Abstract
Background/Aims
Deletion or functional loss of the p53 tumor suppression gene plays a role in oncogenic transformation. The codon 72 polymorphism on exon 4 in the p53 gene produces variant proteins with either arginine (Arg) or proline (Pro), and is associated with an increased susceptibility of cancers of the lung, esophagus, breast, cervix and nasopharynx on a genetic basis. We designed this study to evaluate the influence of the p53 codon 72 polymorphism on gastric cancer in Korea.
Methods
We extracted the peripheral blood samples in 84 patients with gastric cancer, 66 patients with H. pylori-associated chronic gastritis and 43 controls without H. pylori infection. PCR-RFLP analysis was performed to detect p53 codon 72 polymorphism in these patients.
Results
There was no specific genotype of p53 polymorphism in the gastric cancer group compared to the other groups and no difference in genotypes by histologic subtypes. Classified by tumor location, Pro/Pro genotype was associated with an increase in proximal cancer and Arg/Arg genotype with distal cancer. As the frequency of p53 Arg allele increased, the cancer was of a more poorly differentiated type.
Conclusions
The specific genotype of p53 polymorphism seems to correlate with tumor location. Increased frequency of p53 Arg allele is associated with more poorly differentiated cancers.
Keywords: p53, Polymorphism, Gastric cancer
INTRODUCTION
Helicobacter pylori (H. pylori) infection is an established risk factor for the development of gastric cancer, and other environmental factors such as dietary habits1) and smoking2) are known to play a role in gastric carcinogenesis. A multifactorial model of human gastric carcinogenesis is currently accepted.
Recent studies have shown that particular genetic changes may influence the susceptibility to cancer. Individual variation in cancer rates has been associated with specific variant alleles (polymorphism) of different genes that are present in a significant proportion of the normal population. Polymorphisms in a wide variety of genes may modify the effect of environmental exposures3).
The p53 gene, located on the short arm of chromosome 17, encodes a protein that plays a critical role in DNA transcription, cell cycle regulation, and tumor suppression. The p53 expression is stimulated in certain cellular environments such as DNA damage, hypoxia, irradiation, and other cellular stress4). Mutation of the p53 gene represents one of the most common genetic alternations in human cancers, and the acquisition of such defects is strongly related to cancer formation and progression5).
The codon 72 polymorphism on exon 4 of the p53 gene, which produces variant proteins with arginine (Arg) or proline (Pro), has been reported to be associated with the risk of certain cancers - lung, esophagus, breast, cervix and nasopharynx6-8). The specific genotype of the p53 codon 72 polymorphism could be a risk factor for certain tumors and by making an environment favorable for tumor formation. In Japanese patients, the Pro/Pro genotype contributes to susceptibility for diffuse types of gastric cancer9). However, the genotypes of the p53 codon 72 polymorphism varied significantly with race10). There has been no report on the p53 codon 72 polymorphism of gastric cancer patients in Korea.
In the present study, we assessed the role of the p53 codon 72 polymorphism of gastric cancer in Korea. We also examined the p53 polymorphism according to the histologic classification of gastric cancer as defined by Lauren including: tumor location, stage and tumor grading.
MATERIALS AND METHODS
Patients
We studied sera from 193 patients who had undergone upper endoscopy at the Catholic University St. Vincent hospital, Suwon, Korea from Feb. 2002 through Dec. 2003. Eighty-four patients with gastric cancer, 66 H. pylori associated chronic gastritis patients with dyspeptic symptoms and 43 H. pylori negative healthy individuals as normal controls were enrolled in this study. None of the patients had other chronic illness or genetic disorders. Each patient was classified as H. pylori positive or negative according to the serologic and histologic results. Both tests were positive in H. pylori positive patients and negative in H. pylori negative patients. Sixty- four patients with gastric cancer underwent surgery. The surgical specimens of gastric cancer were classified as 40 intestinal type and 24 diffuse type as defined by Lauren11). Tumor stage was based on the TNM tumor classification system12). We determined the tumor stage by surgical pathologic findings in operated patients and by radiologic findings in unoperated patients.
