Abstract
Background
This study was designed to evaluate the clinical, endocrinological and histological characteristics of adrenal incidentalomas.
Methods
Eighty patients (41, males; 38, females; age range 17-80 years) who were diagnosed with adrenal incidentaloma at Korea University Hospital from 1992 to 2003 were studied retrospectively.
Results
Endocrinological investigation revealed 16 pheochromocytomas (20%), nine Cushing's syndromes (11%), eight primary aldosteronism (10%) and 46 non-functioning tumors (58%). Forty-four patients received operations, and biopsies were performed on two patients. Pathologic examination revealed 16 adrenocortical adenomas (20%), five carcinomas (6%), 13 pheochromocytomas (16%), three metastatic cancers (4%), and other tumors (10%). The diameter of the carcinomas (mean: 10.8 cm, range: 5-19 cm) were significantly larger than the diameter of benign adenomas (mean: 2.84 cm, range: 1-6 cm) (p=0.002). According to the receiver operating charactenstic (ROC) curve analysis, the cut-off value of tumor size for discriminate malignant tumor was 4.75 cm (sensitivity 90%, specificity 58%). Twenty-four patients with non-functioning tumors were followed up for a period of 3 to 72 months. During the follow up period, two patients showed an increase in tumor size of more than 1 cm, and one patient developed Cushing's syndrome. Changes in mass size and function were observed only between 10 and 26 months after the initial diagnosis.
Conclusions
These data show that an endocrine evaluation should be performed in all adrenal incidentalomas, and an adrenalectomy is recommended for tumors 5 cm or greater or tumors with adrenocortical hyperfunction. In addition, these tumors should be monitored for changes in mass size and function for a follow up period of approximately 26 months.
Keywords: Adrenocortical adenoma, Carcinoma, Hyperfunction, Adrenalectomy
INTRODUCTION
In the last few years advanced imaging techniques and the increased quality of medical services have resulted in more examinations using abdominal sonography, computerized tomograghy (CT), and magnetic resonance imaging (MRI) than ever before. Consequently, many cases of adrenal tumors have been discovered accidentally during examinations for other diseases. Adrenal masses discovered unexpectedly during examinations for reasons not related to adrenal dysfunction are called adrenal incidentalomas. The frequency of adrenal incidentalomas range from 1.4 to 8.7% on autopsies1, 2), and from 0.5 to 4.4% based on results from imaging studies such as sonography and abdominal CT3, 4). The prevalence of adrenal incidentalomas increases with age, and reaches 7% at the age of 705). Most patients with adrenal incidentalomas report having no clinical problems, but the possibility of a hyperfunctioning tumor or malignancy should be considered.
As the incidence of adrenal incidentalomas increases, many studies investigating clinical manifestations, appropriate tests, indications for treatment, duration of follow up, and prognosis are taking place in other countries, but there have been few studies performed in Korea. Kim et al. reported the characteristics of eight cases observed over a period of 3.5 years in 19886). In 1994, we reported the clinical manifestations of adrenal incidentaloma in eleven cases7). However, these studies had too few cases to make suggestions for surgical treatment or duration of appropriate follow up period, which is an important subject for clinicians.
The object of this study was to evaluate the clinical, endocrinological and imaging characteristics of adrenal incidentalomas by studying a greater number of subjects, and to suggest a strategy for the management of adrenal incidentalomas in Koreans.
MATERIALS AND METHODS
Subjects
Eighty patients who were diagnosed with adrenal incidentaloma at Korea University Hospital from 1992 to 2003 were studied retrospectively. Adrenal incidentaloma was defined as an adrenal tumor detected accidentally on imaging studies such as abdominal sonography, CT and MRI that were performed for reasons other than adrenal problems. Patients with high blood pressure of over 180/110 mmHg, paroxysmal hypertension or obvious clinical signs of hypercortisolism, and patients who were evaluated for the staging of known cancer were excluded from this study.
Methods
Age, gender, reason for examination, presence of hypertension, diabetes mellitus, obesity, and the location, size and characteristics of the tumors on the imaging studies were reviewed. Hypertension was defined as a systolic blood pressure over 140 mmHg or a diastolic blood pressure over 90 mmHg, or if the patient was taking antihypertensive medication. Diabetes mellitus was diagnosed as either a fasting plasma glucose level of ≥ 126 mg/dL or if the patient was taking medication. Obesity was defined as body mass index ≥ 25 kg/m2. As for tumor size, we selected the largest diameter of unilateral tumors and the larger diameter of bilateral tumors. Imaging characteristics such as homogeneity, marginal feature and calcifications were evaluated on the basis of radiologists' descriptions. For the hormonal tests, the results of basal cortisol levels, the results of the 1 mg dexamethasone suppression test, 24 hour urinary cortisol levels, serum adrenocorticotropic hormone (ACTH) levels, plasma renin activity, serum aldosterone levels, 24 hour urinary catecholamine levels, Vanillylmandelic acid (VMA) and metanephrine levels were reviewed. The pathology of surgically removed tumors was also evaluated. For non-functioning tumors that were not operated on, follow up duration, changes in tumor size and the results of endocrine tests were reviewed.
