Abstract
BACKGROUND:
Keloids may complicate wound healing secondary to trauma, inflammation or surgical incision. Although various treatment modalities have been used with variable degrees of success, overall recurrence rates have remained unacceptably high.
METHODS:
The present study involved 80 patients with keloids of at least one-years’ duration. Following total surgical excision of the keloid, a single dose of 5-fluorouracil was injected into the edges of the healing wound on postoperative day 9 together with botulinum toxin. The concentration of 5-fluorouracil used was 50 mg/mL and approximately 0.4 mL was infiltrated per cm of wound tissue, with the total dose <500 mg. The concentration of botulinum toxin was 50 IU/mL with the total dose <140 IU.
RESULTS:
Patients were followed-up for 17 to 24 months and a recurrence rate of 3.75% was found, which was significantly lower than in previously reported studies using other therapeutic modalities.
CONCLUSION:
The author recommends that this treatment be routinely applied to all keloids because it is significantly more effective than those described by other authors.
Keywords: 5-fluorouracil, Botulinum toxin, Keloids, Scars, Wound healing
Abstract
HISTORIQUE:
Les chéloïdes peuvent compliquer la guérison des plaies après un traumatisme, une inflammation ou une incision chirurgicale. Même si diverses modalités thérapeutiques ont déjà été utilisées avec un succès varié, le taux de récurrence global demeure excessif.
MÉTHODOLOGIE:
La présente étude portait sur 80 patients ayant une chéloïde depuis au moins un an. Après une excision chirurgicale totale de la chéloïde, on injectait une dose unique de 5-fluorouracile sur la bordure de la plaie en voie de cicatrisation le neuvième jour postopératoire, conjointement avec de la toxine botulique. Le 5-fluorouracile utilisé avait une concentration de 50 mg/mL, et environ 0,4 mL était infiltré par centimètre de tissu cicatriciel, pour une dose totale de moins de 500 mg. La toxine botulique avait une concentration de 50 UI/mL, pour une dose totale de moins de 140 UI.
RÉSULTATS:
Les patients ont été suivis pendant 17 à 24 mois et ont présenté un taux de récurrence de 3,75 %, ce qui est considérablement plus faible que dans les études antérieures faisant appel à d’autres modalités thérapeutiques.
CONCLUSION:
L’auteur recommande l’utilisation systématique de ce traitement pour toutes les chéloïdes, car il est beaucoup plus efficace que les traitements décrits par d’autres auteurs.
Keloids, described in the Smith papyrus circa 1700 BC, were first discussed by Alibert in 1806. They may result from a variety of cutaneous injuries, inflammatory disorders, burns, trauma or iatrogenic surgical insult (1). Keloids can be differentiated from hypertrophic scars in that excessive scar tissue proliferates beyond the limits of the original lesion (2,3), does not regress over time (1,4) and tends to recur following superficial excision.
Various theories have been advanced to explain the underlying etiology and pathogenesis of keloid formation and, as expected, the less that is known about the pathogenesis of a disease, the more the number of hypotheses suggested to explain it (5). The basic underlying pathology likely lies in excessive proliferation and secretion of abnormal connective tissue fibroblasts, coupled with deficient matrix degradation (6).
Keloid fibroblasts have been found to have intrinsically low levels of plasminogen activator and high levels of inhibitor activity (6), leading to a lower plasmin concentration and a less-than-optimal breakdown of collagen (5–7). Furthermore, when compared with other scars, keloids were found to have higher levels of collagen type III (8–10), indicating high levels of collagen synthesis associated with reduced collagenase activity (11), leading to an increase in collagen content of up to 20-fold (10).
Several growth factors are also involved. Keloid-derived fibroblasts were found to have greater sensitivity to transforming growth factor-beta (TGF-β) (12,13), an increased expression of TGF-β receptors (14), an exaggerated response to platelet-derived growth factor (15) and deficiencies in interferon-alpha and interferon-gamma (16).