p53 codon 72 polymorphism
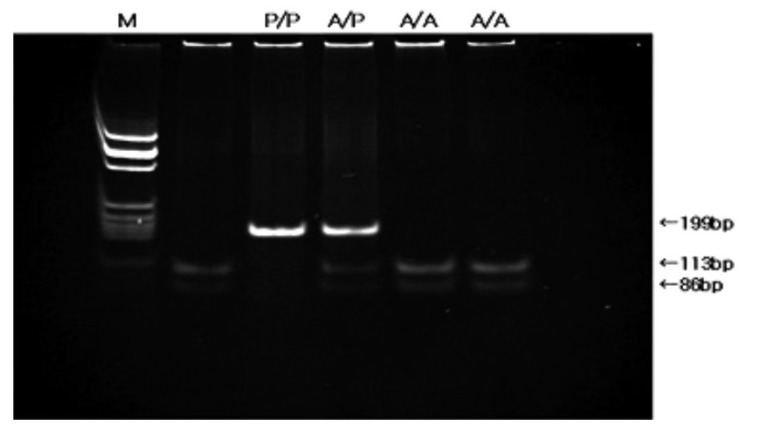
DNA was extracted from 200 L of buffy coat preserved at -40℃ by the use of QIAamp DNA blood mini kit (QIAGEN Inc., Valencia, CA, USA). PCR- restriction fragment length polymorphism (RFLP) analysis of codon 72 of the p53 was used to identify the p53 BstUI genotypes12). The two primers were 5'-TTGCCGTCCCAAGCAATGGATGA-3' and 5'-TCTGGGAA GGGACAGAAGATGAC-3'. Each PCR reaction mixture contained 10 pmol of each primer and genomic DNA. The reaction mixtures were preincubated for 10 minutes at 94℃. The PCR conditions were 94℃ for 30 seconds and 55℃ for 1 minute, followed by 72℃ for 1 minute for 40 rounds. After confirmation of an amplified fragment of the expected size, 199 bp, on agarose gel, the PCR products were digested with 2 units of restriction enzyme BstUI (New England Biolabs, Beverly, MA, USA) at 60℃ for 16 hours. The DNA fragments were electrophoresed through a 2% agarose gel and stained with ethidium bromide. Most significantly,the Pro allele is not cleaved by BstUI at codon 72 and has a single band with a fragment length of 199 bp. The Arg allele is cleaved by BstUI and yields 2 small fragments, 113 bp and 86 bp. The heterozygote has 3 bands, 199, 113 and 86 bp (Figure 1).
Figure 1.
Detection of p53 codon 72 polymorphism by BstUI digestion. The Pro allele is not cleaved and has a single band with a fragment length of 199 bp. The Arg allele is cleaved and has two fragments, 113 and 86 bp. The heterozygote has three bands. (A/A; Arg-Arg, A/P; Arg-Pro, P/P; Pro-Pro genotype, M; molecular marker)
Statistical analysis
The Chi-square test for association was used to test the difference of genotype frequencies between normal controls and gastric cancer patients, and between H. pylori associated chronic gastritis and gastric cancer patients. The other frequency tables were constructed using the SPSS statistical package with statistical significance using Chi-square test.
RESULTS
Eighty-four patients with gastric cancer, 66 H. pylori associated chronic gastritis patients and 43 H. pylori negative healthy controls were analyzed (Table 1). According to the p53 codon 72 genotypes, the age for gastric cancer patients was 58.4±14.1 years (Arg/Arg), 61.5±10.5 years (Arg/Pro) and 64.3±14.0 years (Pro/Pro) respectively. There was no significant age related difference (p=0.30). The genotype frequencies of p53 codon 72 polymorphism in Korean gastric cancer cases and controls were summarized in Table 2. There was not a specific genotype in the gastric cancer group when compared with the other groups. When gastric cancers were classified by histological subtype, there were no statistical differences in the genotypic distribution between the intestinal and diffuse type (Table 3).
Table 1.
Demographic characteristics of patients

M, male; F, female
Table 2.
Frequency of p53 codon 72 genotypes in patients with H. pylori associated chronic gastritis and gastric cancer

*NS, not significant
Table 3.