Statistical analysis
Statistical analysis was performed using the SPSS program (version 10.0, Inc., Chicago, IL). Mann-Whitney U tests, Kruskal-Wallis analysis and Fisher's exact tests were the statistical methods used. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cut-off value of tumor size to discriminate malignant tumors. Statistical significance was determined as p values less than 0.05 for all tests.
RESULTS
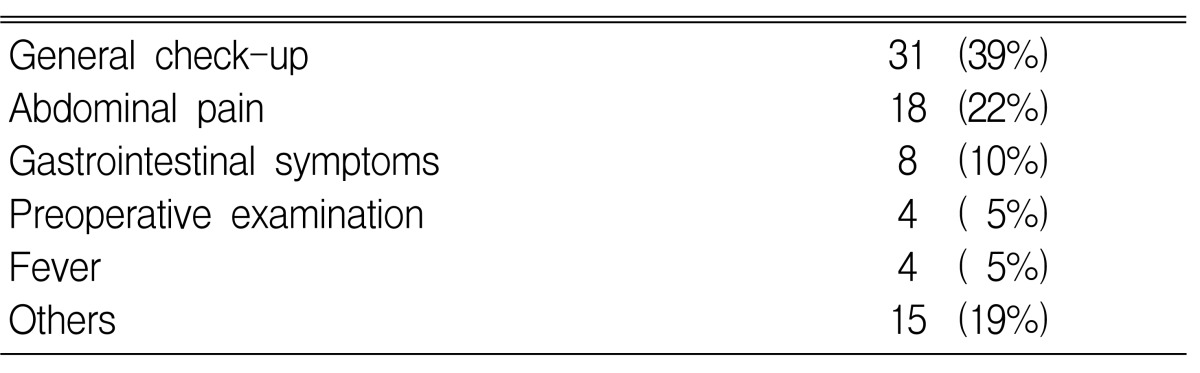
The average age of the patients was 50 years (range: 17-80 years). A total of 80 subjects, (41 males (52%) and 39 females (48%)) were included in this study. Fifty cases (63%) of adrenal incidentalomas were found on the right side of the adrenal gland, 25 (31%) were found on the left, and 5 (6%) were found on both sides. The average size of the tumors was 4.7 cm (range 1~20 cm). Thirty-two patients had hypertension and 11 patients had diabetes (Table 1). Hormonal tests showed 47 cases of non-functioning tumors and 33 cases of functioning tumors, and these functioning tumors included 16 pheochromocytomas (20%, 3 malignancies), 9 Cushing's syndromes (11%, 1 malignancy), and 8 primary aldosteronisms (10%) (Table 1). The most frequent reason for the abdominal imaging screening was general health checks for 31 patients (39%), while other reasons included abdominal pain in 18 patients (22%), gastrointestinal symptoms of dyspepsia or epigastric discomfort in 8 patients (10%), preoperative checks in 4 patients (5%), evaluation of fever in 4 patients (5%) and other reasons in 15 patients (19%) (Table 2).
