Abstract
Background:
Depression is a common neuro-psychiatric consequence of stroke, affecting approximately 40% of the patients. Many studies show that in addition to the psychosocial stress, neurobiological factors such as site of infarct and brain atrophy may also be related to Post Stroke Depression (PSD). There are conflicting results in this area of research and paucity of such data in Indian literature. Thus the aim of this study is to weigh the importance of lesion location in PSD.
Materials and Methods:
Sixty two subjects with their first ever stroke were interviewed using a semi-structured proforma and PSD diagnosed using MINI Plus interview. Scales of Beck Depression Inventory and Montgomery Asberg Depression Rating Scale were used to assess severity of depression. Mini mental state examination was used to assess cognitive impairment and Barthel Index to measure Activities of Daily Living. Neuro-imaging provided information on site and side of lesion. Collected data was analysed using SPSS version 15.0.
Results:
PSD was diagnosed in 28 subjects, amongst who 19 had left sided lesions. Left sided cortical infarcts and sub cortical infarcts showed statistically significant association with PSD.
Conclusion:
Results are in keeping with previous landmark studies. Differences in emotional reactions depending on hemisphere and site of the infarct as shown in this study suggest organic biological basis for post stroke depression. Understanding the etiological basis would allow clinicians to monitor patients at risk of developing PSD, enabling early detection and treatment thus improving their quality of life and rehabilitation.
Keywords: Assessment, lesion location, post-stroke depression
INTRODUCTION
Stroke as the third leading cause of death and one of the most common disabling diseases, has an enormous emotional impact on both patients and their family members.[1] Depression is a common neuro-psychiatric consequence of stroke with quoted rates of post-stroke depressive disorders ranging from 18% to 61%.
Post-stroke depression (PSD) may affect a patient's ability to participate in therapy and is associated with a slower progress in the rehabilitation and increased length of hospital stay.[2] One etiological theory for depression after stroke is that it is a psychological reaction to the clinical consequences of stroke.[3] Another theory was of the biological model proposed by Robinson and co-workers. They hypothesized that the depletion of monoaminergic amines occurring after stroke plays a role in PSD.[4] Norepinephrinergic and serotoninergic pathways are disrupted in the basal ganglia and frontal lobe lesions – sites that are shown to be associated with PSD.
The association between the vascular changes and depression[5] and more specifically the location of brain lesion as a result of stroke and depression has been the topic of much research. Pooling previous results, Morris et al.,[6] identified specific relationships between the locations of brain injury and the character and severity of post-stroke mood disturbances. He suggested that left anterior cerebral lesions were associated with significantly higher depression scores than left posterior lesions and more specifically left frontal lesions.[7] Magnetic resonance imaging (MRI) studies of depressed patients have revealed structural abnormalities in areas related to limbic-cortical-striatal-pallidal-thalamic-cortical pathways, including the frontal lobes, caudate, and putamen.[8,9]
There is paucity of data in this area of research from India. Correlation between the mood changes and the type, location and severity of stroke may provide useful information for improving patient management, including the prediction of functional evaluation and prognosis.
Clinicians need to be watchful and recognize symptoms of depression early, before it interferes with therapy and the patients’ well-being. Pharmacological treatment of post-stroke depression is associated with an improved functional recovery.[10]
In this study, we aimed to assess by standardized means, the prevalence of depression and to study the association of lesion location with PSD.
MATERIALS AND METHODS
The study was approved by the Institutional Human Ethics Committee of Kasturba Medical College, Mangalore. The patients represent the local population of regions in and around Mangalore district of Karnataka.
Consecutive patients with definite history of recent onset of stroke (>2 weeks but <6 months) using the Monitoring of trends and determinants in the cardiovascular disease criteria[11] for diagnosis of stroke, with the ability to communicate verbally, with the investigator and gave informed consent were included in the study. Patients with altered sensorium/aphasia/significant cognitive disturbances interfering with satisfactory communication, past history of stroke or neurological disorders or psychiatric illness were excluded. Patients who suffered disabling conditions with consequences for daily functioning and severe medical illnesses such as uncontrolled diabetes, recent myocardial infarction or severe metabolic disorders were also excluded. The total number of patients excluded was 34, owing to reasons of aphasia (7 patients), duration since onset of stroke being less than 2 weeks (16 patients) and more than 6 months (2 patients), severe medical illness and altered sensorium (4 patients) and past history of stroke (5 patients).