Frequency of p53 codon 72 genotypes according to the Lauren's classification

*operation : 64 patients p=0.13
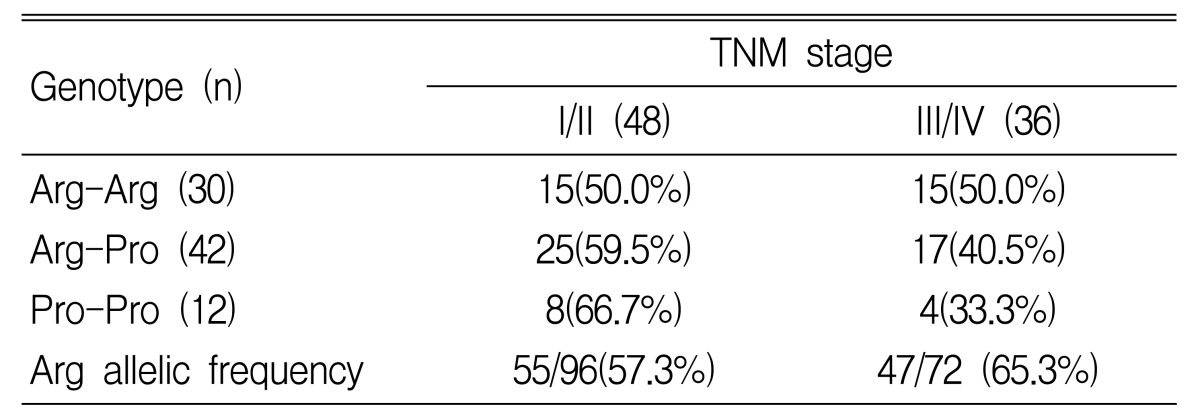
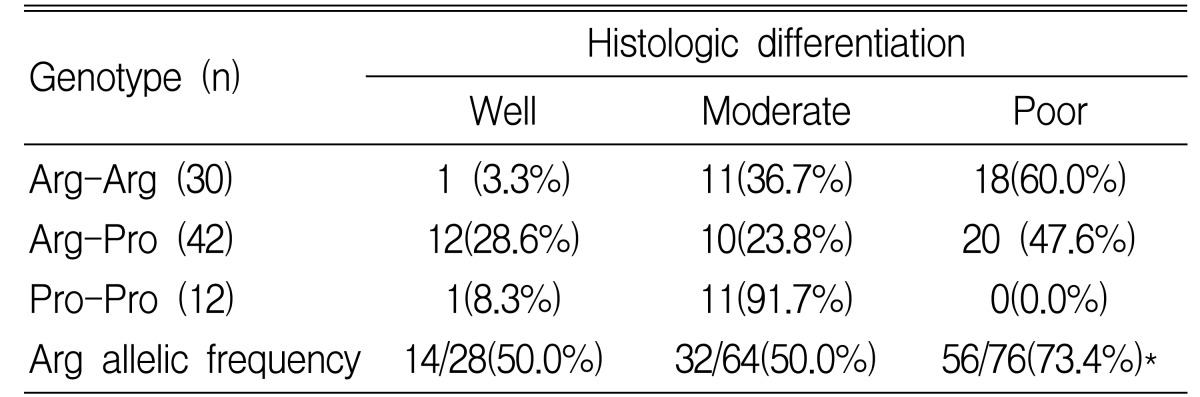
We also examined the differences in genotype by tumor location, stage and tumor grading. In gastric cancer patients with a proximal location (cardia and high body of stomach), the frequencies of the 3 genotypes, Arg-Arg, Arg-Pro and Pro-Pro, were 17.4, 52.2 and 30.4%, respectively. In distal cancer (lower body and antrum), the frequencies of the genotypes were 42.6, 49.2 and 8.2%. The Pro/Pro genotype was associated with proximal location and the Arg/Arg was associated with distal location (p=0.01) (Table 4). In gastric cancer patients who had more than stage III tumor by TNM status, the frequencies of the 3 genotypes, Arg-Arg, Arg-Pro and Pro-Pro, were 50.0, 40.5 and 33.3%. The Arg allelic frequency is 57.3% in stage I/II and 65.3% in stage III/IV. An increased frequency of the Arg allele in p53 codon 72 with advanced stage was observed, but there was no statistical significance (p=0.29) (Table 5). In poorly differentiated tumors, the Arg/Arg genotype was more frequently observed, and the Arg allelic frequency was significantly increased (p=0.007) (Table 6).
Table 4.
Frequency of p53 codon 72 genotypes according to the location of tumors

M, male; F, female
*p=0.01
Table 5.
TNM stage of gastric cancer according to the p53 codon 72 genotypes
p=0.29
Table 6.
Histologic differentiation according to the p53 codon 72 genotypes
*p=0.007
DISCUSSION
The association of p53 with tumorgienesis is mediated by the production of a 21kDa protein (p21/cip1/waf1) that inhibits cyclin dependent kinase (CDK) activity and blocks the normal cell cycle. It plays a rolein apoptosis in repairing damaged DNA14). The polymorphic variants of codon 72 differ in their specific DNA binding affinity, transcriptional activity and induction of apoptosis15).
There is a discrepancy between the p53 immunoreactivity and p53 gene mutation in gastric cancer. Most of the overexpressed p53 protein detected by immunohistochemical stain is a mutant form exhibiting an increased half-life when compared to the wild form. The p53 gene contains 11 exons. Almost all mutations of the p53 gene occur in exon 5~8. Several studies have shown that p53 overexpression was observed in approximately 75% of gastric cancers, while the gene was mutated in only 20% of cases16). To understand this disparity, it is helpful to examine exon 4 polymorphism.