Table 1.
Demographic and clinical data of 80 patients
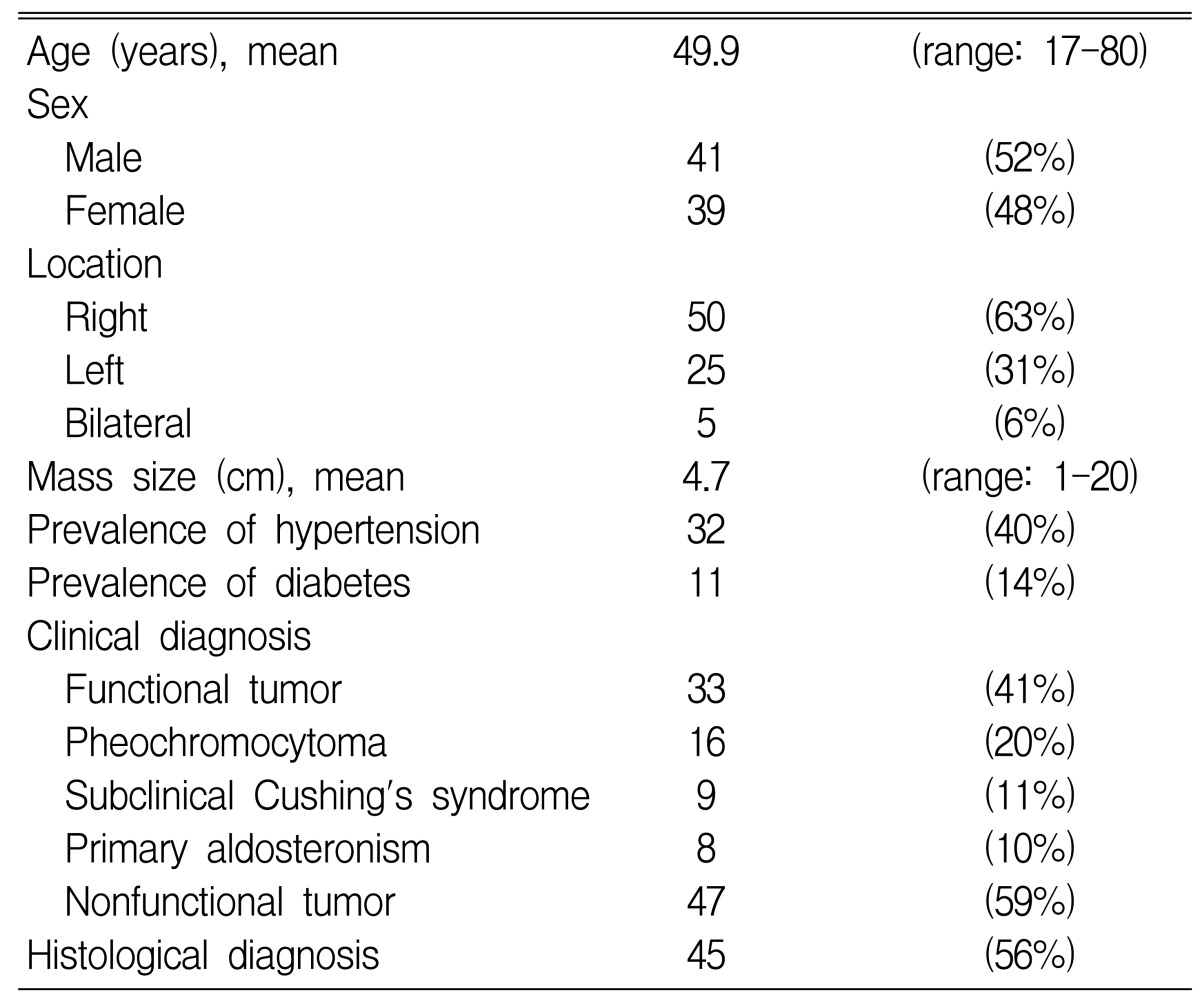
Table 2.
Reasons for abdominal imaging
Adrenal masses were surgically removed in 44 patients, and two patients received fine needle biopsies. The pathological results of the 46 histologically confirmed cases are shown on Table 3. Benign adenoma was the most common finding (16 cases, 19%).
Table 3.
Histological characteristics in relation to gender, age and mass size
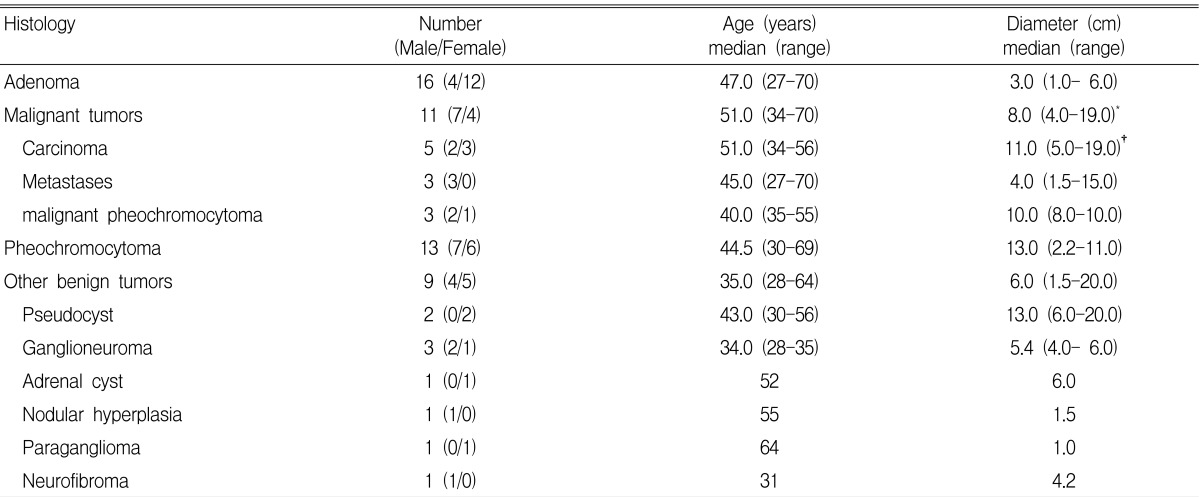
1. Size: adenoma vs. malignant tumor, *p=0.002; adenoma vs. carcinoma, †p=0.042; benign vs. malignant tumor, p=0.007
2. No significant difference between age of adenoma vs. carcinoma (p=0.821); adenoma vs. malignant tumor (p=0.659); benign vs. malignant tumor (p=0.411)
The comparison of characteristics between functioning and non-functioning tumors is shown on Table 4. The average size of non-functioning tumors was 4.5 cm (median: 3.3 cm, range: 1~20 cm), and 21% of patients who had non-functioning tumors had hypertension, 8.5% had diabetes and 25% were obese. Twenty-nine cases of non-functioning tumors were found on the right side, eleven cases were found on the left side, and five cases were found on both sides.
