6(9):e24416. doi: 10.1371/journal.pone.0024416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caughley G, Gunn A. Conservation Biology in Theory and Practice. Cambridge, MA: Blackwell Scientific; 1995. [Google Scholar]
- 9.Drake JM, Baggenstos P, Lodge DM. Propagule pressure and persistence in experimental populations. Biol Lett. 2005;1(4):480–483. doi: 10.1098/rsbl.2005.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lande R. Genetics and demography in biological conservation. Science. 1988;241(4872):1455–1460. doi: 10.1126/science.3420403. [DOI] [PubMed] [Google Scholar]
- 11.Richter-Dyn N, Goel NS. On the extinction of a colonizing species. Theor Popul Biol. 1972;3(4):406–433. doi: 10.1016/0040-5809(72)90014-7. [DOI] [PubMed] [Google Scholar]
- 12.Forsman A, Ahnesjö J, Caesar S, Karlsson M. A model of ecological and evolutionary consequences of color polymorphism. Ecology. 2008;89(1):34–40. doi: 10.1890/07-0572.1. [DOI] [PubMed] [Google Scholar]
- 13.Wennersten L, Forsman A. Population-level consequences of polymorphism, plasticity and randomized phenotype switching: A review of predictions. Biol Rev Camb Philos Soc. 2012;87(3):756–767. doi: 10.1111/j.1469-185X.2012.00231.x. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AR, Inouye BD, Johnson MTJ, Underwood N, Vellend M. Ecological consequences of genetic diversity. Ecol Lett. 2008;11(6):609–623. doi: 10.1111/j.1461-0248.2008.01179.x. [DOI] [PubMed] [Google Scholar]
- 15.Forsman A, Ahnesjö J, Caesar S. Fitness benefits of diverse offspring in pygmy grasshoppers. Evol Ecol Res. 2007;9(8):1305–1318. [Google Scholar]
- 16.Gamfeldt L, Källström B. Increasing intraspecific diversity increases predictability in population survival in the face of perturbations. Oikos. 2007;116(4):700–705. [Google Scholar]
- 17.Caesar S, Karlsson M, Forsman A. Diversity and relatedness enhance survival in colour polymorphic grasshoppers. PLoS One. 2010;5(5):e10880. doi: 10.1371/journal.pone.0010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolnick DI, et al. The ecology of individuals: Incidence and implications of individual specialization. Am Nat. 2003;161(1):1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- 19.Gamfeldt L, Wallen J, Jonsson PR, Berntsson KM, Havenhand JN. Increasing intraspecific diversity enhances settling success in a marine invertebrate. Ecology. 2005;86(12):3219–3224. [Google Scholar]
- 20.Reusch TBH, Ehlers A, Hämmerli A, Worm B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc Natl Acad Sci USA. 2005;102(8):2826–2831. doi: 10.1073/pnas.0500008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glanville PW, Allen JA. Protective polymorphism in populations of computer-simulated moth-like prey. Oikos. 1997;80(3):565–571. [Google Scholar]
- 22.Zhu Y, et al. Genetic diversity and disease control in rice. Nature. 2000;406(6797):718–722. doi: 10.1038/35021046. [DOI] [PubMed] [Google Scholar]
- 23.Mayr E. Animal Species and Evolution. Cambridge, MA: Harvard Univ Press; 1963. [Google Scholar]
- 24.González-Suárez M, Revilla E. Variability in life-history and ecological traits is a buffer against extinction in mammals. Ecol Lett. 2013;16(2):242–251. doi: 10.1111/ele.12035. [DOI] [PubMed] [Google Scholar]
- 25.Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA. 2007;104(10):3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolnick DI, et al. Why intraspecific trait variation matters in community ecology. Trends Ecol Evol. 2011;26(4):183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilpin ME, Soulé ME. Minimum viable populations: Processes of species extinction. In: Soulé ME, editor. Conservation Biology: The Science of Scarcity and Diversity. Sunderland, MA: Sinauer; 1986. pp. 19–34. [Google Scholar]
- 28.Schoener TW. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331(6016):426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- 29.Wang XY, et al. Genotypic diversity enhances invasive ability of Spartina alterniflora. Mol Ecol. 2012;21(10):2542–2551. doi: 10.1111/j.1365-294X.2012.05531.x. [DOI] [PubMed] [Google Scholar]
- 30.Agashe D. The stabilizing effect of intraspecific genetic variation on population dynamics in novel and ancestral habitats. Am Nat. 2009;174(2):255–267. doi: 10.1086/600085. [DOI] [PubMed] [Google Scholar]
- 31.Robinson JD, Wares JP, Drake JM. 2013. Extinction hazards in experimental Daphnia magna populations: Effects of genotype diversity and environmental variation. Ecol Evol 3(2):233–243.