Patients with keloids usually seek treatment for cosmetic reasons, pain, pruritis or restriction of motion. Various treatment modalities – used as isolated treatments or in combination – have been suggested, but all with unsatisfactory results, with reported recurrence rates of between 0% and 100% (5). These include surgical excision, whether intralesional or total (1,17), repeated palliative surgical excision (18), prolonged pressure for four to six months (1,19), pressure with magnets (20), repeated intralesional corticosteroid injections (1,19–21), irradiation (22,23), interstitial brachytherapy (24), silicone gel application (25), pulsed-dye (26,27) and carbon dioxide laser therapy (28), cryotherapy (29), and anecdotal applications of formalin, pepsin, anti-fungals, alpha-tocopherol, putrescine and retinoids (1).
Because the pivotal element in the pathogenesis of keloids appears to be the fibroblast, it is only logical to devise therapeutic protocols focusing on inhibiting its effects. One of these involves the use of 5-fluorouracil (5-FU), a pyrimidine analogue with antimetabolite activity that was shown to inhibit fibroblast proliferation in tissue culture and also following trabeculectomy for glaucoma (30). Initially, repeated applications were used; however, it was later found that single topical applications were sufficient to permanently inhibit fibroblast proliferation (31).
Following the same principle, the use of 5-FU was extrapolated to treat keloids and, in a pilot study performed by Uppal et al (32), irrigation of the wound with 5-FU for 5 min during excision of an existing keloid was found to significantly reduce fibroblast activity, as indicated by the reduction in the levels of the immunohistochemical antigens Ki-67, vascular cell adhesion molecule-1 and TGF-β-1, leading to clinical improvement in the five patients involved in that study. Other studies have used repeated injections of 5-FU and reported improvement in keloids (33–35).
Botulinum toxin type A induces chemodenervation through its action on the presynaptic neuron and was used to improve the appearance of wounds (36), and has also been used as a new treatment for keloids (37).
Combining this scientific base, the present study involved 80 patients with keloids that were surgically excised and injected with 5-FU and botulinum toxin nine days later.
METHODS
Between October 2008 and September 2011, 80 patients with documented keloids of longstanding duration (range one to four years; mean 1.3 years) were involved in the present study. Scars <1 years’ duration (1,4) or not extending beyond the limits of the original lesion (2,3) were excluded from the present study, as well as patients with postburn keloids due to the possible effect of burn blister fluid on fibro-blast contraction (38). The uniform complaint of all patients was cosmetic deformity; 46 (57.5%) patients experienced associated troublesome pruritis. Patients’ age ranged from 16 to 42 years (mean 24.7 years); the ratio of males to females was 3:5.
Twenty-six (32.5%) keloids were presternal, 20 (25%) were facial, 14 (17.5%) were on the trunk, 12 (15%) in the ear lobe and eight (10%) on the arm. Iatrogenic surgical incisions accounted for 40 keloids (50%), traumatic cut wounds for 22 (27.5%), ear lobe puncture for 12 (15%) and skin infections for six (7.5%) (Table 1). All keloids had previously undergone therapeutic attempts by other physicians, with more than one modality used in 30 patients. The most commonly used method was intralesional triamcinolone injections (72 patients), topical silicone gel (32 patients), previous surgical excision (30 patients), laser resurfacing (four patients) and irradiation (34 patients).
TABLE 1.
Site and causative factor of keloids managed in the present study
| Site of keloid | Causative factor | n |
|---|---|---|
| Presternal | Surgical incision | 20 |
| Infection | 4 | |
| Cut wounds | 2 | |
| Face | Cut wounds | 12 |
| Surgical incision | 6 | |
| Infection | 2 | |
| Trunk | Surgical incision | 10 |
| Cut wounds | 4 | |
| Ear lobe | Puncture | 12 |
| Arm | Surgical incision | 4 |
| Cut wounds | 4 |
All patients were unhappy with the results of previous treatment(s), and consented to undergo surgery followed by 5-FU and botulinum toxin injections. Under local anesthesia, total extralesional surgical excision (28) of the keloid was performed and, after minimal undermining, primary closure of the wound in layers was achieved in all cases. A running subcuticular prolene 4/0 stitch was used to suture the skin. The wound was left alone for eight days; on the ninth day, wound edges were injected once with 5-FU and botulinum toxin at concentrations of 50 mg/mL and 50 IU/mL, respectively. A 1 mL Luerlock syringe with a 30-gauge needle was used and injection was both intradermal and subdermal. Repeated alternate punctures were used to bathe the wound edges with the drugs. Approximately 0.4 mL of neat 5-FU (50 mg/mL) (33,34) and 20 IU botulinum toxin (50 IU/mL) were infiltrated alternately per cm of wound tissue, with the total dose injected kept below 500 mg and 140 IU, respectively. The wound was painted with 5% lidocaine prilocaine cream (Emla, Astra, Sweden) 45 min before the injection to achieve local anaesthesia. Laboratory tests, before or after the injection (30,31), were unnecessary. The wound was then resealed until postoperative day 14, at which time the subcuticular stitch was removed.
