Abstract
Background
Inflammatory processes are putative mechanisms underlying the cardio-protective effects of physical activity. An inverse association between physical activity and inflammation has been demonstrated but no long-term prospective data are available. We therefore examined the association between physical activity and inflammatory markers over a 10-year follow-up period.
Methods and Results
Participants were 4289 men and women (mean age 49.2 years) from the Whitehall II cohort study. Self-reported physical activity and inflammatory markers (serum high-sensitivity C-reactive protein [CRP] and interleukin-6 [IL-6]) were measured at baseline (1991) and follow-up (2002). Forty-nine percent of the participants adhered to standard physical activity recommendations for cardiovascular health (2.5 hours per week moderate to vigorous physical activity) across all assessments. Physically active participants at baseline had lower CRP and IL6 levels and this difference remained stable over time. In comparison to participants that rarely adhered to physical activity guidelines over the 10 years follow-up, the high adherence group displayed lower logeCRP (β=−0.07, 95% CI, −0.12, −0.02) and logeIL-6 (β=−0.07, 95% CI, −0.10, −0.03) at follow up after adjustment for a range of covariates. Compared to participants that remained stable, those that reported an increase in physical activity of at least 2.5 hours/wk displayed lower loge CRP (B coefficient =−0.05, 95% CI, −0.10, −0.001) and loge IL-6 (B coefficient =−0.06, 95% CI, −0.09, −0.03) at follow up.
Conclusions
Regular physical activity is associated with lower markers of inflammation over 10 years of follow-up and thus may be important in preventing the pro-inflammatory state seen with ageing.
Keywords: Ageing, C-reactive protein, exercise, physical activity, inflammation
The anti-inflammatory effects of exercise are thought to be one of the mechanisms that explain the well-documented cardio-protective effects of physical activity.1-4 Evidence from epidemiological studies has demonstrated an inverse association between physical activity and markers of low grade systemic inflammation.5 However, the majority of existing evidence is drawn from cross-sectional analyses and few studies have examined the association between long-term physical activity behaviour and low grade inflammation prospectively. Cross-sectional data make it difficult to discount reverse causation effects. For example, some evidence suggests low grade inflammation is a marker of sarcopenia,6 thus functional limitations might explain associations between systemic inflammation and low activity in ageing populations.7 Tracking low-grade inflammation is particularly relevant in an ageing population, as inflammatory markers gradually rise with increasing age and this pro-inflammatory status underlies biological mechanisms responsible for cardiovascular disease (CVD) and other age-related diseases.8-10
Since the majority of health benefits from exercise are established through chronic training adaptations, it is difficult to draw firm conclusions from short-term exercise trials often lasting less than 6 months. Indeed, this might partly explain the equivocal nature of clinical trial data on exercise and inflammatory markers.11 Thus, in the present study we examined the association between physical activity and inflammatory markers over a 10-year follow-up period using a well characterised population based cohort study.
Methods
Participants
Participants were drawn from the Whitehall II population based cohort.12 The Whitehall II study is an on-going prospective cohort study that consists of 10,308 participants (6,895 men and 3,413 women aged 35 to 55) recruited from the British civil service in 1985 in order to investigate social and occupational influences on CVD risk. The baseline medical examination (Phase 1) took place during 1985/88, and subsequent phases have alternated between questionnaire alone (Phases 2, 4, 6 and 8) and phases including both a medical examination and a questionnaire (Phases 1, 3, 5, 7 and 9). For the purposes of the present study, phase 3 (1991/93) was regarded as the baseline when inflammatory markers were first assessed and phase 7 (2002/04) as the follow up. The mean follow-up time between phases 3 and 7 was 11.3 years (range, 9.5–12.9 years). Participants gave full informed written consent to participate in the study and ethical approval was obtained from the University College London Hospital committee on the Ethics of Human Research.