A complete medical, psychiatric, and medication history using a semi-structured case report form was obtained from the 62 patients inducted into the study. In order to adjust the factors that confound the association between stroke and depression, all potentially important co-variates such as age, sex, socio-demographic profile, cognitive deficits, functional disability, and the location of lesion were included in the case report form. Neurologic evaluations in the hospital and at follow-up were carried out by the attending neurologist. The patients were interviewed for depressive symptoms in a private room between 11 am and 2 pm to minimize diurnal mood variation. The patients were interviewed using the Mini International Neuropsychiatric Inventory (MINI) Plus[12] for the diagnosis of psychiatric morbidity as per Diagnostic and Statistical Manual of Mental Disorders Fourth edition Text Revision (DSM-IV-TR) guidelines.[13] Psychiatric examination included two standardized quantitative measures of affective state: Subjective Beck Depression Inventory (BDI)[14] and objective Montgomery Asberg Depression Rating Scale (MADRS).[15] Using information from the care giver, measures in the performance of activities of daily living was calculated with Barthel Index (BI).[16] It is scored out of 100 points with 10 different activities such as feeding, bathing, and walking. Score >75 indicates self-sufficiency. Cognitive function was assessed by Mini Mental Status Examination (MMSE).[17] Data was entered in English keeping the patient identification information confidential. Neuro-imaging in the form of Computed Tomography scans and MRI films brought by the patient on referral were used to elicit information on side and site of lesion. Localization of stroke was divided into right hemispheric, left hemispheric, and bilateral infarcts. Localization was also broadly based on the site of lesion - cortical, sub-cortical, cerebellum and brain stem.
Statistical analysis
Descriptive statistics were used to summarize the demographic, clinical, and the radiological variables. Chi-square/Fisher exact test has been used to find the significance of study parameters on categorical scale between two or more groups. Nearly, 95% confidence interval has been computed to find the significant features. P value was set at ≤0.05. Patient population was categorically divided into diagnostic groups of those with depression, those with adjustment disorder and those with neither. These groups were then analyzed using logistic regression for their correlation with respect to the side and site of lesion. Data collected were analyzed using the Statistical Package for the Social Sciences (SPSS) 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Socio-demographic characteristics are presented in Table 1.
Table 1.
Socio-demographic profile of post-stroke patients
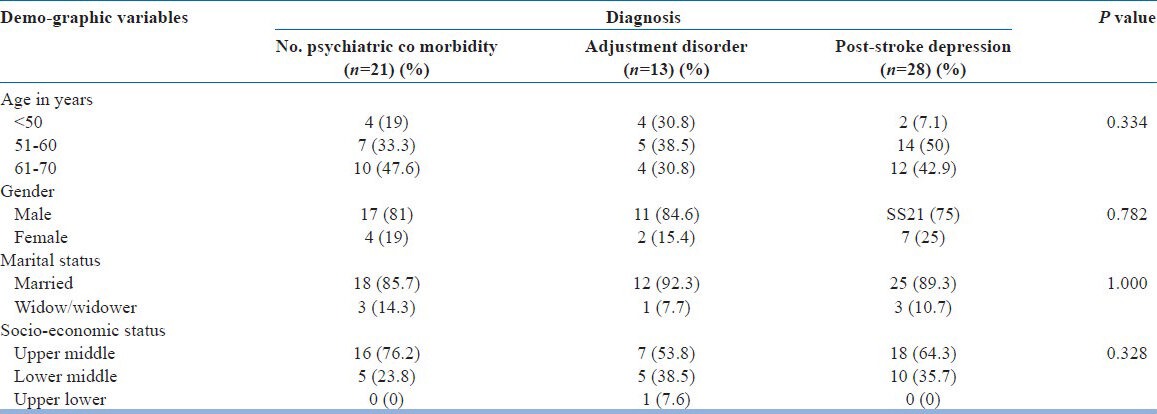
Mean ± SD age of this group was: 57.89 ± 7.12 years. Of the 62 patients, 28 (45.16%) were diagnosed with depression (Mood disorder due to General Medical Condition DSM-IV-TR 293.83), of which 18 qualified for a diagnosis of Major depression. Of the remaining 34 patients, 13 patients qualified for a diagnosis of Adjustment disorder (DSM-IV-TR 309). Comparison of baseline characteristics between the groups of depression, adjustment disorder, and those without any psychiatric morbidity did not show any differences. Cognitive impairment (MMSE scores <24) was almost equally distributed in the study population; hence, no correlation could be made.