Table 4.
Clinical data of functional and nonfunctional tumors
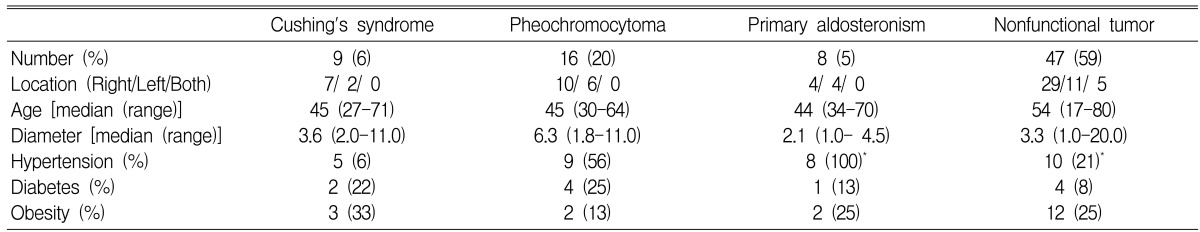
*There was significant difference only in hypertension between primary aldosteronism and nonfunctioning tumor (p=0.001). Besides there were no significant differences in other clinical characteristics between functioning and nonfunctioning tumors (p>0.05)
Among the functioning tumors, nine cases showed clinical manifestations of Cushing's syndrome. The average size of these tumors was 4.1 cm (median: 3.6 cm, range: 2~11 cm), one tumor measuring 11 cm was a carcinoma and the rest were under 4 cm. For the prevalence of accompanying diseases, 56% of the patients had hypertension, 22% had diabetes and 33% were obese; the prevalences were higher than that of the non-functioning tumors, but the difference was not statistically significant. The endocrine tests for Cushing's syndrome included the 24 hour urine free cortisol test, the 1 mg dexamethasone suppression test and serum ACTH level testing. All seven cases of histologically confirmed cortisol producing cortical adenomas showed abnormal findings on more than two tests including the 1 mg dexamethasone suppression test. On the other hand, 38 cases were histologically confirmed as not being Cushing's syndrome and six of them showed abnormal findings on the above mentioned tests. Four of the six cases showed increased levels of 24 hour urinary cortisol of over 100 g/day and two of the six cases showed decreased serum ACTH levels lower than 10 pg/mL, but all six cases were suppressed on the 1 mg dexamethasone suppression test and none of them showed abnormal findings simultaneously on more than two tests.
The average size of pheochromocytomas was 6.5 cm (median: 6.3 cm, range: 1.8~11 cm), and these were the largest tumors except for the carcinomas. The frequency of accompanying hypertension was 56%, diabetes mellitus was 25% and obesity was 13% (Table 4). The sensitivity, specificity andpositive predictive value of several screening tests were then compared. The 24 hour urinary metanephrine test showed the highest sensitivity and positive predictive value at 100% and 80%, respectively. The 24 hour urinary epinephrine test showed the highest specificity at 91% (Table 5).
Table 5.
Characteristic of biochemical tests for pheochromocytoma (%)
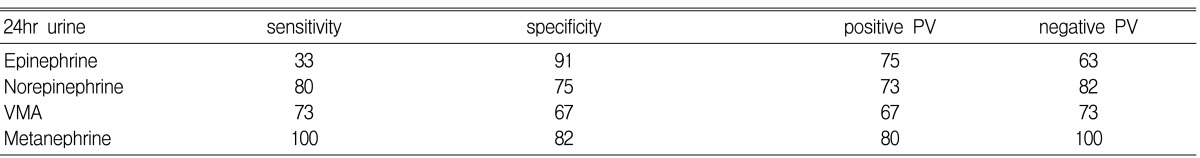
PV, predictive value; VMA, vanillylmandelic acid
Adrenal adenomas with primary aldosteronism were the smallest of the functioning tumors with an average size of 2.1 cm (median: 1.5, range: 1-4.5). All patients with primary aldosteronism had hypertension. Among the 45 histologically confirmed cases, all cases with primary aldosteronism had a plasma aldosterone level/plasma renin activity ratio of over 50, while the other cases without primary aldosteronism had a ratio of under 50 (two cases had a ratio of over 25, but they were ultimately diagnosed as non-functioning tumors and carcinoma after surgery). Hypokalemia was seen in 25% of the patients, and the frequency of hypertension was significantly higher compared with patients with non-functioning tumors (100% vs 21%; p=0.001) (Table 3).