Physical activity assessment
Physical activity was assessed at phases 3, 5 (1997/99), and 7 using a self-reported questionnaire. At phase 3, the physical activity assessment consisted of 3 questions about duration and frequency per week spent at light, moderate and vigorous intensity physical activity. At phases 5 and 7 the physical activity questions consisted of 20 items on frequency and duration of participation in walking, cycling, sports, gardening, housework, and home maintenance.13 Frequency and duration of each activity were combined to compute hours per week of moderate to vigorous physical activity. The 20-item self-reported physical activity questionnaire is a modified version of the previously validated Minnesota leisure-time physical activity questionnaire.14 In addition, the self-reported physical activity measure has demonstrated predictive validity for mortality in the Whitehall II study.15 Although assessed slightly differently, physical activity (moderate-vigorous hrs/wk) measured at phase 3 was correlated with physical activity measured at phases 5 (Spearman's r=0.41, p<0.001) and 7 (r=0.36, p<0.001). Similar correlations were observed between physical activity at phases 5 and 7 (r=0.51, p<0.001) when assessed with an identical questionnaire.
Clinical assessment and Inflammatory markers
The procedures for the clinical examination have been described elsewhere.12 Briefly, measurements included height, weight, waist and hip circumference, blood pressure, and a fasting blood sample taken from the antecubital fossa. Fasting serum was collected between 0800 and 1300 h and was stored at −70 C. Samples from phases 3 and 7 were analyzed at the same time. The inflammatory marker C-reactive protein (CRP) was measured using a high-sensitivity immunonephelometric assay in a BN ProSpec nephelometer (Dade Behring, Milton Keynes, UK). Interleukin-6 (IL-6) was measured using a high-sensitivity ELISA (R&D Systems, Oxford, UK). Values lower than the detection limit (0.154 mg/liter for CRP and 0.08 pg/ml for IL-6) were assigned a value equal to half the detection limit. To measure short-term biological variation and laboratory error, a repeated sample was taken from a subset of 150 participants for CRP and 241 for IL-6 at phase 3 [average elapse time between samples 32 (SD=10.5) days], and 533 for CRP and 329 for IL-6 at phase 7 (average elapse time 24 (SD=11.0) days]. Intra- and inter-assay coefficients of variation were 4.7% and 8.3% for CRP, and 7.5% and 8.9% for IL-6 at phases 3 and 7, respectively. A questionnaire was completed regarding age, civil service employment grade (a measure of socioeconomic status, SES), smoking habits, health status and hormone replace therapy (HRT, women only).
Statistical analysis
The inflammatory markers displayed a skewed distribution, and normality was obtained after natural logarithmic (loge) transformation. Participants were categorised according to whether they adhered to the physical activity guidelines (at least 2.5 hr per week moderate to vigorous physical activity) that are widely used and have been quantitatively validated for cardiovascular outcomes.16,17 In order to examine associations between baseline physical activity and change in inflammatory markers between phases 3 to 7 we adopted a linear mixed models approach and fitted the intercept as a random effect.18 The model included terms for baseline physical activity, time (phase 3 corresponds to time 0, phase 7 to time 1, so that coefficients associated with time correspond to a 10yr change), and an interaction term between physical activity and time to estimate the association between baseline physical activity and change in inflammatory markers over the follow-up. This model also included covariates that were associated with both physical activity and inflammatory markers. In order to examine the effects of long-term physical activity exposure over the three assessments participants were categorised as ‘Rarely’ meeting guideline (once or less through follow-up); ‘Sometimes’ (on two phases); ‘Always’ (on all three follow-up phases). We fitted general linear models to examine the association between long term physical activity exposure (number of times meeting the guideline over follow-up) and inflammatory markers at follow up, adjusting for age, gender, smoking, employment grade, body mass index (BMI), and chronic illness. In separate sensitivity analyses we adjusted for waist to hip ratio instead of BMI, and also modelled BMI change. We also investigated associations between changes in physical activity (calculated as the difference in hours/wk of moderate to vigorous activity between phases 5 and 7) and inflammatory markers using general linear models. Lastly, we used linear mixed models to examine associations between baseline inflammation (categorised as CRP <1mg/l; 1 to < 3 mg/l; ≥ 3mg/l) and change in moderate to vigorous physical activity (hrs/wk) between phases 3 to 7, fitting the intercept as a random effect term and an interaction term between CRP category and time. All analyses were conducted using SPSS version 20 (SPSS, Chicago, IL) using two-sided tests with a significance level p<0.05.