Two significant differences were noted between the groups with PSD and Adjustment disorder. Among those with Adjustment disorder, 61% (n = 8) were diagnosed within the first 5 weeks of stroke onset and the group had lower mean BI scores (59.61) as compared to the group with PSD. This emphasizes the role of time of assessment and functional deficit in those with reactive depressive symptoms not amounting to depression.
Most patients had lesions in one focal area (n = 38, 61.29%). Among those with PSD, left sided lesions were prominent and statistically significant (n = 19, P = 0.002). No statistically significant correlation could be made with the site of lesion. Though, the results do show that patients with sub cortical lesions were more likely to develop PSD (n = 11). Each location lesion was further sub divided based on side of lesion. PSD showed statistically significant association with left sided cortical infarcts and left-sided subcortical infarcts.
These values are represented in Table 2 and graphically in Figures 1-3.
Table 2.
Diagnosis and stroke associated variables
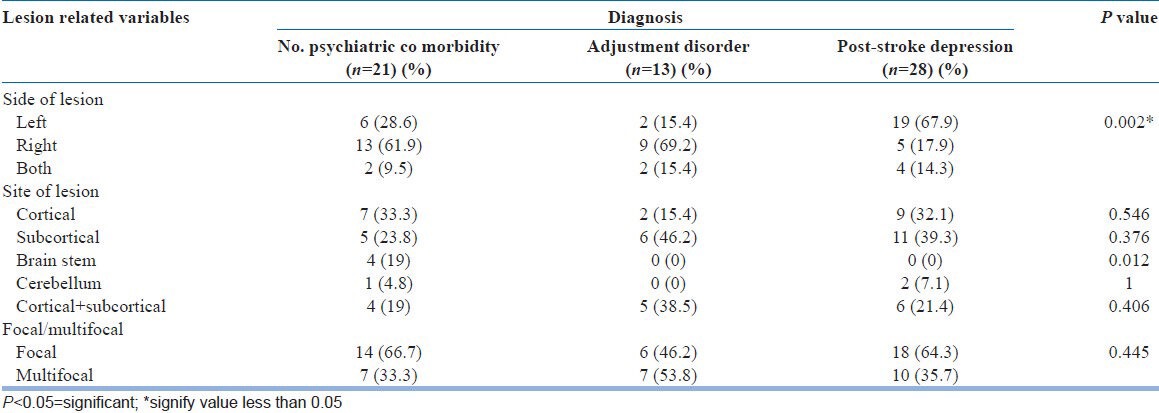
Figure 1.
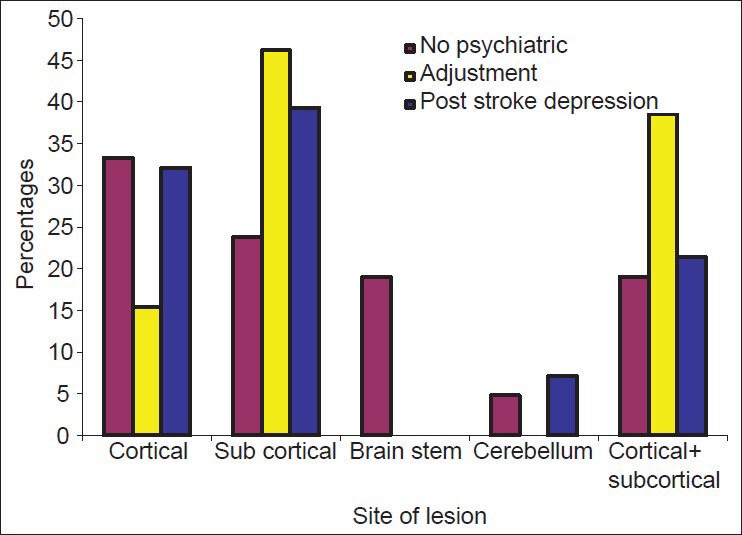
Distribution of diagnosis across lesion location
Figure 3.