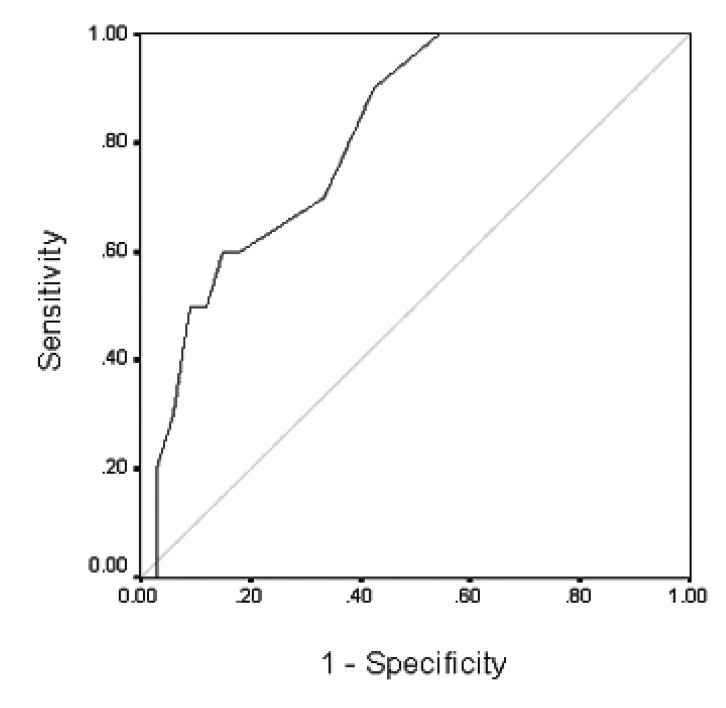
When classified based on pathological diagnosis, the mean size of benign adenomas and carcinomas were 2.9 cm (range 1~6 cm) and 10.8 cm (range 5~19 cm), respectively, which showed that the carcinomas were significantly bigger than the adenomas (p=0.002) (Table 3), and these two types of tumors overlapped at 5~6 cm in size. For the other clinical or laboratory parameters such as age, gender, location of tumor and hormonal tests, no significant differences were found. When all the malignant tumors including metastatic tumors, carcinomas and malignant pheochromocytomas were compared with benign tumors, only the size of the tumor was statistically significant as a predictor for malignancy (p=0.019). On the receiver operating characteristic T(ROC) curve analysis, the tumor size provided high sensitivity (90%) and specificity (58%) for the diagnosis of malignant tumor at a cut-off level of 4.75cm. The area under the curve was 0.803, (95% CI, 0.663 to 0.943 p=0.004) (Figure 1).
Figure 1.
Curve of receiver operating characteristics at different cut off points of mass size for malignant tumors. The area under the curves was 0.803.
Images displaying the characteristics of primary aldosteronism showed homogeneous low density and regular borders, but various densities were noted for the other tumors: no statistical significance was noted between benign and malignant tumors on imaging characteristics.
Seven patients had metastatic carcinomas; there were four cases of lung cancer, one renal cell carcinoma, one cholangiocarcinoma and one metastatic cancer of unknown origin. The average size of the metastatic tumors was 5.35 cm (range: 1.5~15 cm) and the imaging features varied from inhomogeneous density to cystic features with low density. There were three cases bilateral tumors (43%) (Table 3).
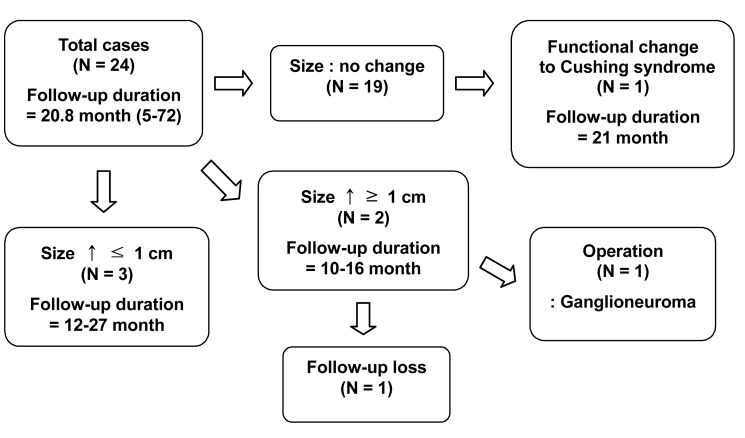
Twenty-four patients who were followed up for an average period of 20.8 months (range 5~72 months) were evaluated. Two of these patients showed an increase in tumor size of more than 1 cm. One patient failed to report for continuous follow up and another case was confirmed to be a ganglioneuroma following surgery. Among the cases that were followed up, one case of a non-functioning tumor developed into Cushing's syndrome and the patient underwent surgery. Changes in size and function of the tumors occurred within 26 months after diagnosis, and no changes were observed afterwards. There was no case of conversion to a malignant tumor (Figure 2).