Results
At baseline 7366 participants had available data on all variables although after excluding participants with missing data through follow-up the final analytic sample comprised 4289 participants (3092 men and 1197 women). Participants excluded were slightly older (50.1 vs. 49.2 yrs, p<0.001), less physically active (3.3 vs. 3.6 hrs/wk moderate to vigorous physical activity, p=0.003), and had higher baseline loge CRP values (0.87 vs. 0.75, p<0.001) compared with those included. However, these absolute differences in characteristics between the groups were trivial, only attaining statistical significance owing to the large sample size. Approximately half the sample (49%) adhered to the physical activity recommendation (2.5 hrs per week moderate to vigorous physical activity) across all assessments (50% at phase 3 [baseline]; 83.7% at phase 5; 83.3% at phase 7). Participants that ‘always’ met the physical activity guidelines were more likely to be men, from higher employment grades, and had lower BMI (Table 1).
Table 1.
Descriptive characteristics of the sample at baseline in relation to habitual physical activity through follow up (n= 4289).
| Variable | Meeting physical activity guidelines through follow up† |
||
|---|---|---|---|
| Rarely (n=681) | Sometimes (n=1503) | Always (n=2105) | |
| Age (yrs) | 48.7 ± 5.7 | 49.4 ± 5.9 | 49.1 ± 6.0 |
| % men | 56.8 | 63.9 | 82.9 |
| % low grade employees | 21.6 | 12.2 | 6.5 |
| % smokers | 13.2 | 10.6 | 10.1 |
| % Chronic illness | 35.8 | 35.2 | 29.9 |
| Average MVPA (hrs/wk) | 1.1 ± 1.9 | 1.3 ± 1.5 | 6.0 ± 3.9 |
| Body Mass Index (kg/m2) | 25.3 ± 3.9 | 25.1 ± 3.6 | 24.9 ± 3.2 |
| CRP‡ (mg/L) | 2.29 ± 1.90 | 2.16 ± 1.81 | 2.05 ± 1.74 |
| IL-6‡ (pg/mL) | 2.70 ± 1.55 | 2.61 ± 1.47 | 2.45 ± 1.42 |
Meeting physical activity guidelines (at least 2.5 hrs moderate to vigorous physical activity [MVPA] per week); “Rarely’ includes meeting guideline once or less through follow-up; ‘sometimes’ on two phases; ‘always’ on all three follow-up phases.
Geometric mean (± SD)
Baseline physical activity and change in inflammatory markers
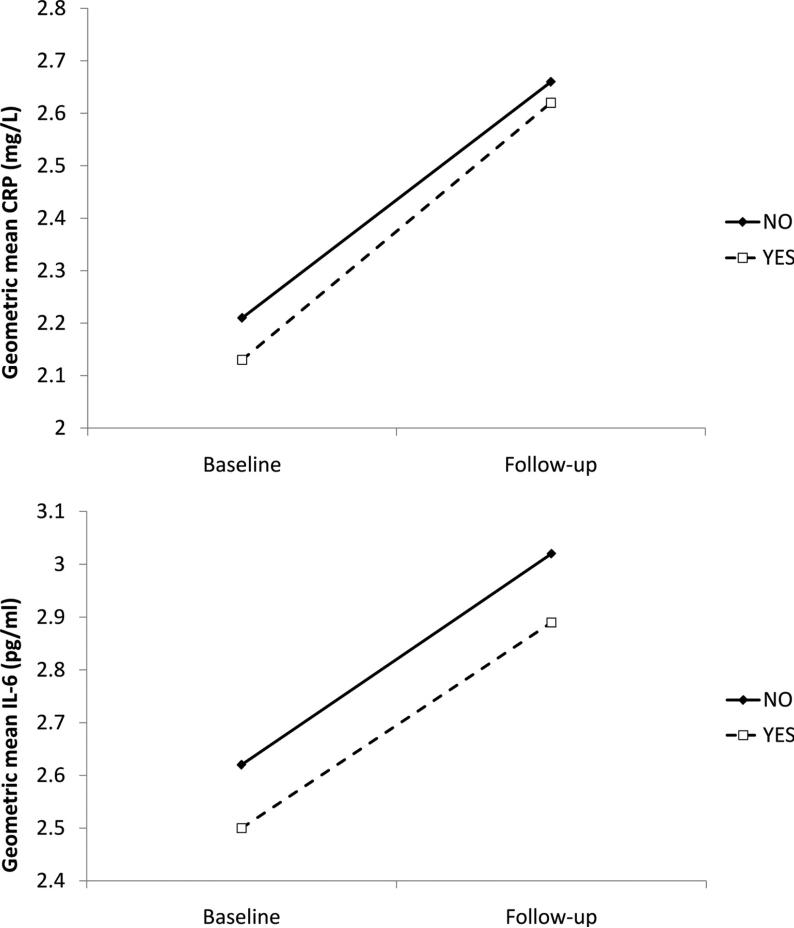
Meeting the physical activity guideline at baseline was inversely associated with baseline loge CRP (coefficient = −0.04, 95% CI, −0.07, −0.01, p=0.007) and loge IL-6 (coefficient = −0.04, 95% CI, −0.06, −0.02, p=0.001) after adjustments for age, gender, smoking, employment grade, BMI, and chronic illness (Table 2). On average, there was an increase in both inflammatory markers from baseline to follow-up: loge CRP increased from 0.75 to 0.94 (p<0.001) and loge IL-6 from 0.93 to 1.08 (p<0.001), corresponding to a change of 0.44 mg/l (21%) in CRP and 0.41 pg/ml (16%) in IL-6 over 10 years. There was no statistically significant association between baseline physical activity and change in loge CRP (p=0.10) or loge IL-6 (p=0.39) over follow-up (Table 2), suggesting that the difference in inflammation levels persisted but did not increase across time (Figure 1).