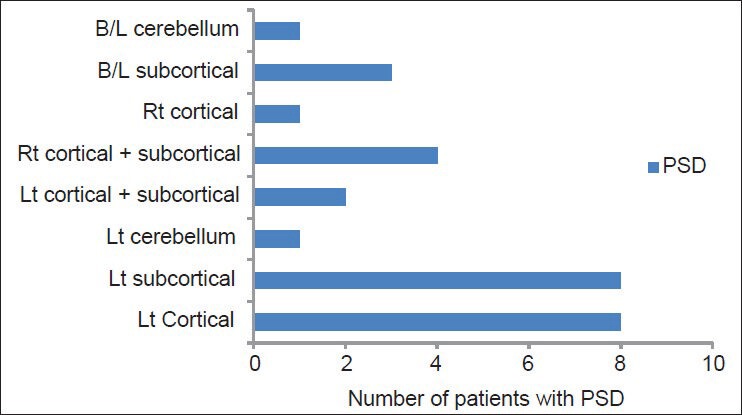
Spread of post-stroke depression across side and site of lesion
Figure 2.
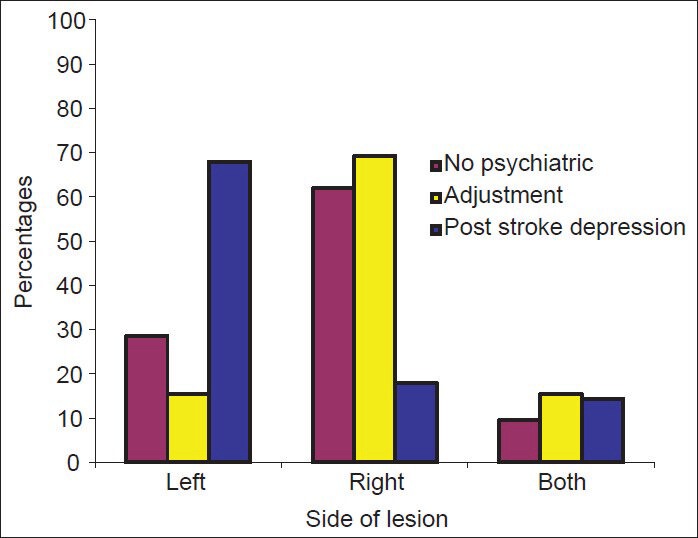
Distribution of diagnosis across left and right hemispherical lesions
DISCUSSION
We examined the frequency and severity of symptoms of depression and its correlation with lesion location, among hospitalized patients with the stroke, enrolled from a tertiary care set-up. Our major finding was the high prevalence of depression in patients with the stroke and its strong association with left sided lesions, more specifically left sided cortical and subcortical lesions.
In our study, the prevalence of major depression was found to be 45.16%, which is in keeping with landmark studies by Robinson and Starkstein,[5] Hackett et al.,[18] and the FINNSTROKE study.[1] Studies by Paolucci et al.,[19] reported a PSD prevalence of 36% among the 1064 patients included in the DESTRO study while Berg et al.,[20] reported prevalence rate as high as 50%. In the Indian study by Srivastava et al.,[2] the prevalence of PSD was found to be 35.29%.
Of the 28 patients with PSD in this study, 18 patients qualified for Major and 10 patients for Minor depression. Of the 62 patients, 20.96% were diagnosed as Adjustment disorder as per the DSM-IV-TR criteria. All together, 66.12% of the study population had developed a depressive reaction within the first 6 months of stroke.