No postoperative medications were given to any of the patients; however, all were advised to avoid direct sun exposure for the following month. No concomitant applications (eg, compression, steroid injections, etc) were used in any of the patients. All patients were reviewed once per month for two years.
RESULTS
The follow-up period ranged from 17 to 24 months (mean 19.6 months) and, in all cases, definite improvement compared with the pretreatment state was apparent. Local adverse reactions included pruritis in eight patients (10%) that gradually subsided over time and with the use of topical antihistaminic creams; pain in seven patients (8.75%); burning sensation in four patients (5%); and residual postinflammatory hyperpigmentation in two patients (2.5%). All of these were evident during the first two to four weeks postinjection, then gradually disappeared over time. Residual redness of the scar was a common problem that occurred in all scars and persisted for almost six months after the injection.
No other complications were encountered in the postoperative period, except in one (1.25%) patient who experienced partial wound dehiscence. The area of gaping was quite small and healed by repeated dressings over a three to six week period, with no need for secondary suture.
Three (3.75%) patients experienced a recurrence: in two patients, the recurrence was incomplete and the extent of keloidal formation was definitely less than in the pretreatment stage; recurrence was complete in one patient with multiple keloids on her back (Figure 1) – in retrospect, however, these were recalled as being sutured under tension.
Figure 1).
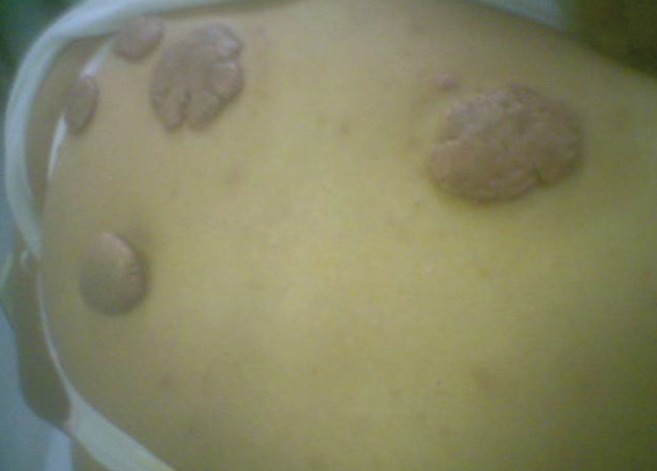
Multiple back keloids in a 29-year-old woman that were sutured under tension and recurred completely
A common complication encountered in 11 (13.75%) keloids was late widening of the scar. This was unattractive to most patients, with one-half opting to undergo corrective surgery at a later date.
Regarding the cosmetic problem, which was the initial complaint of all patients, a previously described subjective scale (39) was used to assess the improvement. Sixty-seven (83.75%) patients rated the improvement as significant, 10 (12.5%) patients as slight and unchanged by the three (3.75%) patients who experienced recurrence. None of the patients rated the appearance to be worse. Representative cases are presented in Figures 1 to 8.
Figure 8).
