Abstract
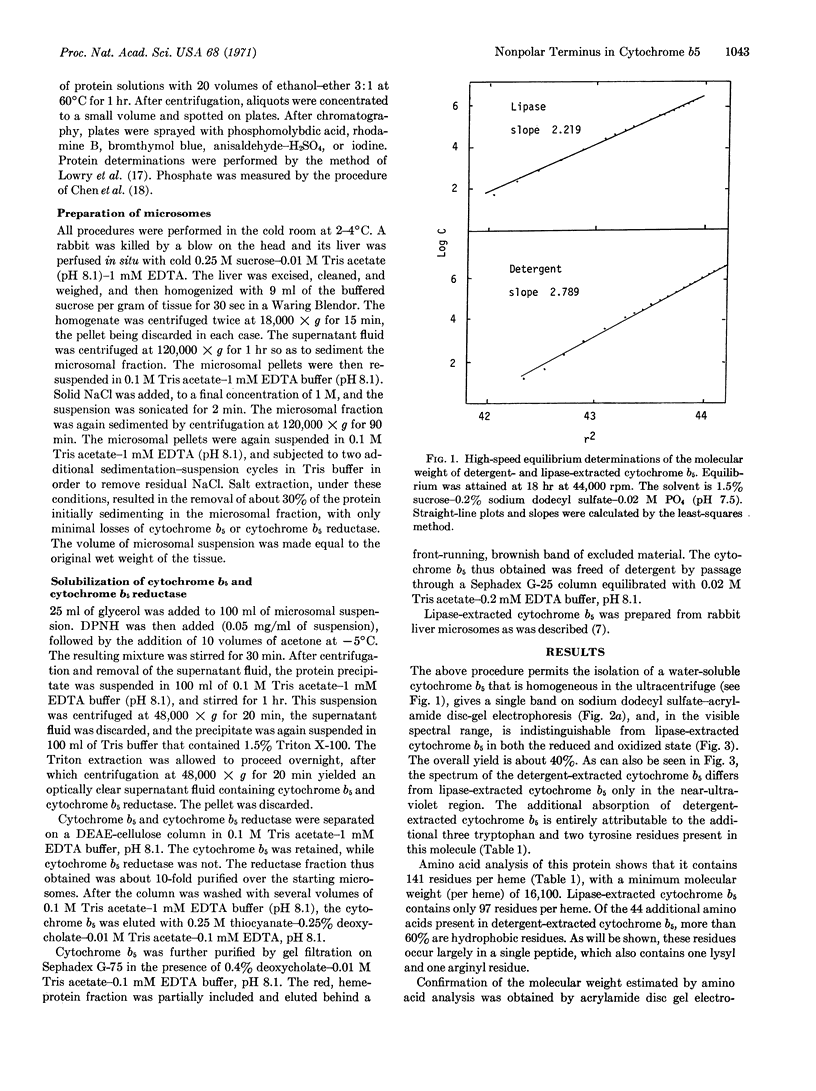
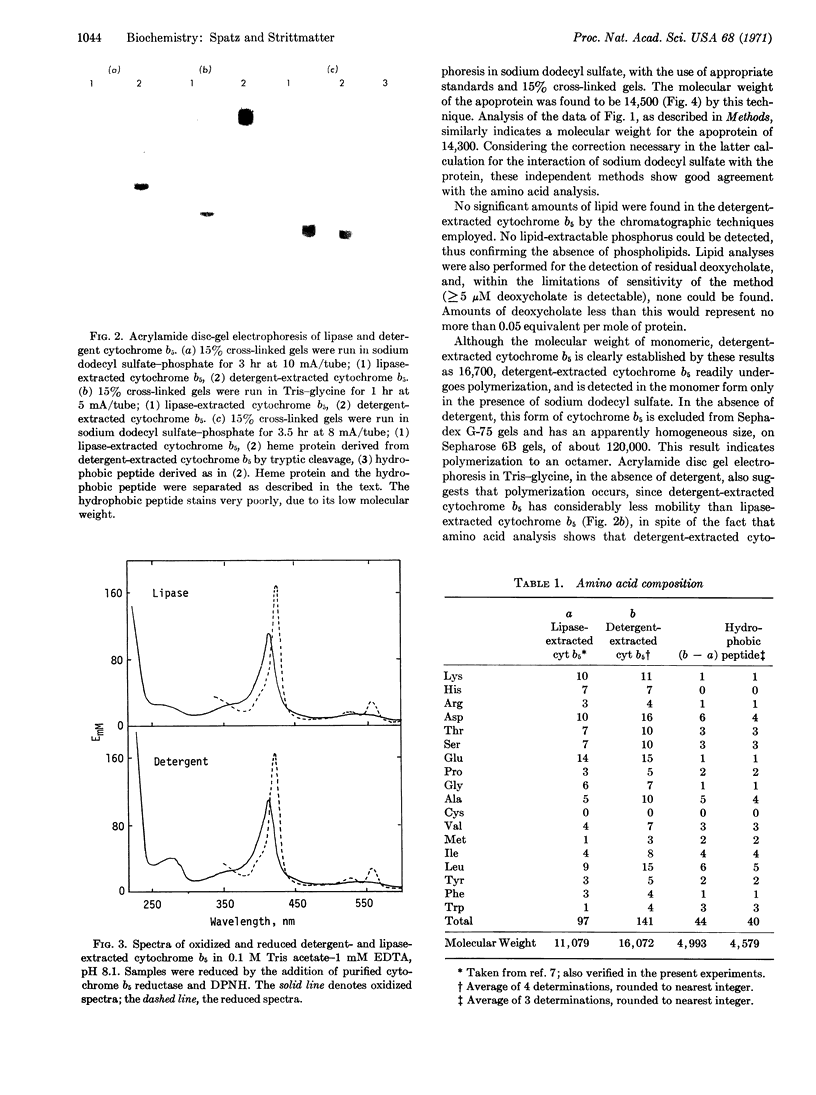
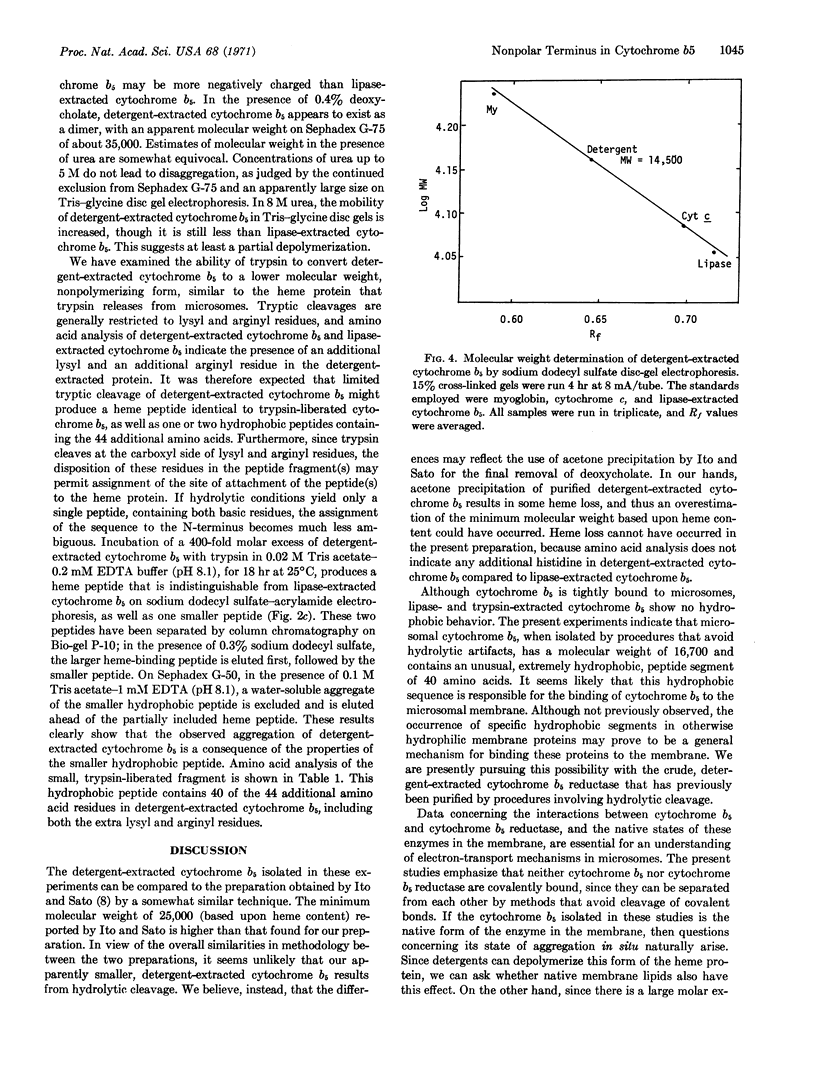
A species of cytochrome b5 with a monomer molecular weight of 16,700 has been isolated from rabbit-liver microsomes by a procedure that uses detergents and avoids the use of any proteolytic or lipolytic enzymes. This detergent-extracted cytochrome b5 is larger than the trypsin- or lipase-extracted enzyme, and appears to contain an extremely hydrophobic appendage of 40 amino acids, probably at the N-terminus. The hydrophobic character of the extra amino acid sequence leads to aggregation in the absence of detergents, and may be of considerable importance in the binding of the enzyme to microsomes. It is suggested that the hydrophilic portion of the cytochrome molecule, which bears the heme and is enzymatically functional, is oriented toward the surface of the membrane where it readily reacts with nonmicrosomal proteins, while the hydrophobic “tail” anchors the heme protein tightly to the membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Ito A., Sato R. Purification by means of detergents and properties of cytochrome b5 from liver microsomes. J Biol Chem. 1968 Sep 25;243(18):4922–4923. [PubMed] [Google Scholar]
- Kajihara T., Hagihara B. Crystalline cytochrome b5. I. Preparation of crystalline cytochrome b5 from rabbit liver. J Biochem. 1968 Apr;63(4):453–461. doi: 10.1093/oxfordjournals.jbchem.a128797. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Omura T., Siekevitz P., Palade G. E. Turnover of constituents of the endoplasmic reticulum membranes of rat hepatocytes. J Biol Chem. 1967 May 25;242(10):2389–2396. [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRITTMATTER P. The nature of the heme binding in microsomal cytochrome b5. J Biol Chem. 1960 Aug;235:2492–2497. [PubMed] [Google Scholar]
- STRITTMATTER P., VELICK S. F. The purification and properties of microsomal cytochrome reductase. J Biol Chem. 1957 Oct;228(2):785–799. [PubMed] [Google Scholar]
- Strittmatter P., Ozols J. The restricted tryptic cleavage of cytochrome b5. J Biol Chem. 1966 Oct 25;241(20):4787–4792. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