Figure 2.
Flow chart of follow-up for adrenal incidentaloma (N=number of patient)
DISCUSSION
Since adrenal incidentalomas can have a hormone-secreting functions or they can be a malignant tumors, a proper evaluation and treatment should be performed. Until now, there have only been a small number of studies investigating adrenal incidentaloma in Koreans, but the number of cases in these studies was small and it was not appropriate to apply these study result to other cases. Thus, the purpose of this study was to determine the cause of adrenal incidentalomas, the frequency of each cause and to establish a treatment policy based on proper endocrinological evaluation and imaging study. In addition, we also wanted to determine the appropriate follow up period when non-surgical treatment is selected.
In this study, there was no significant difference between males and females, and the age distribution ranged from 17 to 80 years with an average age of 50 years. Compared with other studies that showed an increasing prevalence of adrenal incidentalomas with age5, 8), this study is thought to show a different distribution because more cases were discovered via general health checks (38%). In this study, 33 cases (41%) were suspicious for clinically functioning tumors. Therefore, even though there may be no suspicion of a clinically functioning tumor, endocrinological screening is necessary for all patients with adrenal incidentalomas.
According to previous studies, pheochromocytomas account for 4~17% of adrenal incidentalomas1, 8-14). In this study, pheochromocytomas were the most common type of tumor, accounting for 20% of the adrenal incidentalomas. In patients with pheochromocytomas, 56% had hypertension, which is consistent with the results of previous reports8). Since many of the patients who had paroxysmal hypertension were not recognized to have hypertension, the screening test for pheochromocytoma should also be performed on patients without hypertension.
Subclinical Cushing's syndrome has not yet been clearly defined. Each study has shown slightly different definitions, although all of the studies have had similar diagnostic criteria. When there are more than two abnormal findings on the tests evaluating the hypothalamic-pituitary-adrenal axis without specific symptoms or clinical signs, this could be defined as subclinical Cushing's syndrome15-18). These tests include 24 hour urinary free cortisol levels, the 1 mg dexamethasone suppression test, serum ACTH levels and the CRH stimulating test. The 24 hour urinary free cortisol level test and 1 mg dexamethasone suppression test are known to be the most useful screening tests for Cushing's syndrome19). The midnight serum cortisol level test and salivary cortisol levels have recently been suggested as screening tests for Cushing's syndrome, and they could also be considered as screening tests for adrenal incidentaloma22-24). There have been reports that subclinical Cushing's syndrome is related with an increased risk of hypertension, diabetes and obesity22-24), and it may be one of the cause of metabolic syndrome23). In this study, there was no statistically significant difference between various metabolic components when compared with non-functioning tumors.
Tumors with hyperaldosteronism were the smallest tumors (average 2.13 cm). All patients with hyperaldosteronism had hypertension, but only 25% of the patients had hypokalemia. On the other hand, the plasma aldostrone level/plasma renin activity ratio was higher than 50 in all cases, which verifies its usefulness as a screening test. Therefore, as the NIH suggested, for adrenal incidentaloma patients with hypertension, the plasma aldosterone level/plasma renin activity ratio should be used as a screening test instead of potassium levels5). With regards to surgical treatment for functioning adrenal incidentalomas, many physicians agree to removing tumors with hyperaldosteronism and pheochromocytomas, but there is still debate about cortisol-secreting tumors with subclinical Cushing's syndrome. Nevertheless, it is desirable to recommend surgery for hypercortisolemia with accompanying hypertension, diabetes mellitus, impaired glucose tolerance and osteoporosis5).
Approximately 4% of adrenal incidentalomas have been reported to be carcinomas25), but in this study, the prevalence of carcinomas was 6%. A comparison between benign adenomas and carcinomas found in adrenal glands revealed a significant difference only for tumor size (average: 3.0 cm (range: 1~6 cm) vs. 11.0 cm (range: 5~19 cm), p=0.001). Likewise, when considering all the malignant tumors including the metastatic cancer, carcinomas and malignant pheochromocytomas, only the size of the tumors was statistically significant. To identify the diagnostic value of malignant tumors by using tumor size, a ROC curve was constructed; the cut-off value of the tumor size was 4.75 cm. This result shows that surgical treatment should be considered for tumors larger than 5 cm no matter what their function is.