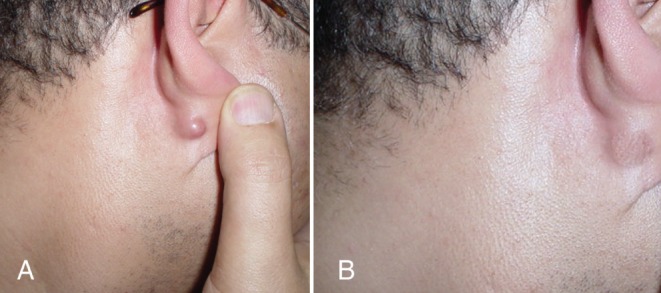
Pretreatment (A) and 21-month post-treatment photograph (B) of an ear lobe keloid following a nonspecified infection
DISCUSSION
In recent decades, multiple studies investigating keloid formation have been conducted, leading to a plethora of therapeutic strategies. However, no clear guidelines have been published, likely due to the poor understanding of the complex underlying mechanisms (5). Published therapeutic modalities involved repeated applications over prolonged periods of time, a process that was cumbersome to both patient and physician. Furthermore, each treatment had its inherent complications and unacceptable recurrence rates. Radiation was insufficient on its own, with recurrence rates of 12.5% to 50%, and posed a risk of malignancy (22,23). Brachytherapy yielded high rates of late recurrence (24) and aesthetic outcome was poor. Intralesional corticosteroids led to the occurrence of telangiectasia, tissue atrophy (1,21), rebound effects and high recurrence rates (27). Repeated debulking surgery was associated with a 100% recurrence rate (18). Cryotherapy required one to 20 sessions (29) and was associated with high recurrence rates. Carbon dioxide laser therapy was tested in small samples and required prolonged treatments (28). Prolonged pressure with magnets and silicone gel sheets had unpredictable outcomes (19,20,25).
In the present study, all of the above-mentioned problems were circumvented following total excision of the keloid, followed by a single application of 5-FU and botulinum toxin; the recurrence rate was only 3.75%. This could be attributed to the specific targeting of the underlying pathophysiology (32–35). Previous studies have demonstrated the inhibitory effect of a single application of 5-FU on fibroblasts, which permanently inhibited all factors promoting keloid formation (30-32). This benefit of a single dose of 5-FU is also attributed to the fact that it was used prophylactically because the keloid was previously excised, contrary to other studies in which previous excision was not performed. The addition of a single application of botulinum toxin would also enhance the inhibitory effect on fibroblasts (7,36).
The treatment protocol in the present study was different from other treatment protocols. Combination of 5-FU with steroids reportedly treated the keloids (40) but repeat injections were required and some untoward effects of steroids were encountered. Addition of pulsed-dye laser to steroid and 5-FU reportedly yielded superior results (27); however, repeated treatments were required and the rates of erythema formation and recurrence were too high. Repeated injections of 5-FU without previous excision improved keloid eradication by 50% but at the cost of higher ulceration, pain, burning sensation, hyperpigmentation, sloughing and higher recurrence (33–35). This is readily explained by the fact that 5-FU merely inhibits fibroblast function in synthesis and secretion of collagen; therefore, it would have no effect on collagen already formed. Thus, existing keloids need to be surgically excised for the 5-FU to have any effect, thus obviating the need for repeat injections in the present study.
5-FU was injected on postoperative day 9 to halt fibroblast function soon after completion of phase 1 of healing but before commencement of phase 2 in which collagen overformation ensues (8,10,39).
There was no need to perform any laboratory tests because only 6% of the topically applied dose of 5-FU is absorbed – a dose insufficient to produce adverse systemic effects (30,31).
Because all keloids in the present series were previously treated by other surgeons, yet all recurred, their preintervention state was considered the control against which the results of our treatment was compared. There was no need in the present study to consider the ethical dilemma of treating only a segment of the keloid and leaving the rest untreated because the results of other treatment modalities were evident in the appearance of the keloid when patients presented for the study.
Regarding the three patients who experienced keloid recurrence, the underlying causative factor was probably increased tension. Not only would it overstimulate fibroblast proliferation and secretion (5,7), but would also make the subsequent injection of 5-FU more guarded (to avoid wound dehiscence). Wound closure without tension appears to be imperative in reducing recurrence rates, and available options may include more extensive undermining or the use of local flaps. Another option would be using alternative techniques in wound closure that reduce dermal tension (39); the author recommends they be used routinely later during the initial stage of keloid resection.
The untoward problems of late scar widening (10%) probably resulted from reduced collagen synthesis and resultant weakening of scar tissue (31,33,34) as a result of excess 5-FU. A delicate balance must be achieved with 5-FU dosing to avoid wound dehiscence and scar widening yet simultaneously to prevent recurrence. Further research and experience in adjusting the dose is needed.