- 32.Martins PS, Jain SK. Role of genetic variation in the colonizing ability of Rose Clover (Trifolium hirtum All.) Am Nat. 1979;114(4):591–595. [Google Scholar]
- 33.Vilas C, San Miguel E, Amaro R, Garcia C. Relative contribution of inbreeding depression and eroded adaptive diversity to extinction risk in small populations of shore campion. Conserv Biol. 2006;20(1):229–238. doi: 10.1111/j.1523-1739.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 34.Hovick SM, Gümüser ED, Whitney KD. Community dominance patterns, not colonizer genetic diversity, drive colonization success in a test using grassland species. Plant Ecol. 2012;213(9):1365–1380. [Google Scholar]
- 35.Fox CW, Reed DH. Inbreeding depression increases with environmental stress: An experimental study and meta-analysis. Evolution. 2011;65(1):246–258. doi: 10.1111/j.1558-5646.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 36.Dalrymple SE, Stewart GB, Pullin AS. 2011. Are Re-introductions an Effective Way of Mitigating Against Plant Extinctions? CEE Review 07-008 (SR32) (Collaboration for Environmental Evidence, Bangor, UK). Available at www.environmentalevidence.org/SR32.html.
- 37.Levins R. Evolution in Changing Environments. Princeton: Princeton Univ Press; 1968. [Google Scholar]
- 38. Ferrière R, Dieckmann U, Couvet D, eds (2004) Evolutionary Conservation Biology (Cambridge Univ Press, Cambridge, UK)
- 39.McClelland E, Naish K. What is the fitness outcome of crossing unrelated fish populations? A meta-analysis and an evaluation of future research directions. Conserv Genet. 2007;8(2):397–416. [Google Scholar]
- 40.Forsman A, Hagman M. Association of coloration mode with population declines and endangerment in Australian frogs. Conserv Biol. 2009;23(6):1535–1543. doi: 10.1111/j.1523-1739.2009.01244.x. [DOI] [PubMed] [Google Scholar]
- 41.Forsman A, Åberg V. Associations of variable coloration with niche breadth and conservation status among Australian reptiles. Ecology. 2008;89(5):1201–1207. doi: 10.1890/07-1670.1. [DOI] [PubMed] [Google Scholar]
- 42.Liow LH. Does versatility as measured by geographic range, bathymetric range and morphological variability contribute to taxon longevity? Glob Ecol Biogeogr. 2007;16(1):117–128. [Google Scholar]
- 43.Kolbe SE, Lockwood R, Hunt G. Does morphological variation buffer against extinction? A test using veneroid bivalves from the Plio-Pleistocene of Florida. Paleobiology. 2011;37(3):355–368. [Google Scholar]
- 44.Delhey K, Smith J, Peters A. Colour-variable birds have broader ranges, wider niches and are less likely to be threatened. J Evol Biol. 2013;26(7):1559–1568. doi: 10.1111/jeb.12157. [DOI] [PubMed] [Google Scholar]
- 45.Jones EI, Gomulkiewicz R. Biotic interactions, rapid evolution, and the establishment of introduced species. Am Nat. 2012;179(2):E28–E36. doi: 10.1086/663678. [DOI] [PubMed] [Google Scholar]
- 46.Bell G. Fluctuating selection: The perpetual renewal of adaptation in variable environments. Philos Trans R Soc Lond B Biol Sci. 2010;365(1537):87–97. doi: 10.1098/rstb.2009.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majerus MEN, Strawson V, Roy H. The potential impacts of the arrival of the harlequin ladybird, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), in Britain. Ecol Entomol. 2006;31(3):207–215. [Google Scholar]
- 48.Savidge JA. Extinction of an island forest avifauna by an introduced snake. Ecology. 1987;68(3):660–668. [Google Scholar]
- 49.Dlugosch KM, Parker IM. Founding events in species invasions: Genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol. 2008;17(1):431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- 50.Krafsur ES, et al. Gene flow in the exotic colonizing ladybeetle Harmonia axyridis in North America. Biol Control. 1997;8(3):207–214. [Google Scholar]
- 51.Johnson LE, Carlton JT. Post-establishment spread in large-scale invasions: Dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology. 1996;77(6):1686–1690. [Google Scholar]
- 52.Phillips BL, Shine R. An invasive species induces rapid adaptive change in a native predator: Cane toads and black snakes in Australia. Proc Biol Sci. 2006;273(1593):1545–1550. doi: 10.1098/rspb.2006.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kolbe JJ, et al. Genetic variation increases during biological invasion by a Cuban lizard. Nature. 2004;431(7005):177–181. doi: 10.1038/nature02807. [DOI] [PubMed] [Google Scholar]