The results of this study revealed that the genotypic frequencies were Arg/Arg (35.7%), Arg/Pro (50.0%), and Pro/Pro (14.3%) in treated patients, and Arg/Arg (36.3%), Arg/Pro (48.5%), and Pro/Pro (15.2%) in controls respectively. There was no specific genotype of p53 polymorphism in the gastric cancer patients. Several studies have reported roles for p53 polymorphic variants in modulating environmental risk factors for cancer. Patients who are smokers and have the Pro/Pro genotype are more likely to develop lung cancer17). In contrast, nonsmokers with lung cancer have an increased frequency of the Arg/Arg genotype18). In women, the Arg/Arg genotype results in a 7-fold increased risk for the development of cervical cancer associated with human papilloma virus (HPV) when compared with other genotypes6). We investigated the distribution of the p53 codon 72 genotypes in controls and gastric cancer patients by H. pylori state, histologic subtypes and tumor locations. There were no significant relationships between H. pylori state, histologic subtypes and the p53 codon 72 genotypes. In the matter of tumor locations, cancers with Pro/Pro genotype were more likely to be located in the proximal part of the stomach. In contrast, cancers with Arg allele had an increased frequency in the distal part. The distal cancers have several different risk factors when compared with the proximal cancers. The distal cancers are associated with H. pylori associated gastritis, atrophy and intestinal metaplasia19) and are more frequently found in patients with high salt and nitrate diets20). Our results do not show a statistically significant association between H. pylori state and the p53 codon 72 genotypes. However, we cannot determine the relationship because the degree of inflammation or atrophy induced by H. pylori infection is not included as one of the variables in our study.
In general, the patient's age, cancer location, histologic subtypes, tumor grading and TNM status could be associated with the prognosis of advanced cancer21, 22). The p53 codon 72 genotypes may affect the prognosis with the protective effects of p53 playing a more important role in early stagedisease23). Miller et al. insisted that the cancer patients with the Pro/Pro allele in p53 had a significantly worse prognosis than others because of the poorer response to treatment24). However, Zhang et al. reported that the frequency of the Arg allele was positively correlated with patient's age at baseline, but that the age-related increase in the percentage of Arg allele was not associated with the prognosis of advanced gastric cancer25). However this assertion is somewhat controvertial. In our study, an increase in the Arg allele was negatively correlated with patient's age, but did not reach it is not statistical significance. Cancers with Arg allele have an increased frequency of the poorly differentiated type. These results stand in contrast to reports from the West24) and China25).
In conclusion, there is acorrelation between the p53 codon 72 genotypes and location of the gastric cancer. Although we have not found a specific genotype of p53 codon 72 to be a significant cause of gastric cancer in Korean patients, we suggest that an increase in Arg allele of p53 codon 72 is correlated with poor tumor differentiation.
Footnotes
Supported by a research grant from the St. Vincent Hospital, College of Medicine, The Catholic University of Korea.
References
- 1.World Cancer Research Fund and American Investigation of Cancer Research. Food, nutrition and the prevention of cancer: a global prospective. Menasha: BANTA Book Group; 1997. [Google Scholar]
- 2.Tredaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. 1997;72:565–573. doi: 10.1002/(sici)1097-0215(19970807)72:4<565::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Perera FP, Weinstein IB. Molecular epidemiology: recent advances and future directions. Carcinogenesis. 2000;21:517–524. doi: 10.1093/carcin/21.3.517. [DOI] [PubMed] [Google Scholar]
- 4.Korsmeyer SJ, Zinkel SS. Cancer: principles and practice of oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. Molecular biology of cancer: apoptosis. [Google Scholar]
- 5.Levine AJ. The p53 tumor suppressor gene. N Engl J Med. 1992;326:1350–1352. doi: 10.1056/NEJM199205143262008. [DOI] [PubMed] [Google Scholar]