It was found that among those patients diagnosed with Adjustment disorder, the lower mean value of BI was 59.61 indicating higher degree of disability in activities of daily living as compared to those with PSD among who the mean BI value was 73.04. This was found to be significant. The difference between adjustment disorder and depression was apparent with the former being indicative of a reaction to the post-stroke state, related predominantly to the functional disability.
In this study, significant correlation was found between left sided lesions and PSD. Significance was also noted with left cortical and left subcortical lesions having independent effects in the development of PSD. According to studies by Robinson et al.,[21] major depression in the post-stroke period showed significant association with the characteristic localization and abnormal dexamethasone suppression test results. A preliminary positron emission tomography scan study demonstrated increased cortical serotonin receptor sensitivity among patients with right as compared with left hemisphere stroke, which could explain the laterality of PSD.[22] All these results are in line with previous studies[18,23,24] and gives credence to the neuro-anatomical model of PSD. This model encompasses several hypotheses suggesting that biogenic amines, differences in post-synaptic receptor sensitivity or serotonin mediated effects on platelet and increased inflammation in the post-stroke state are all individually or in combination associated with risk of depression.
Other studies such as Aben et al., [25] found that no significant association could be found between side of lesion, frontal involvement and development of depression. Sato et al. [26] and Srivastava et al.,[2] in their respective studies, found PSD to have no correlation with lesion location. In some other studies,[27,28] the association was found to change over time. Considering the methodological variations in the study, comparison is difficult and a systematic review[29] shows that due to such limitations no significant conclusion could be reached. However, a recent study[30] cited initial neurological deficit as predictor of depression. Furthermore, a meta-analysis[31] of 111 studies showed that the lesion location is significantly associated with the severity of depressive symptoms in the first 6 months following stroke.
One of the main problems in research of PSD is the diagnostic accuracy of standardized psychiatric assessment techniques in neurologically impaired individuals. The major strength of this study is the use of standardized assessment tools for the diagnosis of PSD. The concurrent use of a structured interview and quantitative measures makes PSD a valid diagnosis and easily replicable. Correlation between the diagnosis obtained by DSM-IV-TR criteria and diagnosis by BDI and MADRS scores was studied, found to be comparable and this improved sensitivity. The MINI was designed as a brief structured interview for major axis I psychiatric disorders and it was found to have acceptably high validation and reliability scores. BDI is a self-rating depression scale that has been extensively studied and found to be useful in the screening of PSD. Self-report measures tend to have low specificity and poor predictive values in patients with stroke because of inclusion of somatic symptoms. Furthermore, patients lack the ability to identify their disability or deficits, predominant causes being denial, anosognosia or cognitive impairment. This was overcome by the concurrent use of an objective measure, which was correlated statistically with BDI and found to be comparable. Its lack of emphasis on physical symptoms makes it more reliable in depressed elderly patients. It has also been found to demonstrate acceptable sensitivity, specificity, and predictive value.[32]
We have also taken into consideration the time of assessment with the primary aim of focusing on lesion location and its association with PSD within the first 6 months of stroke. Different etiological factors have been implicated based on the time of assessment. Here, the patients with duration since onset of stroke less than 2 weeks were excluded to prevent the influence of the acute phase on reactive depression. The first 6 months after stroke, PSD is predominantly associated with the stroke related factors. After 6 months and into the chronic phase, disability related, social and contextual factors come into play.[29] In our study, more than 50% of those diagnosed with PSD were assessed within first 2 months of onset of stroke, which has also been found consistently in other studies. It is logical that with time, brain damage secondary to stroke will decrease and will play a less dominant role in the development of changes in mood and emotion.
To the best of our knowledge, this study is one among the few clinical evaluations that have been carried out in the area of PSD in India, emphasizing on assessment of PSD and its associated etiological variables, in particular lesion location. Unlike few studies where prevalence and correlation were made by using the patients from large databases and reports from published studies; this study was undertaken with strict eligibility criteria where objective analysis was made before documenting the results. Correlation studies in the past have been limited in three areas: Assessment, patient setting, and time since stroke[33] all of which were considered in the design of this study.