It is known that abdominal pain, increased serum DHEAS, irregular boarders on CT, inhomogeneous density and a strong increase in enhancement may predict malignant tumors5, 14, 28, 29). In this study, these markers did not appear to be predictors of malignant tumors. In many cases, DHEAS was not tested, which made a statistical evaluation difficult. Various images failed to show statistically significant differences. In fact, three cases of carcinoma were misdiagnosed as a non-functioning benign adenoma, a pheochromocytoma and a leiomyoma on imaging. Therefore, it is difficult to predict malignancy only with imaging.
The NIH recommends surgical treatment for hormone-secreting tumors or for tumors over 6 cm in size. In addition, the NIH recommends observation of changes in tumor size 6 and 12 months after the initial diagnosis on CT for tumors smaller than 4 cm5). For tumors from 4 to 6 cm, the NIH recommends additional tests including other imaging studies to determine the correct application of surgical treatment.5) When the imaging features of the tumor are consistent with a benign adenoma, the NIH recommends surgical resection for patients under 50 years and follow-up management for patients over 50 years30). Other authors have suggested scintigraphy when determining the need for surgery31).
In this study, twenty-four patients with non-functioning tumors were followed-up on average for a period of 21 months. The size of the tumor increased in seven cases, and two of them showed more than a 1 cm increase at 10 and 26 month, respectively. At 21 months, one case gave the clinical impression of hypersecretion of cortisol and the tumor had to be surgically removed. No cases developed into a malignant tumor. Changes in size and hormone secretion were observed up to 26 months after the initial diagnosis. This result suggests that the follow up period for adrenal incidentaloma should be at least two years long.
Since this study was performed retrospectively, a larger long-term prospective study should be considered to overcome this study's limitations of having a small number of patients and not having a long enough follow-up period.
In conclusion, we suggest that endocrine evaluation should be performed for all adrenal incidentalomas, and adrenalectomy must be recommended for tumors 5 cm or greater in size. In addition, these tumors should be monitored for changes in mass size and function for a follow up period of approximately 26 months.
References
- 1.Kokko JP, Brown TC, Berman MM. Adrenal adenoma and hypertension. Lancet. 1967;1:468–470. doi: 10.1016/s0140-6736(67)91092-6. [DOI] [PubMed] [Google Scholar]
- 2.Hedeland H, Ostberg G, Hokfelt B. On the prevalence of adrenocortical adenomas in an autopsy material in relation to hypertension and diabetes. Acta Med Scand. 1968;184:211–214. doi: 10.1111/j.0954-6820.1968.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 3.Glazer HS, Weyman PJ, Sagel SS, Levitt RG, McClennan BL. Non-functioning adrenal masses: incidental discovery on computed tomography. AJR Am J Roentgenol. 1982;139:81–85. doi: 10.2214/ajr.139.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Peppercorn PD, Grossman AB, Reznek RH. Imaging of incidentally discovered adrenal masses. Clin Endocrinol. 1998;48:379–388. doi: 10.1046/j.1365-2265.1998.00475.x. [DOI] [PubMed] [Google Scholar]
- 5.Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS. Management of the clinically ("inapparent") adrenal mass incidentaloma. Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Lee HD, Chi HS, Kim BR. A clinical review of adrenal "incidentaloma". J Korean Surg Soc. 1988;34:701–707. [Google Scholar]
- 7.Yoo JM, Kim SJ, Choi KM, Lee EJ, Kim YH, Baik SH, Choi DS. A clinical study of 11 cases of adrenal incidentaloma. J Korean Soc Endocrinol. 1994;9:358–365. [Google Scholar]
- 8.Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, Giovagnetti M, Opocher G, Angeli A. A survey on adrenal incidentaloma in Italy. J Clin Endocrinol Metab. 2000;85:637–644. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 9.Bulow B, Ahren B. Adrenal incidentaloma: experience of a standardized diagnostic programme in the Swedish prospective study. J Intern Med. 2002;252:239–246. doi: 10.1046/j.1365-2796.2002.01028.x. [DOI] [PubMed] [Google Scholar]
- 10.Luton JP, Martinez M, Coste J, Bertherat J. Outcome in patients with adrenal incidentaloma selected for surgery: an analysis of 88 cases investigated in a single clinical center. Eur J Endocrinol. 2000;143:111–117. doi: 10.1530/eje.0.1430111. [DOI] [PubMed] [Google Scholar]