- 6.Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, Breuer J, Leigh IM, Matlashewski G, Banks L. Role of p53 polymorphism in the development of human papillomavirusassociated cancer. Nature. 1998;393:229–234. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal AN, Ryan A, Al-Jehani RM, Storay A, Harwood CA, Jacobs IJ. p53 codon 72 polymorphism and risk of cervical cancer in UK. Lancet. 1998;352:871–872. doi: 10.1016/S0140-6736(98)07357-7. [DOI] [PubMed] [Google Scholar]
- 8.Weston A, Ling-Cawley HM, Capaoraso NE, Bowman ED, Hoover RN, Trump BF, Harris CC. Determination of the allelic frequencies of an L-myc and p53 polymorphism in human lung cancer. Carcinogenesis. 1994;15:583–587. doi: 10.1093/carcin/15.4.583. [DOI] [PubMed] [Google Scholar]
- 9.Hiyama T, Tanaka S, Kitadai Y, Ito M, Sumii M, Yoshihara M, Shimamoto F, Haruma K, Chayama K. p53 Codon 72 polymorphism in gastric cancer susceptibility in patients with Helicobacter pylori-associated chronic gastritis. Int J Cancer. 2002;100:304–308. doi: 10.1002/ijc.10483. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd T, Tolbert D, Benedetti J, Macdonald J, Stemmermann G, Wiest J, DeVoe G, Miller MA, Wang J, Noffsinger A, Fenogliio-Preiser C. Alternations in exon 4 of the p53 gene in gastric carcinoma. Gastroenterology. 2000;118:1039–1044. doi: 10.1016/s0016-5085(00)70356-8. [DOI] [PubMed] [Google Scholar]
- 11.Lauren P. The two main histological types of gastric carcinoma: diffuse and so-called intestinal type carcinoma: an attempt at histoclinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 6th ed. Springer; 2002. pp. 99–103. [Google Scholar]
- 13.Ara S, Lee PS, Hansen MF, Saya H. Codon 72 polymorphism of the TP53 gene. Nucleic Acids Res. 1990;18:4961. doi: 10.1093/nar/18.16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx J. How p53 suppresses cell growth. Science. 1993;262:1644–1645. doi: 10.1126/science.8259506. [DOI] [PubMed] [Google Scholar]
- 15.Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999;19:1092–1100. doi: 10.1128/mcb.19.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolbert DM, Noffsinger AE, Miller MA, DeVoe GW, Stemmerman GN, Macdonald JS, Fenoglio-Preiser CM. P53 immunoreactivity and single strand conformational polymorphism analysis often fail to predict p53 mutational status. Mod Pathol. 1999;12:54–60. [PubMed] [Google Scholar]
- 17.Jin X, Wu X, Roth JA, Amos CI, King TM, Branch C, Honn SE, Spitz MR. Higher lung cancer risk for younger African-Americans with the pro-pro p53 genotype. Carcinogenesis. 1995;16:2205–2208. doi: 10.1093/carcin/16.9.2205. [DOI] [PubMed] [Google Scholar]
- 18.Murata M, Tagawa M, Kimura M, Kimura H, Watanabe S, Saisho H. Analysis of a germ line polymorphism of the p53 gene in lung cancer patients: discrete results with smoking history. Carcinogenesis. 1996;17:261–264. doi: 10.1093/carcin/17.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Drumm B, Sherman P, Cutz E, Karmali M. Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med. 1987;316:1557–1561. doi: 10.1056/NEJM198706183162501. [DOI] [PubMed] [Google Scholar]
- 20.Kono S, Hirohata T. Nutrition and stomach cancer. Cancer Causes Control. 1996;7:41–55. doi: 10.1007/BF00115637. [DOI] [PubMed] [Google Scholar]
- 21.Msika S, Benhamiche AM, Tazi MA, Rat P, Faivre J. Improvement of operative mortality after curative resection for gastric cancer: population-based study. World J Surg. 2000;24:1137–1142. doi: 10.1007/s002680010185. [DOI] [PubMed] [Google Scholar]
- 22.Moriwaki Y, Kunisaki C, Kobayashi S, Harada H, Imai S, Kido Y, Kasaoka C. Progressive improvement of prognosis for patients with gastric cancer (dynamic stage grouping) with increasing survival interval from initial staging: how much longer can a given survivor expect to live. Surgery. 2003;133:135–140. doi: 10.1067/msy.2003.95. [DOI] [PubMed] [Google Scholar]
- 23.Benard J, Douc-Rasy S, Ahomadegbe JC. TP53 family members and human cancers. Hum Mutat. 2003;21:182–191. doi: 10.1002/humu.10172. [DOI] [PubMed] [Google Scholar]
- 24.Miller BA, Ries LA, Hankey BF, Kosary CL, Edwards BK. Cancer statistics review 1973-1989. National Cancer Institution NIH Publication 92-2789. 1992. pp. XXIII 1–XXIII 9. [Google Scholar]
- 25.Zhang ZW, Newcomb P, Hollowood A, Feakings R, Moorghen M, Storey A, Farthing MJ, Alderson D, Holly J. Age-associated increase of codon 72 arginine p53 frequency in gastric cardia and non-cardia adenocarcinoma. Clin Cancer Res. 2003;9:2151–2156. [PubMed] [Google Scholar]