Table 2.
Linear mixed models to examine the association between meeting physical activity guidelines at baseline on inflammatory markers over phases 3 to 7.
| Meeting physical activity guidelines at baseline | Loge C-reactive protein |
Loge interleukin-6 |
||
|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | |||
| Model 1 | Model 2 | Model 1 | Model 2 | |
| No | Reference | Reference | Reference | Reference |
| Yes | −0.06 (−0.09, −0.04) | −0.04 (−0.07, −0.01) | −0.05 (−0.07, −0.04) | −0.04 (−0.06, −0.02) |
| Interaction term: | ||||
| PA * time | 0.03 (−0.01, 0.06) | 0.03 (−0.01, 0.06) | 0.01 (−0.01, 0.03) | 0.01 (−0.01, 0.03) |
Model 1; adjusted for age, gender.
Model 2; adjusted for age, gender, smoking, employment grade, BMI, chronic illness.
Physical activity (PA) by time interaction term calculated from meeting the PA recommendation (no=0, yes=1) and time (phase 3 corresponds to time 0, phase 7 to time 1).
Figure 1.
The association between physical activity at baseline in relation to change in C-reactive protein (upper panel) and interleukin-6 (lower panel) over 10 years. Solid and dashed lines represent participants that do not and do adhere to physical activity guidelines, respectively. Participants are 4289 men and women from the Whitehall II cohort assessed during 1991 – 2002. The geometric means are adjusted for age, sex, smoking, employment grade, BMI, chronic illness.
Habitual physical activity over 10 years and inflammatory markers at follow up
In comparison to participants that rarely adhered to physical activity guidelines through follow up, the high adherence group displayed lower loge CRP (B coefficient=−0.07, 95% CI, −0.12, −0.02) and loge IL-6 (B coefficient =−0.07, 95% CI, −0.10, −0.03) at follow up after adjustment for age, gender, smoking, employment grade, BMI, and chronic illness (Table 3). These coefficients corresponded to a fully adjusted difference of 0.18 mg/l in CRP and 0.20 pg/ml in IL-6 between individuals who adhered consistently compared to those that did not adhere to physical activity guidelines over 10 years. When we adjusted for waist circumference as a marker of central adiposity (instead of BMI) the effect estimate was slightly attenuated for loge CRP (B coefficient=−0.04, 95% CI, −0.10, 0.01) but changed little for loge IL-6 (B coefficient =−0.06, 95% CI, −0.09, −0.02). Participants that were consistently physically active over follow up gained less weight compared to those rarely active (average BMI increase, 1.4 ± 1.8kg/m2 vs. 1.6 ± 2.2kg/m2, p=0.04). However, when we adjusted for change in BMI during follow-up (instead of BMI at baseline) this did not alter the association between physical activity and inflammatory markers.
Table 3.