Concomitant use of corticosteroids with 5-FU (40) is not recommended because there would be no scientific rationale to support this combination and, given the above-mentioned complications, would make it unjustifiable. However, topical application of silicone gels after the injection of 5-FU, which the author has started using in a few recent cases (unpublished data), may improve the cosmetic outcome (25).
The 3.75% recurrence rate in the present series is the lowest recurrence rate reported among similar studies (17–29). The author believes that minimizing tension on the wound will further reduce this recurrence rate.
Given the results of the present series, which to the author’s knowledge involved the largest number of patients published to date, it is strongly recommended that 5-FU and botulinum toxin be routinely injected in wounds following resection of keloids.
CONCLUSION
Keloids occasionally complicate wound healing and cause marked disfigurement. Different techniques have been used to treat keloids, but failure and recurrence rates remain unacceptably high. The author has used single injections of 5-FU and botulinum toxin nine days after keloid excision. The permanent inhibitory effect of 5-FU on fibro-blasts following a single application led to a recurrence rate of 3.75%. Given the results of the present study, the authors recommend that 5-FU and botulinum toxin be routinely injected into scar tissue following resection of keloids.
Figure 2).
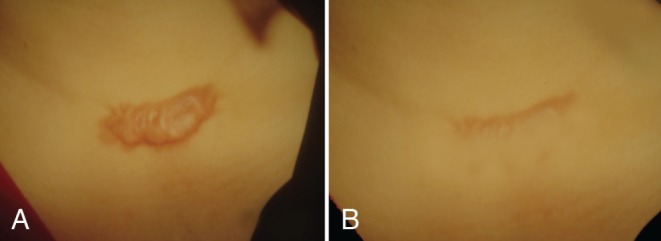
Pretreatment (A) and 18-month post-treatment photographs (B) of a presternal keloid in a 38-year-old woman. Note the resulting linear scar
Figure 3).
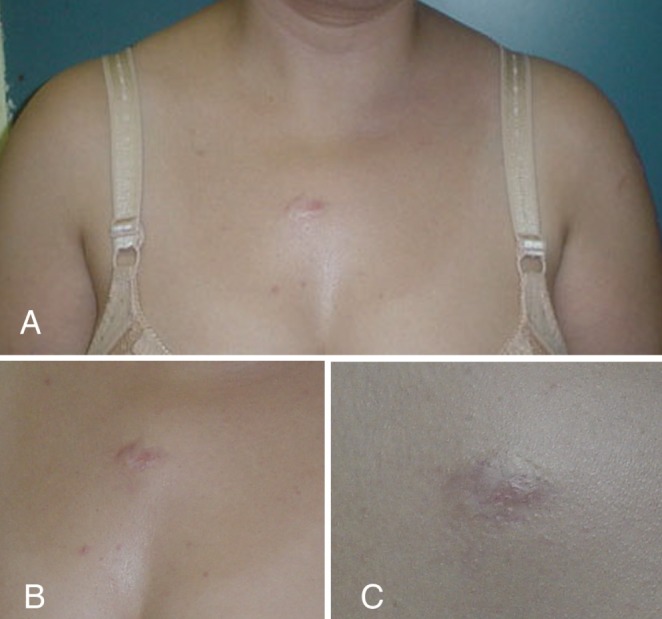
Pretreatment (A) and 19-month post-treatment photographs (B) of a presternal keloid in a 43-year-old woman. Note the complete regression of the keloid indicated by the close-up photograph (C)
Figure 4).
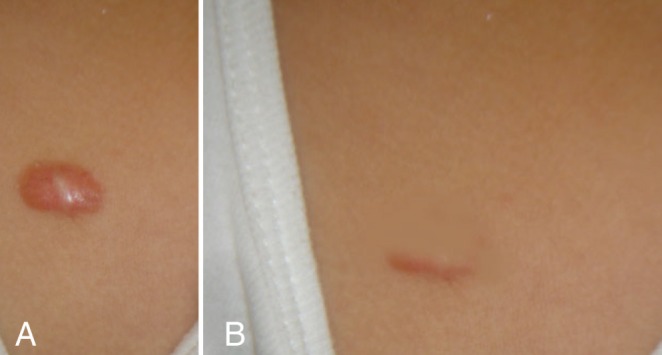
Pretreatment (A) and 18-month post-treatment photographs (B) of a presternal keloid in a 26-year-old patient following a nonspecified childhood infection. Note the complete regression
Figure 5).
