Abstract
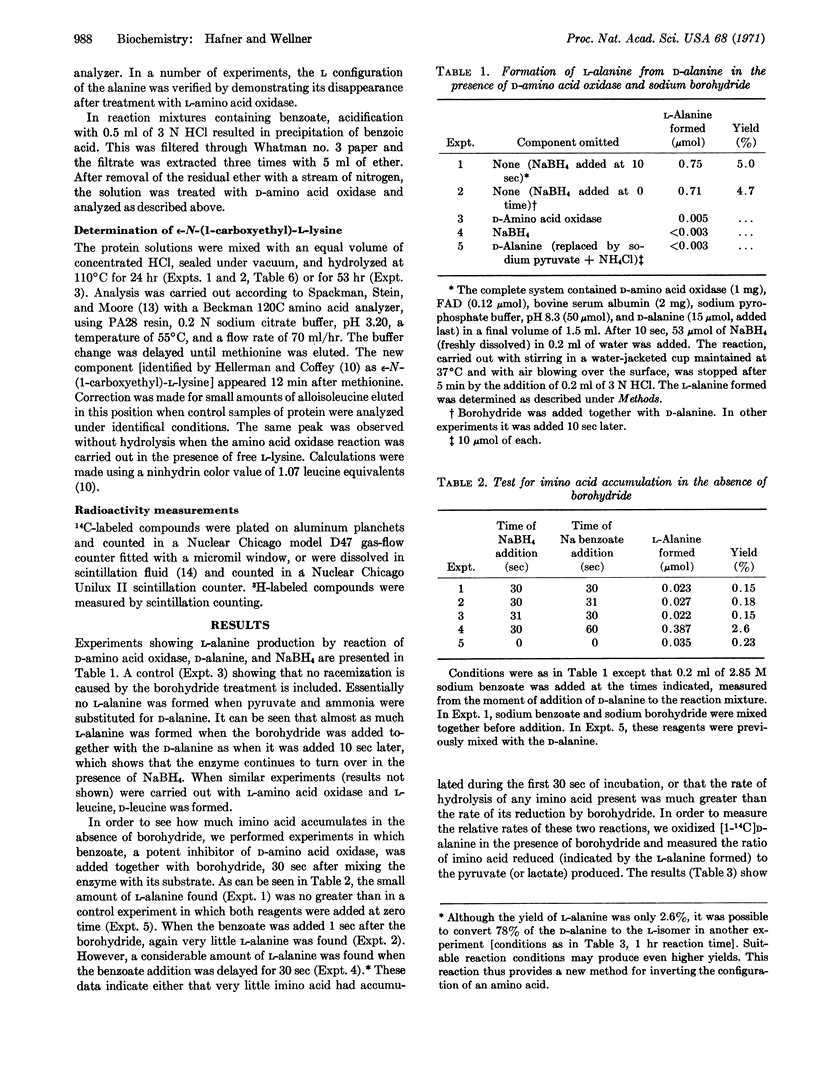
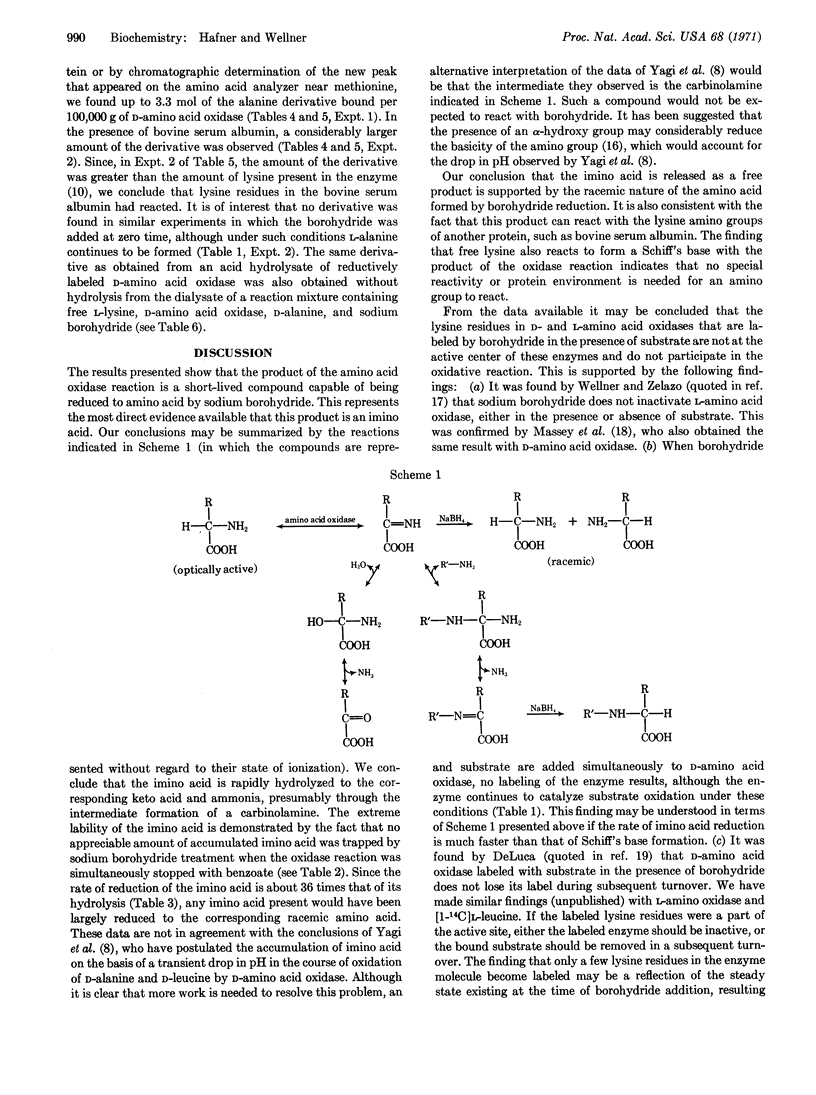
It had long been thought, but never demonstrated, that imino acids are formed in the reactions catalyzed by D- and L-amino acid oxidases (EC 1.4.3.3 and 1.4.3.2). The formation of imino acids is now shown directly by allowing the amino acid oxidase reaction to proceed in the presence of NaBH4, when the imino acid is reduced to the corresponding racemic amino acid. Thus, when NaBH4 is added to a mixture of D-amino acid oxidase and D-alanine, a significant amount of L-alanine is formed. Analogous results are obtained using L-amino acid oxidase and L-leucine. Since D-amino acid oxidase is active in the presence of NaBH4, L-alanine continues to be formed until most of the D-isomer is oxidized by the enzyme. This reaction provides a new method for inverting the configuration of an amino acid.
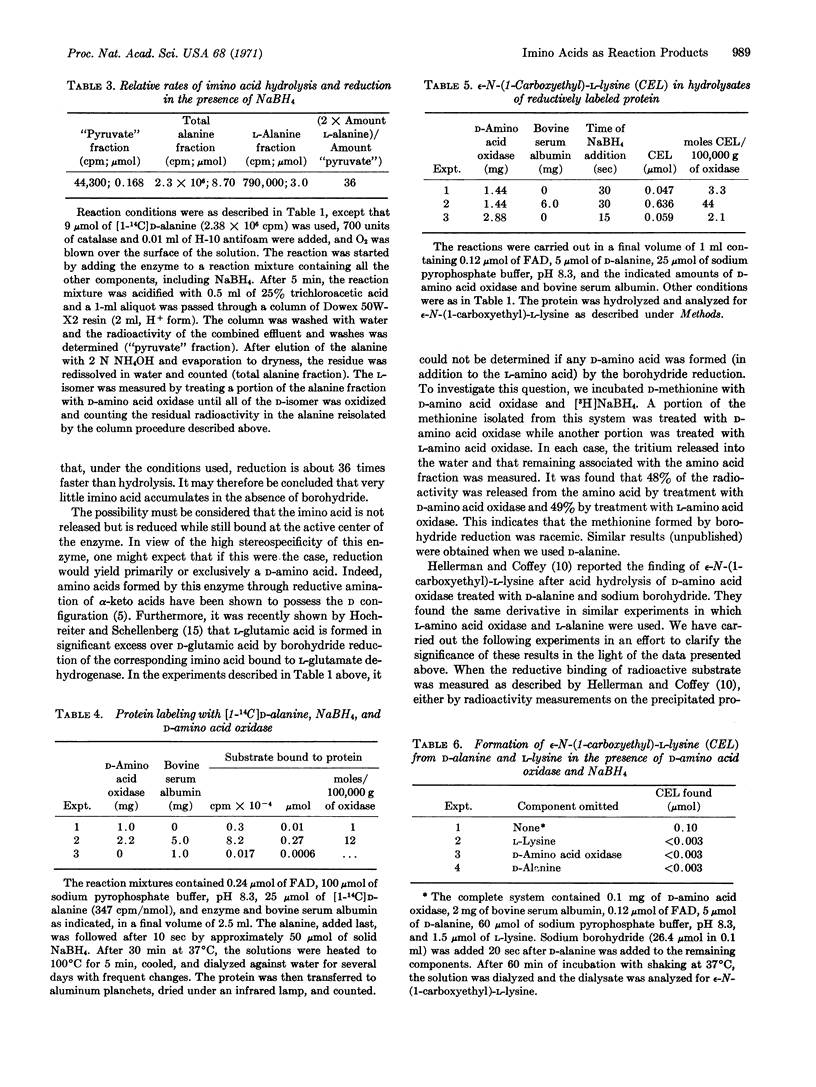
When NaBH4 is added to a system containing D-amino acid oxidase plus D-alanine and L-lysine, free ε-N-(1-carboxyethyl)-L-lysine is formed. When bovine serum albumin is substituted for L-lysine, the same compound results upon acid hydrolysis. It is concluded that the amino acid