A prominent limitation is the small sample size with the exclusion of an important sub set of patients with aphasia and those in acute phase of stroke. Paolucci et al. (2005)[19] noted in his study that a history of stroke and depression in the past are factors likely to facilitate the development of depression following stroke. To minimize this, these patients were excluded. In addition, the findings of neuro-imaging were at best superficial and further research is needed to delve deeper into the characteristics of the lesion especially in multi-infarct states.
CONCLUSION
The results of our study show the high prevalence of PSD and its correlation with left sided cortical and sub cortical lesions. A clearer understanding of the relationship between neurological damage and depression after stroke would provide insight into the neurobiology of mood disorders and assist clinicians in early identification of patients at the highest risk for mood disturbance and those most likely to benefit from prophylactic[34] and treatment interventions. This, in turn, will lead to shortened hospital stays, improved quality of life, and reduced morbidity and mortality.
ACKNOWLEDGMENT
The authors thank the Staff of Department of Neurology and Psychiatry, Kasturba Medical College, Mangalore, for their support and co-operation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kotila M, Numminen H, Waltimo O, Kaste M. Depression after stroke: Results of the Finnstroke Study. Stroke. 1998;29:368–72. doi: 10.1161/01.str.29.2.368. [DOI] [PubMed] [Google Scholar]
- 2.Srivastava A, Taly AB, Gupta A, Murali T. Post-stroke depression: Prevalence and relationship with disability in chronic stroke survivors. Ann Indian Acad Neurol. 2010;13:123–7. doi: 10.4103/0972-2327.64643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.House A, Dennis M, Mogridge L, Warlow C, Hawton K, Jones L. Mood disorders in the year after first stroke. Br J Psychiatry. 1991;158:83–92. doi: 10.1192/bjp.158.1.83. [DOI] [PubMed] [Google Scholar]
- 4.Santos M, Kövari E, Gold G, Bozikas VP, Hof PR, Bouras C, et al. The neuroanatomical model of post-stroke depression: Towards a change of focus? J Neurol Sci. 2009;283:158–62. doi: 10.1016/j.jns.2009.02.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson RG, Starkstein SE. Current research in affective disorders following stroke. J Neuropsychiatry Clin Neurosci. 1990;2:1–14. doi: 10.1176/jnp.2.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Morris PL, Robinson RG, Raphael B, Hopwood MJ. Lesion location and post stroke depression. J Neuropsychiatry Clin Neurosci. 1996;8:399–403. doi: 10.1176/jnp.8.4.399. [DOI] [PubMed] [Google Scholar]
- 7.Robinson RG, Starr LB, Kubos KL, Price TR. A two-year longitudinal study of post-stroke mood disorders: Findings during the initial evaluation. Stroke. 1983;14:736–41. doi: 10.1161/01.str.14.5.736. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan KR, McDonald WM, Doraiswamy PM, Tupler LA, Husain M, Boyko OB, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243:41–6. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 9.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;4:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 10.Robinson RG, Jorge RE, Clarence-Smith K. Double-blind randomized treatment of poststroke depression using nefiracetam. J Neuropsychiatry Clin Neurosci. 2008;20:178–84. doi: 10.1176/jnp.2008.20.2.178. [DOI] [PubMed] [Google Scholar]
- 11.The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): A major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41:105–14. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 13.Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. [Google Scholar]
- 14.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 16.Mahoney FI, Barthel DW. Functional evaluation: The barthel index. Md State Med J. 1965;14:61–5. [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Hackett ML, Anderson CS. Predictors of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:2296–301. doi: 10.1161/01.STR.0000183622.75135.a4. [DOI] [PubMed] [Google Scholar]