- 54.McKinnon JS, Pierotti MER. Colour polymorphism and correlated characters: Genetic mechanisms and evolution. Mol Ecol. 2010;19(23):5101–5125. doi: 10.1111/j.1365-294X.2010.04846.x. [DOI] [PubMed] [Google Scholar]
- 55.True JR. Insect melanism: The molecules matter. Trends Ecol Evol. 2003;18(12):640–647. [Google Scholar]
- 56.Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA. 2000;97(13):7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verhoeven KJF, Macel M, Wolfe LM, Biere A. Population admixture, biological invasions and the balance between local adaptation and inbreeding depression. Proc Biol Sci. 2011;278(1702):2–8. doi: 10.1098/rspb.2010.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tallmon DA, Luikart G, Waples RS. The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol. 2004;19(9):489–496. doi: 10.1016/j.tree.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Ganz HH, Ebert D. Benefits of host genetic diversity for resistance to infection depend on parasite diversity. Ecology. 2010;91(5):1263–1268. doi: 10.1890/09-1243.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imura D, Toquenaga Y, Fujii K. Genetic variation can promote system persistence in an experimental host-parasitoid system. Popul Ecol. 2003;45(3):205–212. [Google Scholar]
- 61.Baer B, Schmid-Hempel P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature. 1999;397(6715):151–154. [Google Scholar]
- 62.Davies CM, Fairbrother E, Webster JP. Mixed strain schistosome infections of snails and the evolution of parasite virulence. Parasitology. 2002;124(Pt 1):31–38. doi: 10.1017/s0031182001008873. [DOI] [PubMed] [Google Scholar]
- 63.Jokela J, Schmid-Hempel P, Rigby MC. Dr. Pangloss restrained by the Red Queen—steps towards a unified defence theory. Oikos. 2000;89(2):267–274. [Google Scholar]
- 64.Andersson M, Scherman K, Råberg L. Multiple-strain infections of Borrelia afzelii: A role for within-host interactions in the maintenance of antigenic diversity? Am Nat. 2013;181(4):545–554. doi: 10.1086/669905. [DOI] [PubMed] [Google Scholar]
- 65.Dugan VG, et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008;4(5):e1000076. doi: 10.1371/journal.ppat.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharp GB, et al. Coinfection of wild ducks by influenza A viruses: Distribution patterns and biological significance. J Virol. 1997;71(8):6128–6135. doi: 10.1128/jvi.71.8.6128-6135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garten RJ, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson DL, et al. Genetic diversity in Campylobacter jejuni is associated with differential colonization of broiler chickens and C57BL/6J IL10-deficient mice. Microbiology. 2010;156(Pt 7):2046–2057. doi: 10.1099/mic.0.035717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson HM, Read AF. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc Biol Sci. 2002;269(1497):1217–1224. doi: 10.1098/rspb.2002.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson HM, Rivero A, Read AF. The influence of malaria parasite genetic diversity and anaemia on mosquito feeding and fecundity. Parasitology. 2003;127(Pt 1):9–19. doi: 10.1017/s0031182003003287. [DOI] [PubMed] [Google Scholar]
- 71.Karvonen A, Rellstab C, Louhi K-R, Jokela J. Synchronous attack is advantageous: Mixed genotype infections lead to higher infection success in trematode parasites. Proc Biol Sci. 2012;279(1726):171–176. doi: 10.1098/rspb.2011.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ISI . Web of Knowledge. New York: Thomson Reuters; 2012. [Google Scholar]
- 73.Crow JF. Mutation, mean fitness, and genetic load. Oxf Surv Evol Biol. 1993;9:3–42. [Google Scholar]
- 74.Hunter JE, Schmidt FL. Methods of Meta-Analysis—Correcting Error and Bias in Research Findings. 2nd Ed. London: Sage Publications; 2004. [Google Scholar]
- 75.Rosenthal R. The “file-drawer problem” and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- 76.Hedges LV, Olikin I. Statistical Methods for Meta-Analysis. London: Academic; 1985. [Google Scholar]
- 77.Scargle JD. Publication bias: The “File-Drawer” problem in scientific inference. J Sci Explor. 2000;14(1):91–106. [Google Scholar]
- 78.Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol. 2005;58(9):894–901. doi: 10.1016/j.jclinepi.2005.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.