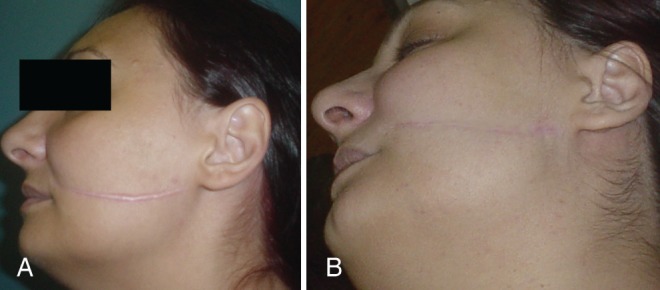
Pretreatment (A) and 19-month post-treatment photgraphs (B) of a keloid in the left cheek of a 22-year-old woman. Note the resulting linear scar
Figure 6).
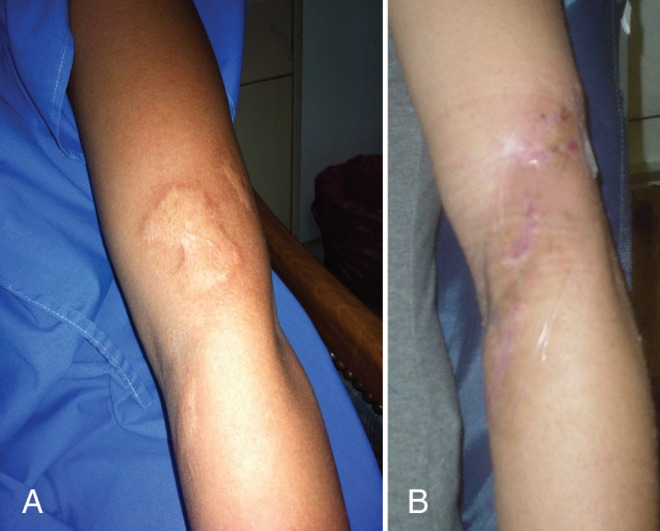
Pretreatment (A) and 17-month post-treatment photographs (B) of a keloid on the left arm of a 17-year-old woman. Note the resulting linear scar, which remains red
Figure 7).
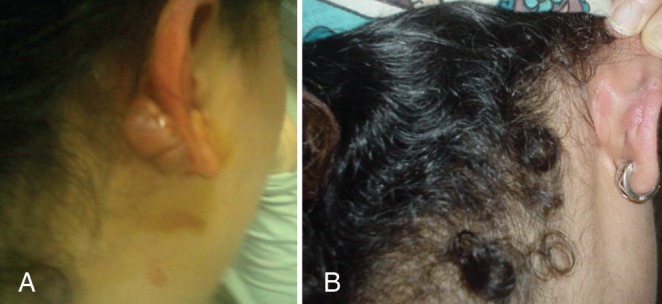
Pretreatment (A) and 24-month post-treatment photographs (B) of an ear lobe keloid. Note that when the ear was pierced again, there was no recurrence
Footnotes
DISCLOSURES: This study was funded by the author, and there are no financial disclosures or conflict of interest to declare. This study was approved by the Ethics Committee of Cairo University and did not violate the Declaration of Helsinki in any way. All patients signed informed consent.