- 19.Paolucci S, Gandolfo C, Provinciali L, Torta R, Sommacal S, Toso V, et al. Quantification of the risk of post stroke depression: The Italian multicenter observational study DESTRO. Acta Psychiatr Scand. 2005;112:272–8. doi: 10.1111/j.1600-0447.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 20.Berg A, Lönnqvist J, Palomäki H, Kaste M. Assessment of depression after stroke: A comparison of different screening instruments. Stroke. 2009;40:523–9. doi: 10.1161/STROKEAHA.108.527705. [DOI] [PubMed] [Google Scholar]
- 21.Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood disorders in stroke patients. Importance of location of lesion. Brain. 1984;107:81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Mayberg HS, Robinson RG, Wong DF, Parikh R, Bolduc P, Starkstein SE, et al. PET imaging of cortical S2 serotonin receptors after stroke: Lateralized changes and relationship to depression. Am J Psychiatry. 1988;145:937–43. doi: 10.1176/ajp.145.8.937. [DOI] [PubMed] [Google Scholar]
- 23.Robinson RG, Lipsey JR, Rao K, Price TR. Two-year longitudinal study of post-stroke mood disorders: Comparison of acute-onset with delayed-onset depression. Am J Psychiatry. 1986;143:1238–44. doi: 10.1176/ajp.143.10.1238. [DOI] [PubMed] [Google Scholar]
- 24.Parikh RM, Lipsey JR, Robinson RG, Price TR. A two year longitudinal study of poststroke mood disorders: Prognostic factors related to one and two year outcome. Int J Psychiatry Med. 1988;18:45–56. doi: 10.2190/lw46-3e9f-kyjm-wxgq. [DOI] [PubMed] [Google Scholar]
- 25.Aben I, Lodder J, Honig A, Lousberg R, Boreas A, Verhey F. Focal or generalized vascular brain damage and vulnerability to depression after stroke: A 1-year prospective follow-up study. Int Psychogeriatr. 2006;18:19–35. doi: 10.1017/S104161020500270X. [DOI] [PubMed] [Google Scholar]
- 26.Sato R, Bryan RN, Fried LP. Neuroanatomic and functional correlates of depressed mood: The Cardiovascular Health Study. Am J Epidemiol. 1999;150:919–29. doi: 10.1093/oxfordjournals.aje.a010100. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda K, Robinson RG. The relationship between poststroke depression and lesion location in long-term follow-up. Biol Psychiatry. 1999;45:187–92. doi: 10.1016/s0006-3223(98)00178-4. [DOI] [PubMed] [Google Scholar]
- 28.Singh A, Black SE, Herrmann N, Leibovitch FS, Ebert PL, Lawrence J, et al. Functional and neuroanatomic correlations in poststroke depression: The Sunnybrook Stroke Study. Stroke. 2000;31:637–44. doi: 10.1161/01.str.31.3.637. [DOI] [PubMed] [Google Scholar]
- 29.Bhogal SK, Teasell R, Foley N, Speechley M. Lesion location and poststroke depression: Systematic review of the methodological limitations in the literature. Stroke. 2004;35:794–802. doi: 10.1161/01.STR.0000117237.98749.26. [DOI] [PubMed] [Google Scholar]
- 30.Bour A, Rasquin S, Aben I, Boreas A, Limburg M, Verhey F. A one-year follow-up study into the course of depression after stroke. J Nutr Health Aging. 2010;14:488–93. doi: 10.1007/s12603-010-0033-x. [DOI] [PubMed] [Google Scholar]
- 31.Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra-and inter-hemispheric lesion location using meta-analysis. J Neuropsychiatry Clin Neurosci. 2003;15:422–30. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- 32.Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook Stroke Study: A prospective study of depressive symptoms and functional outcome. Stroke. 1998;29:618–24. doi: 10.1161/01.str.29.3.618. [DOI] [PubMed] [Google Scholar]
- 33.Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother. 2008;9:1291–8. doi: 10.1517/14656566.9.8.1291. [DOI] [PubMed] [Google Scholar]
- 34.Ramasubbu R. Therapy for prevention of post-stroke depression. Expert Opin Pharmacother. 2011;12:2177–87. doi: 10.1517/14656566.2011.596149. [DOI] [PubMed] [Google Scholar]