oxidase reaction produces a free imino acid, which may be reduced by NaBH4 to a racemic amino acid or may form Schiff's bases by reaction with the ε-amino groups of proteins and of free lysine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coffey D. S., Neims A. H., Hellerman L. Studies on crystalline D-amino acid oxidase. II. Isolation of a reduced 14C-labeled substrate-enzyme intermediate after the action of sodium borohydride. J Biol Chem. 1965 Oct;240(10):4058–4064. [PubMed] [Google Scholar]
- Hellerman L., Coffey D. S. Studies on crystalline D-amino acid oxidase. V. Characterization of borohydride-reduced enzyme-subtrate intermediate. Synthesis of epsilon-N-(1-carboxyethyl)-L-lysine. J Biol Chem. 1967 Feb 25;242(4):582–589. [PubMed] [Google Scholar]
- Hochreiter M. C., Schellenberg K. A. Alpha-iminoglutarate formation by beef liver L-glutamate dehydrogenase. Detection by borohydride or dithionite reduction to glutamate. J Am Chem Soc. 1969 Nov 5;91(23):6530–6531. doi: 10.1021/ja01051a084. [DOI] [PubMed] [Google Scholar]
- Massey V., Curti B., Müller F., Mayhew S. G. On the reaction of borohydride with D- and L-amino acid oxidases. J Biol Chem. 1968 Mar 25;243(6):1329–1330. [PubMed] [Google Scholar]