REFERENCES
- 1.Berman B, Bieley HC. Keloids. J Am Acad Dermatol. 1995;33:117–21. doi: 10.1016/0190-9622(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 2.Peacock EE, Jr, Madden JW, Trier WC. Biological basis for the treatment of keloids and hypertrophic scars. South Med J. 1970;63:755–83. doi: 10.1097/00007611-197007000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mancini RE, Quaife JW. Histogenesis of experimentally produced keloids. J Invest Dermatol. 1962;38:142–57. doi: 10.1038/jid.1962.29. [DOI] [PubMed] [Google Scholar]
- 4.Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: A comprehensive review. Plast Reconstr Surg. 1989;84:827–33. doi: 10.1097/00006534-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Tredget EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars, keloids and contractures: The cellular and molecular basis of therapy. Surg Clin North Am. 1997;77:701–39. doi: 10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 6.Tuan TL, Zhu JY, Sun B, Nichter LS, Nimni ME, Laug WE. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol. 1996;106:1007–10. doi: 10.1111/1523-1747.ep12338552. [DOI] [PubMed] [Google Scholar]
- 7.Kischer CW, Thies AC, Chvapil M. Perivascular myofibroblasts and microvascular occlusion in hypertrophic scars and keloids. Hum Pathol. 1982;13:819–23. doi: 10.1016/s0046-8177(82)80078-6. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DW, Boyd CD, Mackenzie JW, Norton P, Olson RM, Deak SB. Regulation of collagen gene expression in keloids and hypertrophic scars. J Surg Res. 1993;55:214–9. doi: 10.1006/jsre.1993.1132. [DOI] [PubMed] [Google Scholar]
- 9.Uitto J, Perejda AJ, Abergel RP, Chu ML, Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci USA. 1985;82:5935–41. doi: 10.1073/pnas.82.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abergel RP, Pizzurro D, Meeker CA, et al. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985;84:384–8. doi: 10.1111/1523-1747.ep12265471. [DOI] [PubMed] [Google Scholar]
- 11.McCoy BJ, Cohen IK. Collagenase in keloid biopsies and fibroblasts. Connect Tissue Res. 1982;9:181–5. doi: 10.3109/03008208209160259. [DOI] [PubMed] [Google Scholar]
- 12.Russell SB, Trupin KM, Rodriguez Eaton S, Russell JD, Trupin JS. Reduced growth-factor requirement of keloid-derived fibroblasts may account for tumor growth. Proc Natl Acad Sci USA. 1988;85:587–91. doi: 10.1073/pnas.85.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner WL, Karmiol S, Rodriguez JL, Smith DJ, Jr, Phan SH. Phenotypic differences in cytokine responsiveness of hypertrophic scars versus normal dermal fibroblasts. J Invest Dermatol. 1993;101:875–80. doi: 10.1111/1523-1747.ep12371710. [DOI] [PubMed] [Google Scholar]
- 14.Chin GS, Liu W, Peled Z, et al. Differential expression of transforming growth factor-B receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423–8. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Haisa M, Okochi H, Grotendorst GR. Elevated levels of PDGF alpha receptors in keloid fibroblasts contribute to an enhanced response to PDGF. J Invest Dermatol. 1994;103:560–6. doi: 10.1111/1523-1747.ep12396856. [DOI] [PubMed] [Google Scholar]
- 16.Larrabee WF, Jr, East CA, Jaffe HS, Stephenson C, Peterson KE. Intralesional interferon gamma treatment for keloids and hypertrophic scars. Arch Otolaryngol Head Neck Surg. 1990;116:1159–64. doi: 10.1001/archotol.1990.01870100053011. [DOI] [PubMed] [Google Scholar]
- 17.Cosman B, Wolff M. Correlation of keloid recurrence with completeness of local excision: A negative report. Plast Reconstr Surg. 1972;50:163–6. doi: 10.1097/00006534-197208000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Marsh DJ, Pacifico MD, Gault DT. Keloids: When excision is the better part of valor. Plast Reconstr Surg. 2008;121:214–5. doi: 10.1097/01.prs.0000305379.97906.68. [DOI] [PubMed] [Google Scholar]
- 19.Page RE, Robertson GA, Pettigrew NM. Microcirculation in hypertrophic burn scars. Burns Incl Therm Inj. 1983;10:64–8. doi: 10.1016/0305-4179(83)90130-4. [DOI] [PubMed] [Google Scholar]