- 11.Libe R, Dall'Asta C, Barbetta L, Baccarelli A, Beck-Peccoz P, Ambrosi B. Long-term follow-up study of patients with adrenal incidentalomas. Eur J Endocrinol. 2002;147:489–494. doi: 10.1530/eje.0.1470489. [DOI] [PubMed] [Google Scholar]
- 12.Osella G, Terzolo M, Borretta G, Magro G, Ali A, Piovesan A, Paccotti P, Angeli A. Endocrine evaluation of incidentally discovered adrenal masses (incidentalomas) J Clin Endocrinol Metab. 1994;79:1532–1539. doi: 10.1210/jcem.79.6.7989452. [DOI] [PubMed] [Google Scholar]
- 13.Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110:1014–1021. [PubMed] [Google Scholar]
- 14.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460–484. doi: 10.1210/edrv-16-4-460. [DOI] [PubMed] [Google Scholar]
- 15.Reincke M, Nieke J, Krestin GP, Saeger W, Allolio B, Winkelmann W. Preclinical Cushing's syndrome in adrenal "incidentalomas": comparison with adrenal Cushing's syndrome. J Clin Endocrinol Metab. 1992;75:826–832. doi: 10.1210/jcem.75.3.1517373. [DOI] [PubMed] [Google Scholar]
- 16.Gross MD, Shapiro B. Clinical review 50: clinically silent adrenal masses. J Clin Endocrinol Metab. 1993;77:885–888. doi: 10.1210/jcem.77.4.8408461. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosi B, Peverelli S, Passini E, Re T, Ferrario R, Colombo P, Sartorio A, Faglia G. Abnormalities of endocrine function in patients with clinically "silent" adrenal masses. Eur J Endocrinol. 1995;132:422–428. doi: 10.1530/eje.0.1320422. [DOI] [PubMed] [Google Scholar]
- 18.Terzolo M, Osella G, Ali A, Borretta G, Cesario F, Paccotti P, Angeli A. Subclinical Cushing's syndrome in adrenal incidentaloma. Clin Endocrinol (Oxf) 1998;48:89–97. doi: 10.1046/j.1365-2265.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 19.Orth DN. Cushing's syndrome. N Engl J Med. 1995;332:791–803. doi: 10.1056/NEJM199503233321207. [DOI] [PubMed] [Google Scholar]
- 20.Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing's syndrome. J Clin Endocrinol Metab. 1998;83:2681–2686. doi: 10.1210/jcem.83.8.4936. [DOI] [PubMed] [Google Scholar]
- 21.Putignano P, Toja P, Dubini A, Pecori Giraldi F, Corsello SM, Cavagnini F. Midnight salivary cortisol versus urinary free and midnight serum cortisol as screening tests for Cushing's syndrome. J Clin Endocrinol Metab. 2003;88:4153–4157. doi: 10.1210/jc.2003-030312. [DOI] [PubMed] [Google Scholar]
- 22.Rossi R, Tauchmanova L, Luciano A, di Martino M, Battista C, del Viscovo L, Nuzzo V, Lombardi G. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85:1440–1448. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 23.Terzolo M, Pia A, Ali A, Osella G, Reimondo G, Bovio S, Daffara F, Procopio M, Paccotti P, Borretta G, Angeli A. Adrenal incidentaloma: a new cause of the metabolic syndrome? J Clin Endocrinol Metab. 2002;87:998–1003. doi: 10.1210/jcem.87.3.8277. [DOI] [PubMed] [Google Scholar]
- 24.Tauchmanova L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri EA, Fazio S, Lombardi G. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. 2002;87:4872–4878. doi: 10.1210/jc.2001-011766. [DOI] [PubMed] [Google Scholar]
- 25.Siren JE, Haapiainen RK, Huikuri KT, Sivula AH. Incidentalomas of the adrenal gland: 36 operated patients and review of literature. World J Surg. 1993;17:634–639. doi: 10.1007/BF01659129. [DOI] [PubMed] [Google Scholar]
- 26.Abecassis M, McLoughlin MJ, Langer B, Kudlow JE. Serendipitous adrenal masses: prevalence, significance, and management. Am J Surg. 1985;149:783–788. doi: 10.1016/s0002-9610(85)80186-0. [DOI] [PubMed] [Google Scholar]
- 27.Copeland PM. The incidentally discovered adrenal mass. Ann Intern Med. 1983;98:940–945. doi: 10.7326/0003-4819-98-6-940. [DOI] [PubMed] [Google Scholar]
- 28.Pender SM, Boland GW, Lee MJ. The incidental nonhyperfunctioning adrenal mass: an imaging algorithm for characterization. Clin Radiol. 1998;53:796–804. doi: 10.1016/s0009-9260(98)80189-x. [DOI] [PubMed] [Google Scholar]
- 29.Barzon L, Scaroni C, Sonino N, Fallo F, Paoletta A, Boscaro M. Risk factors and long-term follow-up of adrenal incidentalomas. J Clin Endocrinol Metab. 1999;84:520–526. doi: 10.1210/jcem.84.2.5444. [DOI] [PubMed] [Google Scholar]
- 30.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass ("incidentaloma") NIH Consens State Sci Statements. 2002;19:1–25. [PubMed] [Google Scholar]
- 31.Barzon L, Boscaro M. Diagnosis and management of adrenal incidentalomas. J Urol. 2000;163:398–407. [PubMed] [Google Scholar]