- Müller F., Massey V., Heizmann C., Hemmerich P., Lhoste J. M., Gould D. C. The reduction of flavins by borohydride: 3,4-dihydroflavin. Struction, absorption and luminescence. Eur J Biochem. 1969 Jun;9(3):392–401. doi: 10.1111/j.1432-1033.1969.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Neims A. H., Hellerman L. Flavoenzyme catalysis. Annu Rev Biochem. 1970;39:867–888. doi: 10.1146/annurev.bi.39.070170.004251. [DOI] [PubMed] [Google Scholar]
- RADHAKRISHNAN A. N., MEISTER A. Amino acid synthesis by reversal of the amino acid oxidase reaction. J Biol Chem. 1958 Aug;233(2):444–450. [PubMed] [Google Scholar]
- TABORSKY G. Mechanism of the oxidation of tyrosine by amino acid oxidase of snake venoms. Yale J Biol Med. 1955 Feb;27(4):267–278. [PMC free article] [PubMed] [Google Scholar]
- WELLNER D., MEISTER A. Crystalline L-amino acid oxidase of Crotalus adamanteus. J Biol Chem. 1960 Jul;235:2013–2018. [PubMed] [Google Scholar]
- Yagi K., Nishikimi M., Oishi N., Takai A. Release of alpha imino acid as primary product in D-amino-acid oxidase reaction. Biochim Biophys Acta. 1970 Aug 15;212(2):243–247. doi: 10.1016/0005-2744(70)90204-4. [DOI] [PubMed] [Google Scholar]