- 20.Park TH, Seo SW, Kim JK, Chang CH. Outcomes of surgical excision with pressure therapy using magnets and identification of risk factors for recurrent keloids. Plast Reconstr Surg. 2011;128:431–6. doi: 10.1097/PRS.0b013e31821e7006. [DOI] [PubMed] [Google Scholar]
- 21.Jemec GB. Linear atrophy following intralesional steroid injections. J Dermatol Surg Oncol. 1988;14:88–92. doi: 10.1111/j.1524-4725.1988.tb03345.x. [DOI] [PubMed] [Google Scholar]
- 22.Doornbos JF, Stoffel TJ, Hass AC, et al. The role of kilovoltage irradiation in the treatment of keloids. Int J Radiat Oncol Biol Phys. 1990;18:833–9. doi: 10.1016/0360-3016(90)90405-9. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa R, Yoshitatsu S, Yoshida K, Miyashita T. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–7. doi: 10.1097/PRS.0b013e3181b5a3ae. [DOI] [PubMed] [Google Scholar]
- 24.Emmanuel R, Etienne B, Patrick P, Michel P, Brigitte D. Perioperative interstitial brachytherapy for recurrent keloid scars. Plast Reconstr Surg. 2009;124:180–1. doi: 10.1097/PRS.0b013e3181a83b7e. [DOI] [PubMed] [Google Scholar]
- 25.Fulto JE., Jr Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatol Surg. 1995;21:947–55. doi: 10.1111/j.1524-4725.1995.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 26.Dierickx C, Goldman MP, Fitzpatrick RE. Laser treatment of erythematous/hyertrophic and pigmented scars in 26 patients. Plast Reconstr Surg. 1995;95:84–90. [PubMed] [Google Scholar]
- 27.Asilian A, Darougheh A, Shariati F. New combination of triamcinolone, 5-fluorouracil, and pulsed-dye laser for treatment of keloid and hypertrophic scars. Dermatol Surg. 2006;32:907–12. doi: 10.1111/j.1524-4725.2006.32195.x. [DOI] [PubMed] [Google Scholar]
- 29.Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111:1841–52. doi: 10.1097/01.PRS.0000056868.42679.05. [DOI] [PubMed] [Google Scholar]
- 30.The Fluorouracil Filtering Surgery Study Group: 3-year follow-up of the fluorouracil filtering surgery study. Am J Ophthalmol. 1993;115:82–8. doi: 10.1016/s0002-9394(14)73529-9. [DOI] [PubMed] [Google Scholar]
- 31.Lanignan L, Sturmer J, Baez KA, Hitchings RA, Khaw PT. Single intraoperative applications of 5-fluorouracil during filteration. Br J Ophthalmol. 1994;78:33–40. doi: 10.1136/bjo.78.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uppal RS, Khan Kakar S, Talas G, Chapman P, McGrouther DA. The effects of a single dose of 5-fluorouracil on keloid scars: A clinical trial of timed wound irrigation after extralesional excision. Plast Reconstr Surg. 2001;108:1218–24. doi: 10.1097/00006534-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick R. Treatment of inflamed hypertrophic scars using intralesional 5-FU. Dermatol Surg. 1999:25224–30. doi: 10.1046/j.1524-4725.1999.08165.x. [DOI] [PubMed] [Google Scholar]
- 34.Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, et al. Intralesional 5-fluorouracil in the treatment of keloids: An open clinical and histopathologic study. J Am Acad Dermatol. 2005;52:474–81. doi: 10.1016/j.jaad.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Nanda S, Reddy BS. Intralesional 5-fluorouracil as a treatment modality of keloids. Dermatol Surg. 2004;30:54–60. doi: 10.1111/j.1524-4725.2004.29382.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AM. Use of botulinum toxin type A to prevent widening of facial scars. Plast Reconstr Surg. 2006;117:1758–66. doi: 10.1097/01.prs.0000209944.45949.d1. [DOI] [PubMed] [Google Scholar]
- 37.Zhibo X, Miaobo Z. Intralesional botulinum toxin type A injection as a new treatment measure for keloids. Plast Reconstr Surg. 2009;124:276–7. doi: 10.1097/PRS.0b013e3181b98ee7. [DOI] [PubMed] [Google Scholar]
- 38.Wilson AM, McGrouther DA, Eastwood M, Brown RA. The effect of burn blister fluid on fibroblast contraction. Burns. 1997;23:306–12. doi: 10.1016/s0305-4179(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 39.Wilson AM. Widening of scars: Foe coaxed into friend? Plast Reconstr Surg. 2000;106:1488–93. doi: 10.1097/00006534-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Davison SP, Dayan JH, Clemens MW, Sonni S, Wang A, Crane A. Efficacy of intralesional 5-fluorouracil and triamcinolone in the treatment of keloids. Aesthetic Surg J. 2009;29:40–6. doi: 10.1016/j.asj.2008.11.006. [DOI] [PubMed] [Google Scholar]