Adjusted coefficients (95% CI) for habitual physical activity over 10 years on inflammatory markers at follow up (n= 4289).
| Meeting physical activity guidelines‡ | Loge C-reactive protein |
Loge interleukin-6 |
||
|---|---|---|---|---|
| Model 1 β (95% CI) | Model 2 β (95% CI) | Model 1 β (95% CI) | Model 2 β (95% CI) | |
| Rarely (n=681) | Reference | Reference | Reference | Reference |
| Sometimes (n=1503) | −0.10 (−0.16, −0.04) | −0.08 (−0.13, −0.02) | −0.06 (−0.10, −0.03) | −0.05 (−0.08, −0.01) |
| Always (n=2105) | −0.11 (−0.17, −0.05) | −0.07 (−0.12, −0.02) | −0.09 (−0.12, −0.06) | −0.07 (−0.10, −0.03) |
| p-trend | 0.001 | 0.02 | 0.001 | 0.001 |
Meeting physical activity guidelines (at least 2.5 hrs MVPA per week); ‘Rarely’ includes meeting guideline once or less through follow-up; ‘sometimes’ on two phases; ‘always’ on all three follow-up phases.
Model 1; adjusted for age, gender.
Model 2; adjusted for age, gender, smoking, employment grade, BMI, chronic illness.
We examined the associations for change in physical activity (Table 4). In order to retain consistency we calculated changes in activity between phases 5 and 7 when the same questionnaire was used. Compared to participants that remained stable, those that reported an increase in physical activity of at least 2.5 hrs/wk displayed lower loge CRP (B coefficient =−0.05, 95% CI, −0.10, −0.001) and loge IL-6 (B coefficient =−0.06, 95% CI, −0.09, −0.03) at follow up after adjustment for age, gender, hours/week of moderate to vigorous physical activity at phase 5, smoking, employment grade, BMI, and chronic illness. There was no difference in inflammatory markers between participants that reported a reduction in physical activity compared with those remaining stable.
Table 4.
Adjusted coefficients (95% CI) for physical activity change on inflammatory markers at follow up.
| Physical activity Change‡ | Loge C-reactive protein |
Loge interleukin-6 |
||
|---|---|---|---|---|
| Model 1 β (95% CI) | Model 2 β (95% CI) | Model 1 β (95% CI) | Model 2 β (95% CI) | |
| Stable (n=989) | Reference | Reference | Reference | Reference |
| Decrease (n=1636) | −0.04 (−0.09, 0.02) | −0.02 (−0.07, 0.03) | −0.02 (−0.05, 0.01) | −0.01 (−0.04, 0.02) |
| Increase (n=1664) | −0.06 (−0.12, −0.01) | −0.05 (−0.10, −0.001) | −0.08 (−0.10, −0.04) | −0.06 (−0.09, −0.03) |
| p-trend | 0.04 | 0.12 | <0.001 | <0.001 |
Physical activity change calculated from phases 5 through 7. A decrease/increase represents a change of at least 2.5 hrs/wk of moderate to vigorous physical activity.
Model 1; adjusted for age, gender, and hrs/wk of moderate to vigorous physical activity at phase 5.
Model 2; adjusted for age, gender, hrs/wk of moderate to vigorous physical activity at phase 5, smoking, employment grade, BMI, chronic illness.
Sensitivity analyses
In order to account for non-specific inflammatory responses we re-ran the analysis after removing 823 participants who had reported acute infections such as cold or influenza 2 weeks prior to the phase 7 clinical assessment. This did not, however, change the results; for example, compared with participants rarely meeting physical activity guidelines, those that always met the guidelines had significantly lower loge CRP at follow-up (fully adjusted B coefficient = −0.06, 95% CI, −0.11, −0.005). We ran additional analyses in order to account for potential effects of HRT in women. 586 women never used HRT, 211 constantly used HRT, 26 stopped, and 336 started HRT through follow-up (n=38 missing data). In comparison to the women that never used HRT, only those that started using HRT through follow up displayed elevated loge CRP at follow up (age adjusted coefficient = 0.11, 95% CI, 0.03, 0.19). We re-ran the analyses for physical activity and inflammatory markers in women making additional adjustments for HRT use (as categorised above; never/ constant/ stopped/ started). The results still showed that in comparison to women that rarely adhered to physical activity guidelines, the high adherence group displayed lower loge CRP (fully adjusted B coefficient=−0.10, 95% CI, −0.20, −0.01, p=0.04) and loge IL-6 (B coefficient =−0.07, 95% CI, −0.13, −0.01, p=0.02) at follow up.
Association of basal inflammatory markers with physical activity change
We also examined the association between baseline inflammatory markers and change in moderate to vigorous physical activity from phase 3 to phase 7 using linear mixed models. Participants with CRP≥3mg/l at baseline demonstrated decreased moderate to vigorous physical activity (hrs/wk) at phase 7 (estimate for CRP * time interaction = −1.14, 95% CI, −0.35, −1.92; p=0.004) compared to those with CRP<1mg/l, after adjustments for age, gender, smoking, employment grade, BMI, and chronic illness.
Discussion
Given that the majority of existing data on physical activity and markers of systemic inflammation is cross-sectional, the aim of this study was to explore the longitudinal association between physical activity and inflammatory markers over a 10-year follow-up period. The main findings show that physically active participants at baseline had lower CRP and IL-6 levels and this difference remained stable over time. Secondly, maintenance of physical activity over the 10 years follow-up period was associated with lower levels of both inflammatory markers at follow-up. An increase in physical activity was also associated with lower levels of both inflammatory markers at follow up. Crucially, the associations observed between physical activity and inflammatory markers were independent of adiposity, which is an important confounder of the association between physical activity and inflammatory markers as physically active participants tend to have lower levels of adiposity, and adipose tissue is a key production site for several inflammatory markers.19 Previous data from the Whitehall II study have demonstrated that increases in BMI and waist circumference over time were associated with higher levels of inflammatory markers at follow up,20 although the present findings were independent of changes in body composition. Another important finding showed that basal systemic inflammation was associated with reduction in physical activity over follow up, after adjusting for confounders such as BMI and chronic illness. Given that inflammatory processes are thought to be involved in sarcopenia and functional decline,6,7 this explains why systemic inflammation may result in decreased activity in ageing populations.
Physical activity, inflammation and health are linked together in a complex fashion. Cytokines are secreted transiently in large doses by several metabolically active tissues during exercise; namely from the muscle during contraction and adipose tissue via exercise-related mechanisms.
Paradoxically, regular (chronic) exercise training has been consistently associated with lower levels of systemic inflammatory markers5 and reduced adipose tissue inflammation.21 The expression of exercise-regulated muscle genes, such as the transcriptional co-activator PGC1α, is thought to promote anti-inflammatory effects through a transient release of cytokines,22 and possibly explains some of the systemic and beneficial effects of exercise in non-muscle tissue.21-25 In contrast, chronically elevated levels of low grade systemic inflammation have been linked to the development of many diseases associated with inflammation including CVD, sarcopenia, neurodegeneration and depression.6-10, 26, 27 Thus, the transient fluctuations of cytokines following exercise might contribute to the beneficial effects of exercise on organs other than muscle in a hormone-like fashion, whereas chronic, low grade elevation of many of these same molecules is almost certainly pro-inflammatory and detrimental.
A notable strength of this study is the repeated serial measures taken over a 10-year follow-up period in a well characterised cohort. This allowed us to track changes in physical activity, inflammatory markers and other important clinical variables. Self-reported measures of physical activity are prone to reporting bias although the questionnaire used in the present study is well validated and has demonstrated convergent validity in predicting mortality in the Whitehall II study.15 In addition, among a sub-cohort of 394 Whitehall II participants, we recently demonstrated that self-reported physical activity was associated with objectively (accelerometry) assessed activity at 10-year follow-up across various activity categories.28 Although there was only modest correlation between physical activity measures at different phases of data collection, we did observe an upward trend in physical activity. This might be explained by the fact many participants from Whitehall II were in the transition to retirement during this period. This is consistent with recent data from the GAZEL cohort 4 years before and 4 years after retirement showing that leisure-time physical activity increased by 36% in men and 61% in women during the transition to retirement.29 Our findings on the association between baseline inflammatory markers and change in physical activity over follow up should be interpreted with caution as we were unable to account for presence of sarcopenia. Nevertheless, the analyses were adjusted for chronic illness that incorporates factors such as functional limitations and history of CVD.
In summary, the results show that physically active participants maintain lower levels of inflammatory markers over a 10 year period. Thus, physical activity may be important in preventing the pro-inflammatory state seen with ageing.
Acknowledgements
We thank all participating civil service departments and their welfare personnel, and establishment officers; the Occupational Health and Safety Agency; the Council of Civil Service Unions; all participating civil servants in the Whitehall II study; all members of the Whitehall II study team. The Whitehall II Study team comprises research scientists, statisticians, study coordinators, nurses, data managers, administrative assistants and data entry staff, who make the study possible.
Sources of Funding
The Whitehall II study has been supported by grants from the Medical Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (R01HL36310), US, NIH: National Institute on Aging (R01AG013196; R01AG034454), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D and Catherine T MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. MH and MJS are supported by the British Heart Foundation (RE/10/005/28296) and (RG/07/008/23674); MK is supported by the EU New OSH ERA research programme and the Academy of Finland; GDB is a Wellcome Trust Research Fellow; SS is supported by the NIH (grant R01AG034454); AS-M is supported by a ‘EURYI’ award from the European Science Foundation. The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr Hamer had full access to the data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
Disclosures
None of the authors report any conflicts of interest.
References
- 1.Hamer M, Stamatakis E. Physical activity and risk of cardiovascular disease events events: inflammatory and metabolic mechanisms. Med Sci Sports Exerc. 2009;41:1206–11. doi: 10.1249/MSS.0b013e3181971247. [DOI] [PubMed] [Google Scholar]
- 2.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rana JS, Arsenault BJ, Després JP, Côté M, Talmud PJ, Ninio E, Wouter Jukema J, Wareham NJ, Kastelein JJ, Khaw KT, Matthijs Boekholdt S. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur Heart J. 2011;32:336–44. doi: 10.1093/eurheartj/ehp010. [DOI] [PubMed] [Google Scholar]
- 4.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 5.Hamer M. The relative influence of fitness and fatness on inflammatory factors. Prev Med. 2007;44:3–11. doi: 10.1016/j.ypmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Molloy GJ. Association of C-reactive protein and muscle strength in the English Longitudinal Study of Ageing. Age (Dordr) 2009;31:171–7. doi: 10.1007/s11357-009-9097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–61. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G, Caruso C. Inflammation, ageing and cancer. Mech Ageing Dev. 2009;130:40–5. doi: 10.1016/j.mad.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a Mendelian randomisation analysis. Lancet. 2012;379:1214–24. doi: 10.1016/S0140-6736(12)60110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–93. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 13.Singh-Manoux A, Hillsdon M, Brunner E, Marmot M. Effects of physical activity on cognitive functioning in middle age: evidence from the Whitehall II prospective cohort study. Am J Public Health. 2005;95:2252–8. doi: 10.2105/AJPH.2004.055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 15.Sabia S, Dugravot A, Kivimaki M, Brunner E, Shipley MJ, Singh-Manoux A. Effect of intensity and type of physical activity on mortality: Results from the Whitehall II cohort study. Am J Public Health. 2012;102:698–704. doi: 10.2105/AJPH.2011.300257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, Fox KR, Gately P, Giles-Corti B, Gill JM, Hamer M, McDermott I, Murphy M, Mutrie N, Reilly JJ, Saxton JM, Stamatakis E. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci. 2010;28:573–91. doi: 10.1080/02640411003671212. [DOI] [PubMed] [Google Scholar]
- 17.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124:789–95. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. [9th May 2012];SPSS Technical report: Linear Mixed-Effects Modeling in SPSS: An Introduction to the MIXED Procedure. http://www.spss.ch/upload/1126184451_Linear%20Mixed%20Effects%20Modeling%20in%20SPSS.pdf.
- 19.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 20.Fransson EI, Batty GD, Tabák AG, Brunner EJ, Kumari M, Shipley MJ, Singh-Manoux A, Kivimäki M. Association between change in body composition and change in inflammatory markers: an 11-year follow-up in the Whitehall II Study. J Clin Endocrinol Metab. 2010;95:5370–4. doi: 10.1210/jc.2010-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab. 2009;296:E1164–71. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–9. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811–16. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–10. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz M, Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat Rev Neurol. 2010;6:405–10. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 27.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamer M, Kivimaki M, Steptoe A. Longitudinal patterns in physical activity and sedentary behaviour from mid-life to early old age: A sub-study of the Whitehall II cohort. J Epidemiol Community Health. 2012 doi: 10.1136/jech-2011-200505. [e-pub ahead of print] doi:10.1136/jech-2011-200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjösten N, Kivimäki M, Singh-Manoux A, Ferrie JE, Goldberg M, Zins M, Pentti J, Westerlund H, Vahtera J. Change in physical activity and weight in relation to retirement: the French GAZEL Cohort Study. BMJ Open. 2012;2:e000522. doi: 10.1136/bmjopen-2011-000522. [DOI] [PMC free article] [PubMed] [Google Scholar]